Chitosan-Based Gastric Dressing Materials Loaded with Pomegranate Peel as Bioactive Agents: Pharmacokinetics and Effects on Experimentally Induced Gastric Ulcers in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Gelatin
2.2. The Gastric Dressing (CH-GEL-PP) Formulation
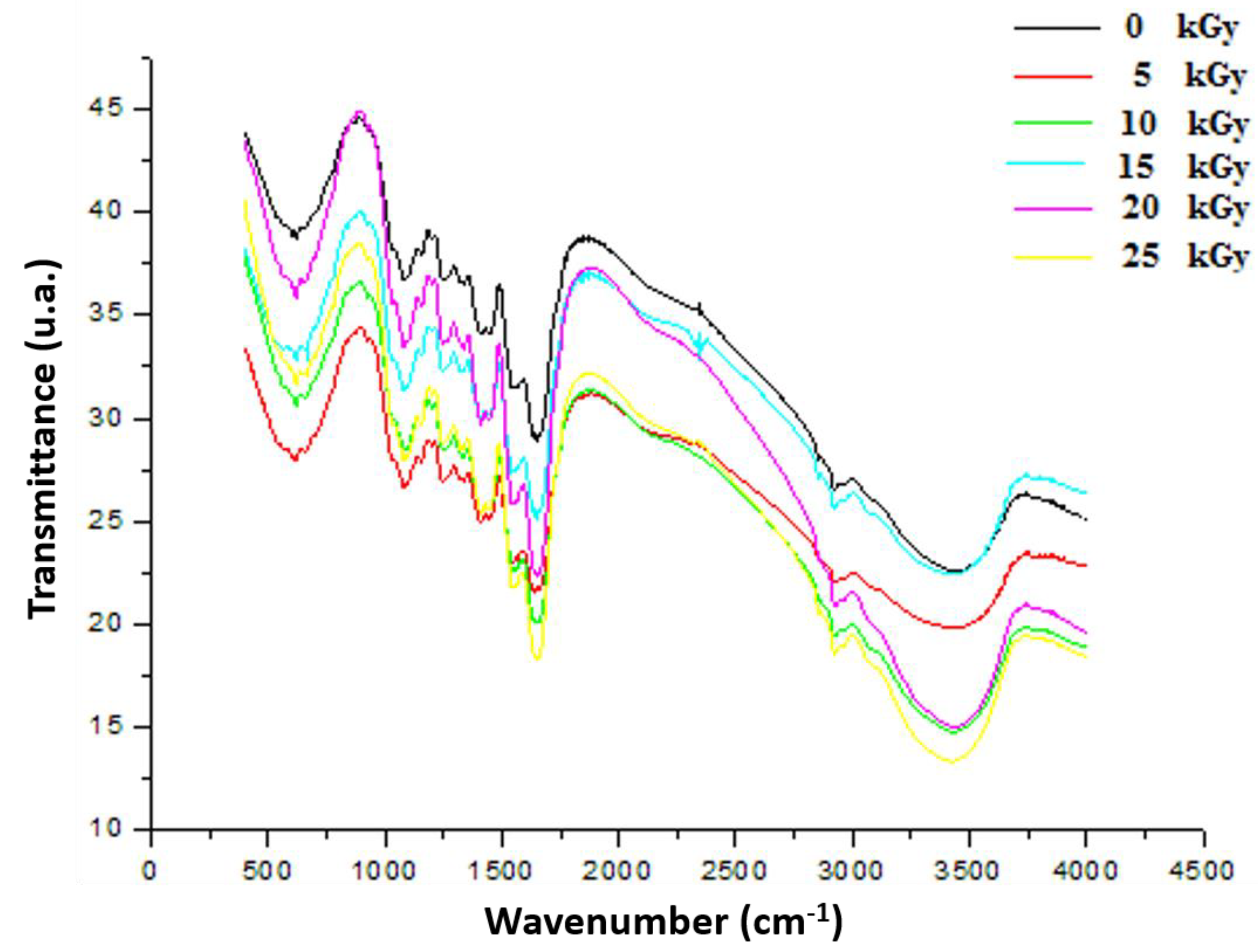
2.3. Fourier Transform Infrared (FTIR)
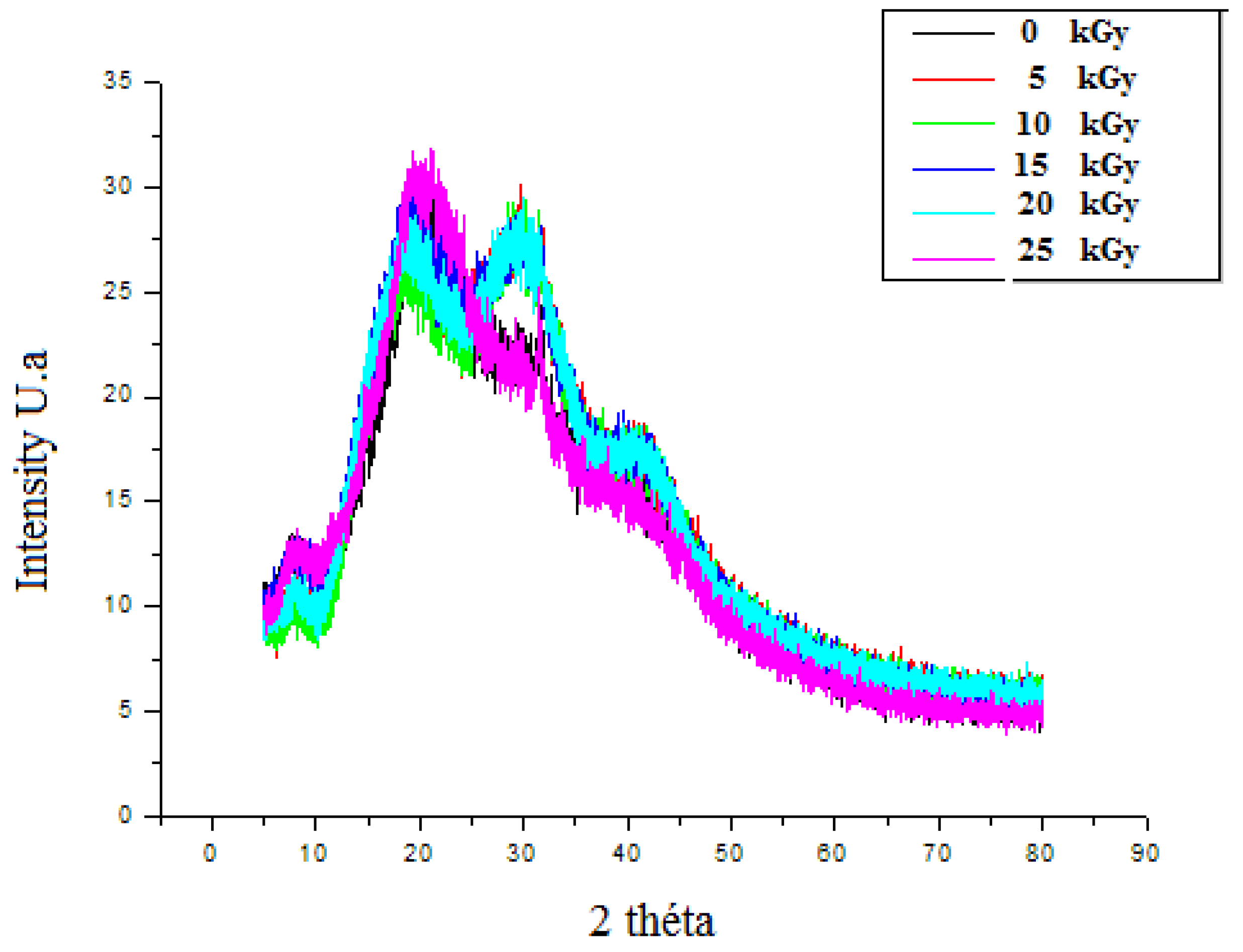
2.4. X-ray Diffraction (XRD)
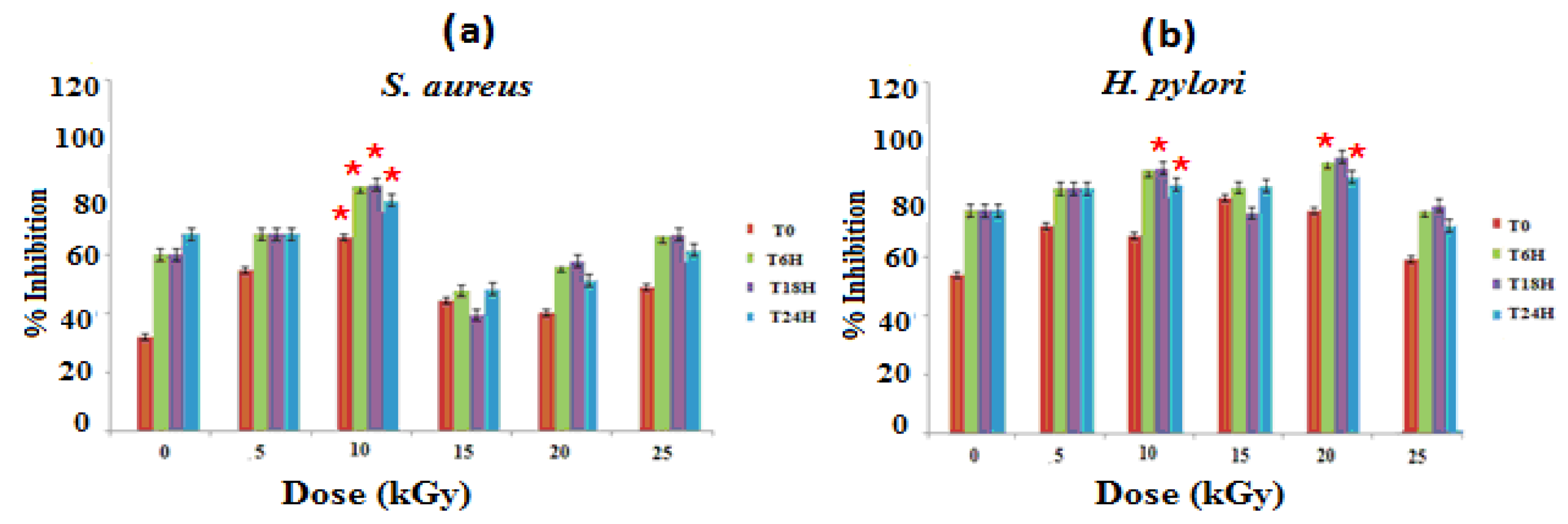
2.5. Antimicrobial Activity Assay
2.5.1. Microbial Strains
2.5.2. Liquid Medium Test
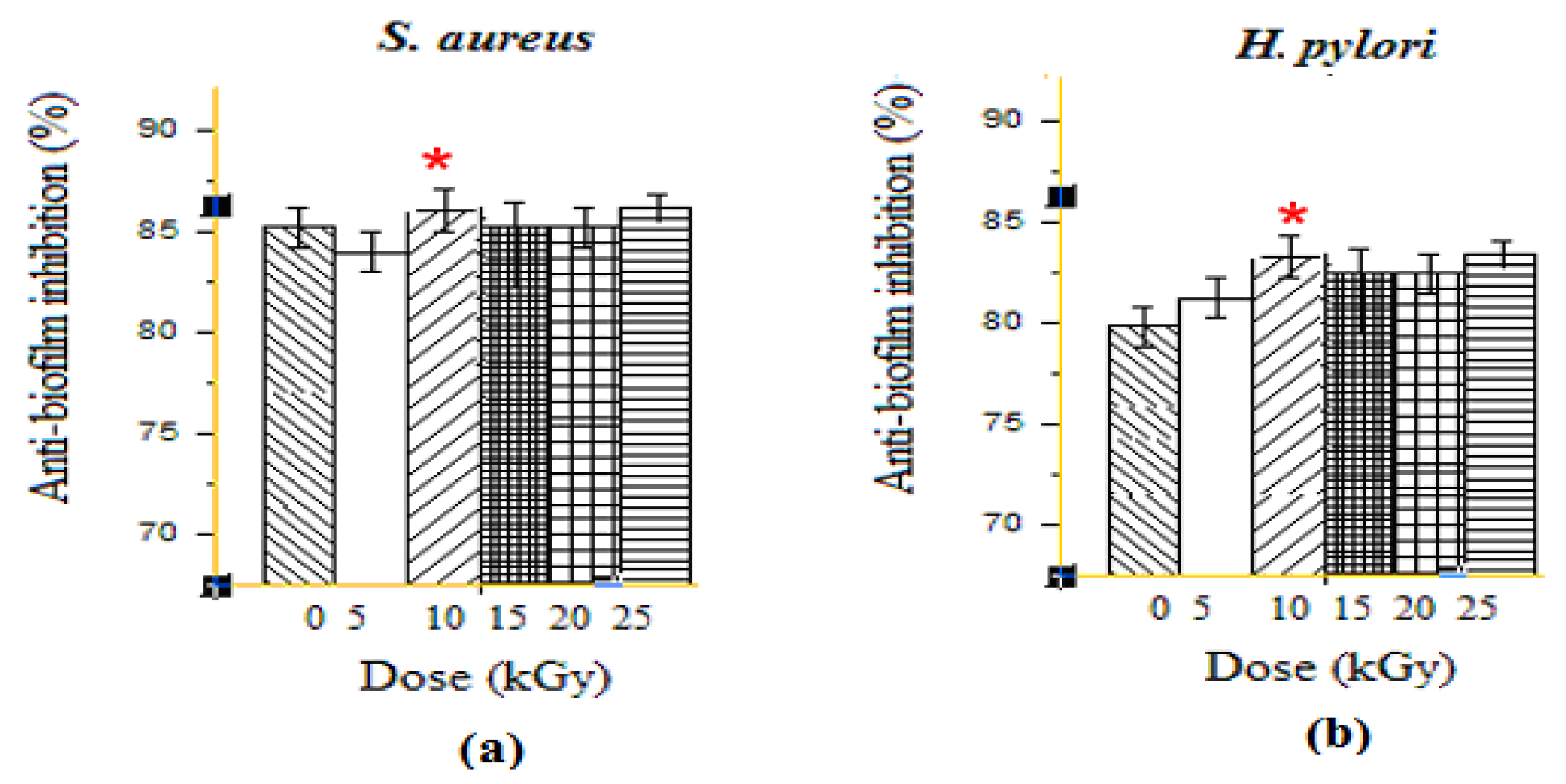
2.6. Cristal Violet Biofilm Assay
2.7. Antioxidant Activities
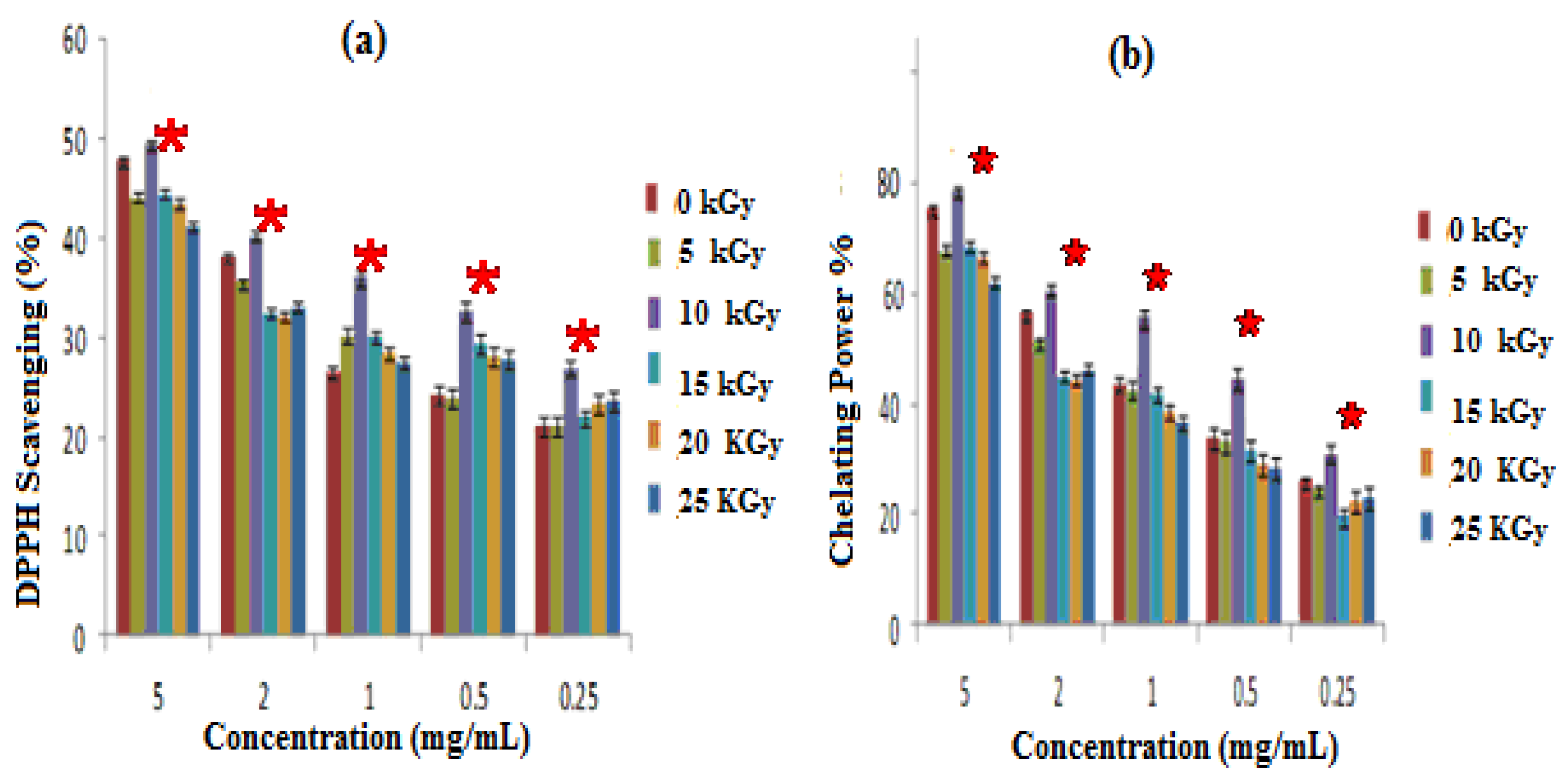
2.7.1. DPPH Radical-Scavenging Activity
2.7.2. Ferrous Chelating Activity
2.8. Hemolysis
2.9. Blood Anticoagulant Index
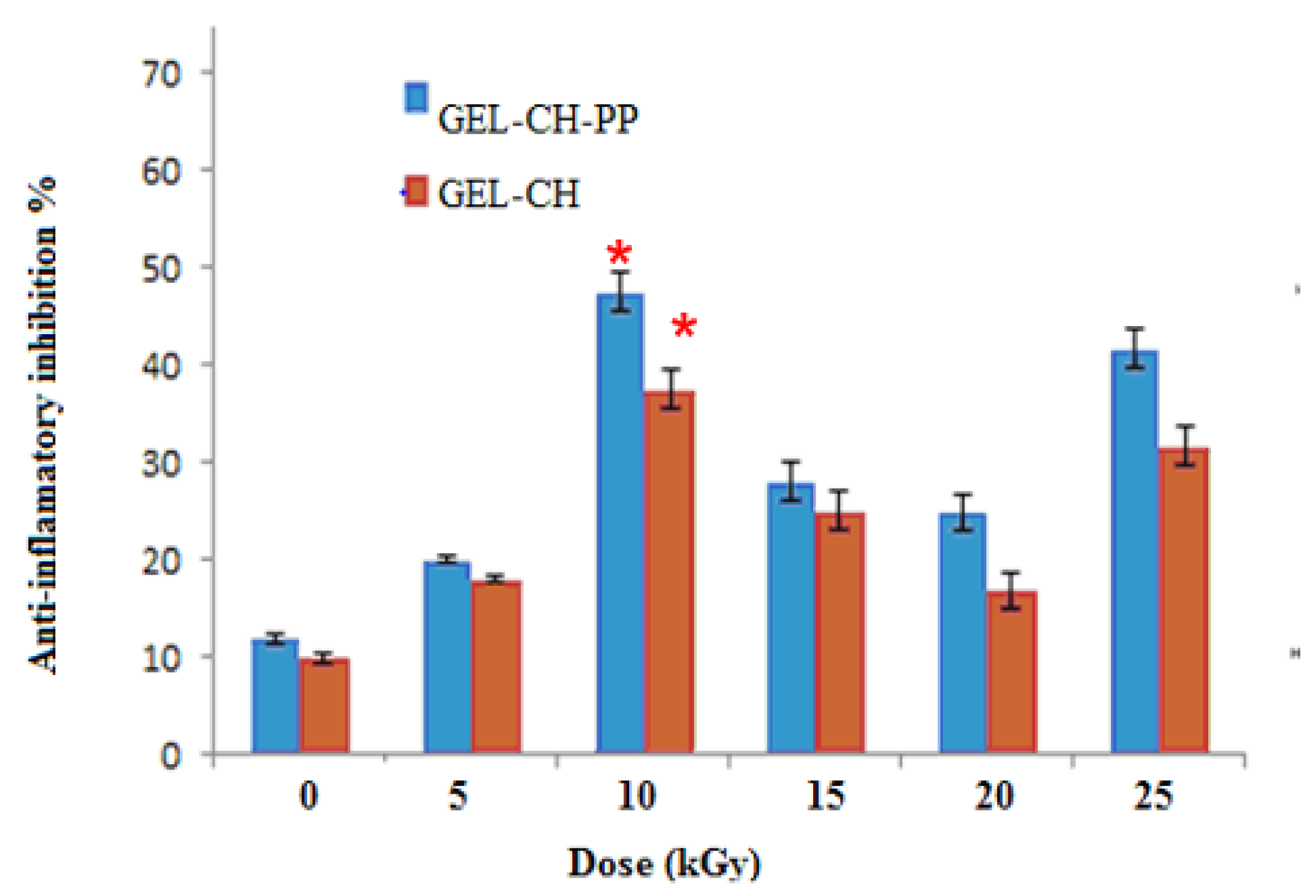
2.10. Evaluation of In Vitro Anti-Inflammation Activity
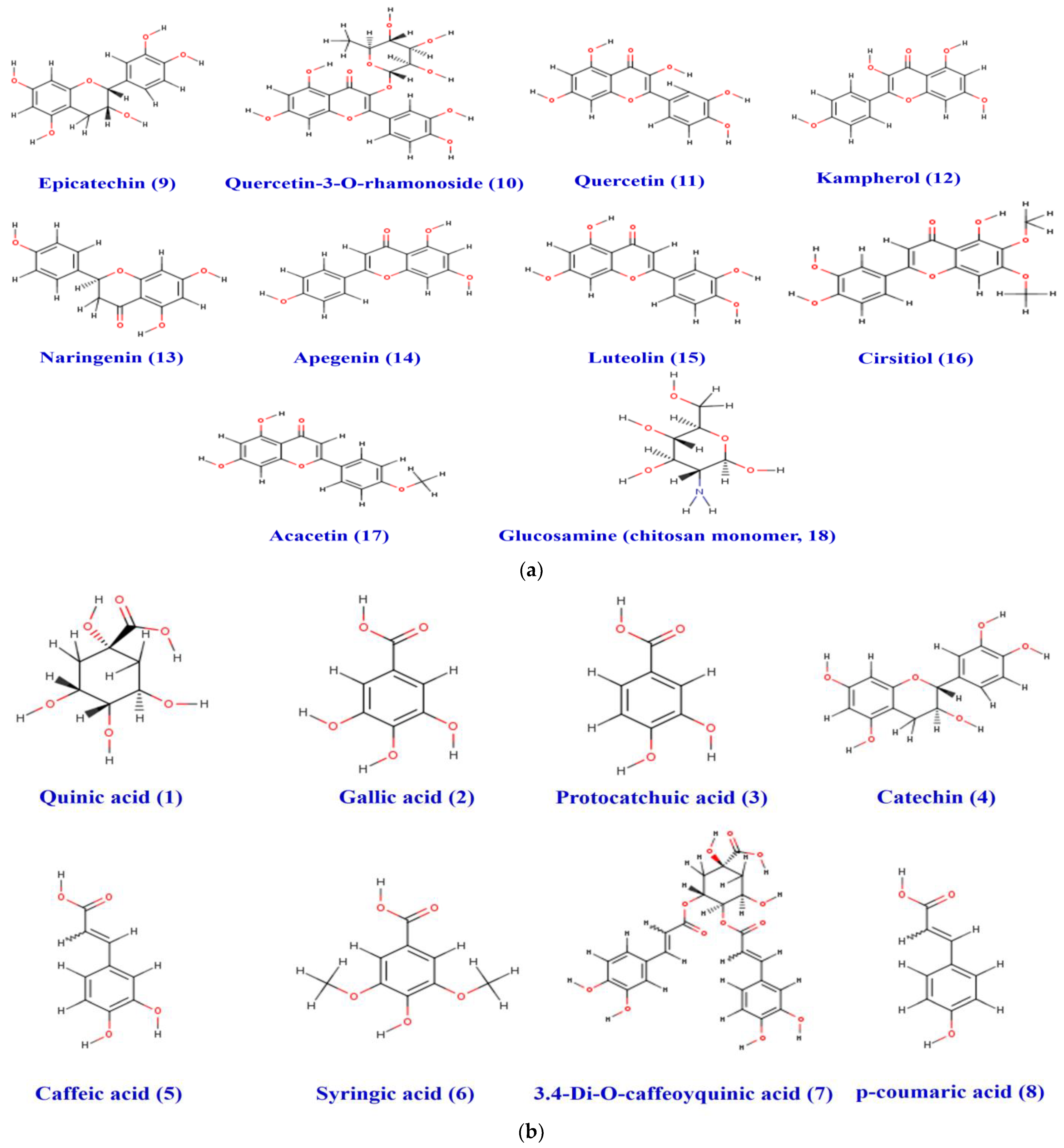
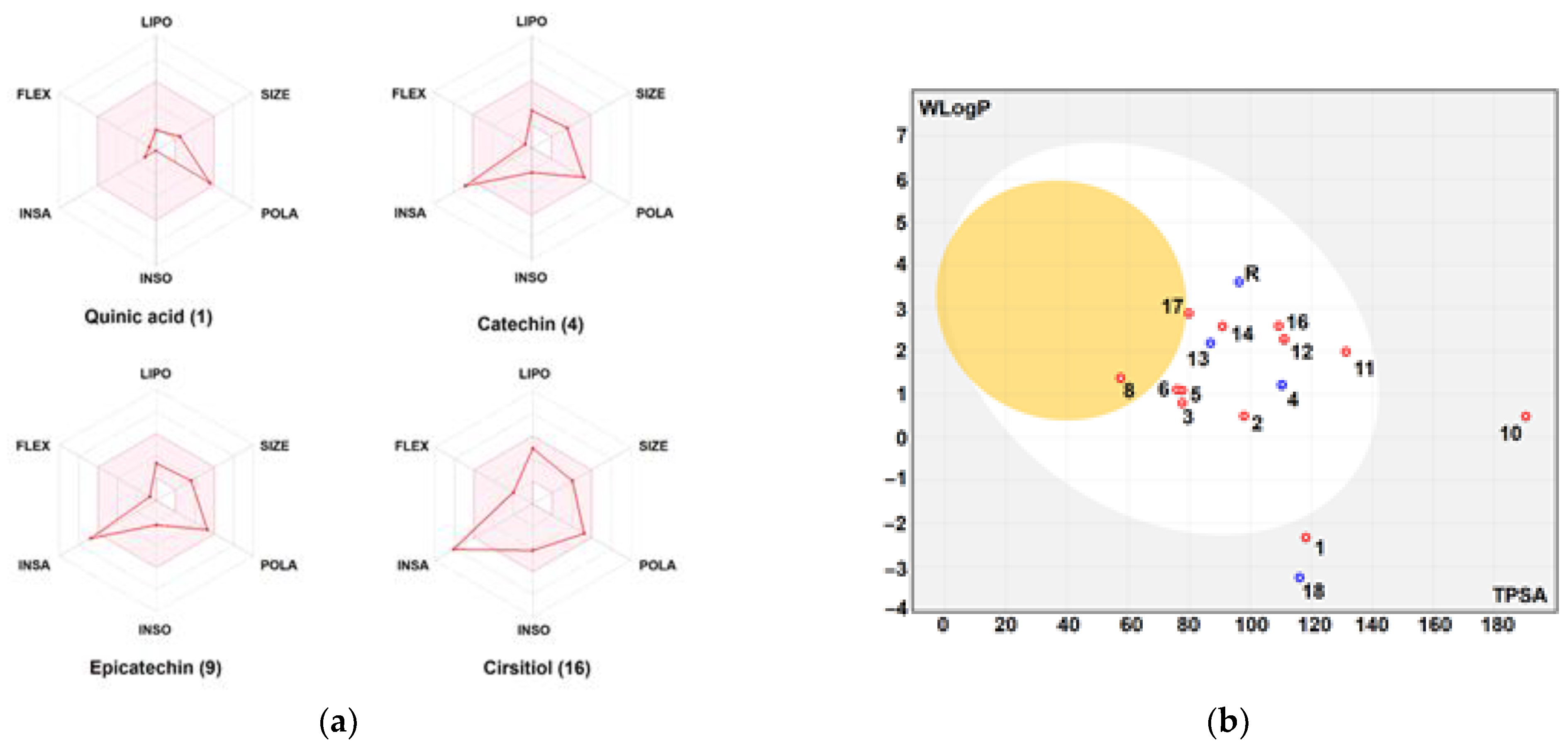
2.11. Drug-Likeness and Pharmacokinetics Study
2.12. In Vivo Study Animal Model
2.12.1. Rabbits Manipulation and Ethical Considerations
2.12.2. Induction of Gastric Ulcer and Experimental Design
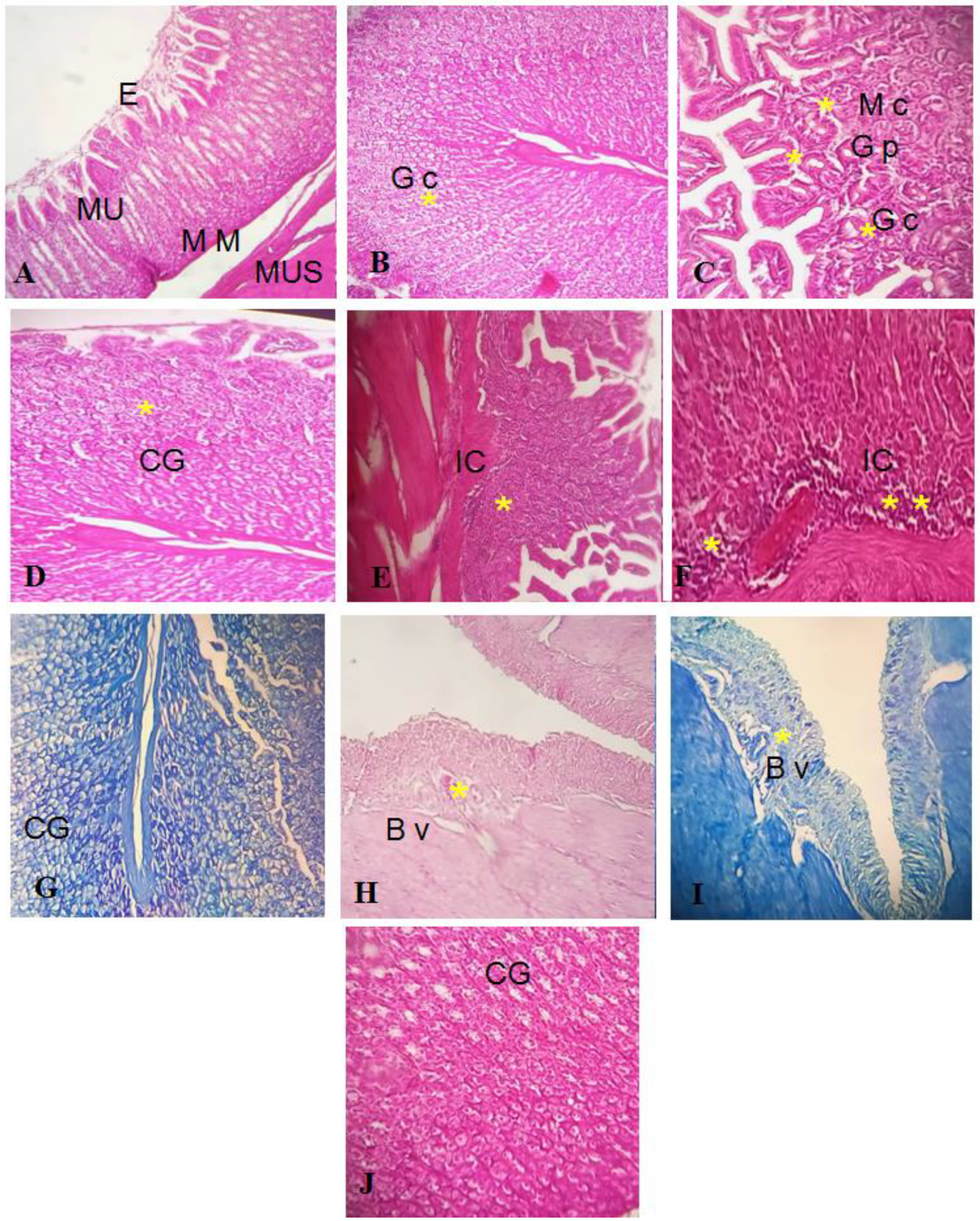
2.12.3. Histological Studies
2.13. Statistical Analyses
3. Results
3.1. In Vitro Biological Characterization
3.1.1. Antibacterial Activities
3.1.2. Anti-Biofilm Activities
3.1.3. Antioxidant Activities
- Antioxidant activity assessed by DPPH radical scavenging test
- Iron-chelating ability Iron (FeII) Chelation
3.2. Biocompatibility
3.2.1. Anti-Hemolytic Activities
3.2.2. In Vitro Anti-Inflammation Assays
3.3. Physico-Chemical Analyses
3.3.1. Electron Paramagnetic Resonance Analysis (EPR)
3.3.2. Fourier Transform Infrared (FTIR)
3.3.3. The X-ray Diffraction (XRD)
3.4. Druggability and Pharmacokinetics of Gastric Dressing GEL–CH–PP
3.5. In Vivo Study
Effect of GEL–CH–PP Dressing on Acid Acetic-Induced Histopathological Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vale, F.F.; Oleastro, M. Overview of the phytomedicine approaches against Helicobacter pylori. World J. Gastroenterol. 2014, 20, 5594–5609. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.; Peng, J.; Zhang, Z.; Jiang, D.J.; Li, Y.J. Role of endogenous nitric oxide synthase inhibitor in gastric mucosal injury. Can. J. Physiol. Pharmacol. 2008, 86, 97–104. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Lu, B.; Dai, J. Helicobacter pylori and Antibiotic Resistance, A Continuing and Intractable Problem. Helicobacter 2016, 21, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Jebahi, S.; Oudadessse, H.; Saoudi, M.; Kallabi, F.; Pascal, P.; Rebai, T.; Elfeki, A.; Keskes, H. Cytocompatibility, gene-expression profiling, apoptotic, mechanical and 29Si, 31P solid-state nuclear magnetic resonance studies following treatment with a bioglass-chitosan composite. Biotechnol. Lett. 2014, 36, 2571–2579. [Google Scholar] [CrossRef]
- Jebahi, S.; Ben Salah, G.; Saoudi, M.; Besaleh, S.; Oudadesse, H.; Mhadbi, M.; Rebai, T.; Keskes, H.; El Feki, A. Genotoxicity effect, antioxidant and biomechanical correlation: Experimental study of agarose-chitosan bone graft substitute in New Zealand white rabbit model. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 800–809. [Google Scholar] [CrossRef]
- Jebahi, S.; Saoudi, M.; Farhat, L.; Oudadesse, H.; Rebai, T.; Kabir, A.; El Feki, A.; Keskes, H. Effect of novel curcumin-encapsulated chitosan-bioglass drug on bone and skin repair after gamma radiation: Experimental study on a Wistar rat model. Cell Biochem. Funct. 2015, 33, 150–159. [Google Scholar] [CrossRef]
- Kim, G.; Dasagrandhi, C.; Kang, E.H.; Eom, S.H.; Kim, Y.M. In vitro antibacterial and early stage biofilm inhibitory potential of an edible chitosan and its phenolic conjugates against Pseudomonas aeruginosa and Listeria monocytogenes. 3 Biotech 2018, 8, 439. [Google Scholar] [CrossRef]
- Tripathi, S.; Singh, B.N.; Singh, D.; Kumar, G.; Srivastava, P. Optimization and evaluation of ciprofloxacin-loaded collagen/chitosan scaffolds for skin tissue engineering. 3 Biotech 2021, 11, 160. [Google Scholar] [CrossRef]
- Haque, M.A.; Hossain, M.S.; Sayed, N.M.A.; Islam, M.T.; Khan, M.R.; Ahmmed, F.; Zohora, F.T.; Ağagündüz, D.; Ming, L.C.; Capasso, R. Abelmoschus esculentus (L.) Moench Pod Extract Revealed Antagonistic Effect against the Synergistic Antidiabetic Activity of Metformin and Acarbose upon Concomitant Administration in Glucose-Induced Hyperglycemic Mice. Biologics 2022, 2, 128–138. [Google Scholar] [CrossRef]
- Akacha, A.; Badraoui, R.; Rebai, T.; Zourgui, L. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: A biochemical, docking and histological study. J. Biomol. Struct. Dyn. 2022, 40, 4341–4351. [Google Scholar] [CrossRef]
- Mechchate, H.; Ouedrhiri, W.; Es-safi, I.; Amaghnouje, A.; Jawhari, F.z.; Bousta, D. Optimization of a New Antihyperglycemic Formulation Using a Mixture of Linum usitatissimum L., Coriandrum sativum L., and Olea europaea var. sylvestris Flavonoids: A Mixture Design Approach. Biologics 2021, 1, 9. [Google Scholar] [CrossRef]
- Badraoui, R.; Rebai, T.; Elkahoui, S.; Alreshidi, M.; Veettil, V.N.; Noumi, E.; Al-Motair, K.A.; Aouadi, K.; Kadri, A.; De Feo, V.; et al. Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study. Antioxidants 2020, 9, 1003. [Google Scholar] [CrossRef]
- Bouasla, A.; Bouasla, I.; Boumendjel, A.; Abdennour, C.; El Feki, A.; Messarah, M. Prophylactic effects of pomegranate (Punica granatum) juice on sodium fluoride induced oxidative damage in liver and erythrocytes of rats. Can. J. Physiol. Pharmacol. 2016, 94, 709–718. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Saftić Martinović, L.; Malenica, M.; Gobin, I.; Pedisić, S.; Dragović-Uzelac, V.; Kraljević Pavelić, S. Assessment of the Biological Activity and Phenolic Composition of Ethanol Extracts of Pomegranate (Punica granatum L.) Peels. Molecules 2020, 25, 5916. [Google Scholar] [CrossRef]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Lim, M.M.; Sultana, N. In vitro cytotoxicity and antibacterial activity of silver-coated electrospun polycaprolactone/gelatine nanofibrous scaffolds. 3 Biotech 2016, 6, 211. [Google Scholar] [CrossRef]
- Sharma, A.; Mittal, A.; Puri, V.; Kumar, P.; Singh, I. Curcumin-loaded, alginate-gelatin composite fibers for wound healing applications. 3 Biotech 2020, 10, 464. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; Abd El Aziz, A.E.; Abdel Raouf, B.M.; Mohamed, S.A.; Nada, D. Antioxidant-biocompatible and stable catalase-based gelatin-alginate hydrogel scaffold with thermal wound healing capability: Immobilization and delivery approach. 3 Biotech 2022, 12, 73. [Google Scholar] [CrossRef]
- Sakai, S.; Hashimoto, I.; Kawakami, K. Agarose-gelatin conjugate for adherent cell-enclosing capsules. Biotechnol. Lett. 2007, 29, 731–735. [Google Scholar] [CrossRef]
- Frasca, G.; Cardile, V.; Puglia, C.; Bonina, C.; Bonina, F. Gelatin tannate reduces the proinflammatory effects of lipopolysaccharide in human intestinal epithelial cells. Clin. Exp. Gastroenterol. 2012, 5, 61–67. [Google Scholar]
- Vargas, M.H.; Del-Razo-Rodríguez, R.; López-García, A.; Lezana-Fernández, J.L.; Chávez, J.; Furuya, M.E.Y.; Marín-Santana, J.C. Effect of oral glycine on the clinical, spirometric and inflammatory status in subjects with cystic fibrosis: A pilot randomized trial. BMC Pulm. Med. 2017, 17, 206. [Google Scholar] [CrossRef] [PubMed]
- Escárcega-Galaz, A.A.; Cruz-Mercado, J.L.; López-Cervantes, J.; Sánchez-Machado, D.I.; Brito-Zurita, O.R.; Ornelas-Aguirre, J.M. Chitosan treatment for skin ulcers associated with diabetes. Saudi J. Biol. Sci. 2018, 25, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, P.; Zang, J.; Liu, J. Enhanced hemostatic performance of tranexamic acid-loaded chitosan/alginate composite microparticles. J. Biomed. Biotechnol. 2012, 2012, 981321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro antiinflammatory activity of coffee against the denaturation of protein. Asian Pac. J Trop. Biomed. 2012, 2, 178–180. [Google Scholar] [CrossRef]
- Badraoui, R.; Adnan, M.; Bardakci, F.; Alreshidi, M.M. Chloroquine and Hydroxychloroquine Interact Differ-ently with ACE2 Domains Reported to Bind with the Coronavirus Spike Protein: Mediation by ACE2 Polymorphism. Molecules 2021, 26, 673. [Google Scholar] [CrossRef]
- Mhadhbi, N.; Issaoui, N.; Hamadou, W.S.; Alam, J.M.; Elhadi, A.S.; Adnan, M.; Naϊli, H.; Badraoui, R. Physico-Chemical Properties, Pharmacokinetics, Molecular Docking and In-Vitro Pharmacological Study of a Cobalt (II) Complex Based on 2-Aminopyridine. ChemistrySelect 2022, 7, 3. [Google Scholar] [CrossRef]
- Jebahi, S.; Oudadesse, H.; El Feki, A.; Keskes, H.; Rebai, T.; El Feki, H. Repair of bone defect using bioglass-chitosan as pharmaceutical drug; experimental study in ovariectomised rat model. Afr. J. Pharm. Pharmacol. 2012, 6, 1276–1287. [Google Scholar] [CrossRef]
- Costa, P.; Somensi, L.B.; da Silva, R.C.M.V.A.F.; Mariano, L.N.B.; Boeing, T.; Longo, B.; Perfoll, E.; de Souza, P.; Gushiken, L.F.s.; Pellizzon, C.H.; et al. Role of the antioxidant properties in the gastroprotective and gastric healing activity promoted by Brazilian green propolis and the healing efficacy of Artepillin C. Inflammopharmacology 2020, 28, 1009–1025. [Google Scholar] [CrossRef]
- Svistunenko, D.A. An EPR study of the peroxyl radicals induced by hydrogen peroxide in the haem proteins. Biochim. Biophys. Acta 2001, 1546, 365–378. [Google Scholar] [CrossRef]
- Vidal, C.M.; Zhu, W.; Manohar, S.; Aydin, B.; Keiderling, T.A.; Messersmith, P.B.; Bedran-Russo, A.K. Collagen-collagen interactions mediated by plant-derived proanthocyanidins: A spectroscopic and atomic force microscopy study. Acta Biomater. 2016, 41, 110–118. [Google Scholar] [CrossRef]
- De Paula, G.A.; Costa, N.N.; da Silva, T.M.; Bastos, K.A.; Ignacchiti, M.D.C.; Severi, J.A.; Oréfice, R.L.; Carreira, L.G.; Villanova, J.C.O.; Resende, J.A. Polymeric film containing pomegranate peel extract as a promising tool for the treatment of candidiasis. Nat. Prod. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Jebahi, S.; Saoudi, M.; Badraoui, R.; Rebai, T.; Oudadesse, H.; Ellouz, Z.; Keskes, H.; El Feki, A.; El Feki, H. Biologic Response to Carbonated Hydroxyapatite Associated with Orthopedic Device: Experimental Study in a Rabbit Mode. Korean J. Pathol. 2012, 46, 48–54. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. Evaluation of the information content in infrared spectra for protein secondary structure determination. Biophys J. 2006, 90, 2946–2957. [Google Scholar] [CrossRef]
- Bessalah, S.; Jebahi, S.; Faraz, A.; Raoufi, A.; Tırınk, C.; Dridi, W.; Daouad, M.; Keskes, H.; Khorchani, T.; Farah, K.; et al. Effect of Gamma Radiation on Novel Gelatin Extracted from Camel Skin for Pharmaceutical Application. Pak. J. Zool. 2022, 1–12. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Das, H.; Mukherjee, A.; Kundu, R. Nanopriming with zero-valent iron synthesized using pomegranate peel waste: A "green" approach for yield enhancement in Oryza sativa L. cv. Gonindobhog. Plant Physiol. Biochem. 2021, 163, 261–275. [Google Scholar] [CrossRef]
- Dziedzic, S. Polyhydroxy chalcones and flavanones as antioxidants for edible oils. Food Chem. 1983, 12, 205–212. [Google Scholar] [CrossRef]
- Jing, L.; Durand, E.M.; Ezzio, C.; Pagliuca, S.M.; Zon, L.I. In situ hybridization assay-based small molecule screening in zebrafish. Curr. Protoc. Chem. Biol. 2012, 4, 110236. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Und-Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Flavonoids Other Polyphen. 2001, 335, 190–203. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Pourahmad, J.; Bui, H.; Siraki, A.; O’Brien, P.J. Dietary flavonoid iron complexes as cytoprotective superoxide radical scavengers. Free Radic. Biol. Med. 2003, 34, 243–253. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structureantioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Van Besien, E.; Milhazes, N.; Borges, F.; Marques, M.P.M. Conformational analysis of a trihydroxylated derivative of cinnamic acid –a combined Raman spectroscopy and ab initio study. J. Mol. Struct. 2004, 693, 103–118. [Google Scholar] [CrossRef]
- Marinova, E.M.; Yanishlieva, N.V. Effect of temperature on the antioxidative action of inhibitors in lipid autoxidation. J. Sci. Food Agric. 1992, 60, 313–318. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Haile, T.G.; Sibhat, G.G.; Molla, F. Physicochemical Characterization of Grewia ferruginea Hochst. ex A. Rich Mucilage for Potential Use as a Pharmaceutical Excipient. Biomed. Res. Int. 2020, 2020, 4094350. [Google Scholar] [CrossRef]
- Colombo, E.; Sangiovanni, E.; Dell’agli, M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid. Based Complement. Alternat. Med. 2013, 2013, 247145. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019, 82, 445–454. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern Med. 2012, 12, 200. [Google Scholar] [CrossRef]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.-P.; Carlson, E.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and anti-inflammatory effects of Zingiber officinale roscoe and Allium subhirsutum: In silico, biochemical and histological study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef]
- Berdowska, I.; Matusiewicz, M.; Fecka, I. Punicalagin in Cancer Prevention—Via Signaling Pathways Targeting. Nutrients 2021, 13, 2733. [Google Scholar] [CrossRef]
- Saoudi, M.; Badraoui, R.; Rahmouni, F.; Jamoussi, K.; El Feki, A. Antioxidant and protective effects of Artemisia campestris essential oil against chlorpyrifos-induced kidney and liver injuries in rats. Front. Physiol. 2021, 12, 194. [Google Scholar] [CrossRef]
- Badraoui, R.; Saeed, M.; Bouali, N.; Hamadou, W.S.; Elkahoui, S.; Alam, M.J.; Siddiqui, A.J.; Adnan, M.; Saoudi, M.; Rebai, T. Expression profiling of selected immune genes and trabecular microarchitecture in breast cancer skeletal metastases model: Effect of α–tocopherol acetate supplementation. Calcif. Tissue Int. 2022, 110, 475–488. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Chao, Z. A review on phytochemicals, metabolic profiles and pharmacokinetics studies of the different parts (juice, seeds, peel, flowers, leaves and bark) of pomegranate (Punica granatum L.). Food Chem. 2022, 395, 133600. [Google Scholar] [CrossRef]
- Li, H.M.; Kouye, O.; Yang, D.S.; Zhang, Y.Y.Q.; Ruan, J.Y.; Han, L.F.; Zhang, Y.; Wang, T. Polyphenols from the Peels of Punica granatum L. and Their Bioactivity of Suppressing Lipopolysaccharide-Stimulated Inflammatory Cytokines and Mediators in RAW 264.7 Cells via Activating p38 MAPK and NF-κB Signaling Pathways. Molecules 2022, 27, 4622. [Google Scholar] [CrossRef]
- Zammel, N.; Jedli, O.; Rebai, T.; Hamadou, W.S.; Elkahoui, S.; Jamal, A.; Alam, J.M.; Adnan, M.; Siddiqui, A.J.; Alreshidi, M.M.; et al. Kidney injury and oxidative damage alleviation by Zingiber officinale: Pharmacokinetics and protective approach in a combined murine model of osteoporosis. 3 Biotech 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Morimoto, N.; Yoshimura, K.; Niimi, M.; Ito, T.; Aya, R.; Fujitaka, J.; Tada, H.; Teramukai, S.; Murayama, T.; Toyooka, C.; et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: Clinical trial for chronic skin ulcers. Tissue Eng. 2013, 19, 1931–1940. [Google Scholar] [CrossRef]
- Jedli, O.; Ben-Nasr, H.; Zammel, N.; Rebai, T.; Saoudi, M.; Elkahoui, S.; Jamal, A.; Siddiqui, A.J.; Sulieman, A.E.; Alreshidi, M.M.; et al. Attenuation of ovalbumin-induced inflammation and lung oxidative injury in asthmatic rats by Zingiber officinale extract: Combined in silico and in vivo study on antioxidant potential, STAT6 and TNF-α pathways. 3 Biotech 2022, 12, 191. [Google Scholar] [CrossRef]
- Darakhshan, S.; Malmir, M.; Bagheri, F.; Safaei, M.; Sharifi, R.; Sadeghi, M.; Hatami, M.; Mozaffari, H.; Tahvilian, R. The effects of pomegranate peel extract on recurrent aphthous stomatitis. Curr. Issues Pharm. Med. Sci. 2019, 32, 115–120. [Google Scholar] [CrossRef]


| Entry | 1 (A1) | 2 (A2) | 3 (A3) | 4 (A4) | 5 (A5) | 6 (A6) | 7 (A8) | 8 (A9) | 9 (F1) | 10 (F3) | 11 (F6) | 12 (F7) | 13 (F8) | 14 (F9) | 15 (F10) | 16 (F11) | 17 (F12) | 18 (Chito-san) | 19 (Ref) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicochemical properties | |||||||||||||||||||
| Molecular weight (g/mol) | 192.17 | 170.12 | 154.12 | 290.27 | 180.16 | 198.17 | 516.45 | 164.16 | 290.27 | 448.38 | 302.24 | 286.24 | 272.25 | 270.24 | 286.24 | 330.29 | 284.26 | 179.17 | 345.42 |
| Solubility/Lipophilicity/Druggability | |||||||||||||||||||
| Lipinski | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Biovailability Score | 0.56 | 0.56 | 0.56 | 0.55 | 0.56 | 0.56 | 0.11 | 0.85 | 0.55 | 0.17 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Pharmacokinetics | |||||||||||||||||||
| GI Absorption | Low | High | High | High | High | High | Low | High | High | Low | High | High | High | High | High | High | High | Low | High |
| BBB Permeant | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No |
| P-gp Substrate | No | No | No | Yes | No | No | Yes | No | Yes | No | No | No | Yes | No | No | No | No | Yes | Yes |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes | Yes | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| CYP3A4 inhibitor | No | No | Yes | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Log Kp (cm/s) | −9.15 | −6.84 | −6.42 | −7.82 | −6.58 | −6.77 | −8.37 | −6.26 | −7.82 | −8.42 | −7.05 | −6.70 | −6.17 | −5.80 | −6.25 | −6.14 | −5.66 | −9.88 | −6.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebahi, S.; Ben Salah, G.; Jarray, S.; Naffati, M.; Ahmad, M.A.; Brahmi, F.; Saeed, M.; Siddiqui, A.J.; Abdelmajid, K.; Badraoui, R. Chitosan-Based Gastric Dressing Materials Loaded with Pomegranate Peel as Bioactive Agents: Pharmacokinetics and Effects on Experimentally Induced Gastric Ulcers in Rabbits. Metabolites 2022, 12, 1158. https://doi.org/10.3390/metabo12121158
Jebahi S, Ben Salah G, Jarray S, Naffati M, Ahmad MA, Brahmi F, Saeed M, Siddiqui AJ, Abdelmajid K, Badraoui R. Chitosan-Based Gastric Dressing Materials Loaded with Pomegranate Peel as Bioactive Agents: Pharmacokinetics and Effects on Experimentally Induced Gastric Ulcers in Rabbits. Metabolites. 2022; 12(12):1158. https://doi.org/10.3390/metabo12121158
Chicago/Turabian StyleJebahi, Samira, Ghada Ben Salah, Soufien Jarray, Mounir Naffati, Mohammad Ayaz Ahmad, Faten Brahmi, Mohd Saeed, Arif J. Siddiqui, Khabir Abdelmajid, and Riadh Badraoui. 2022. "Chitosan-Based Gastric Dressing Materials Loaded with Pomegranate Peel as Bioactive Agents: Pharmacokinetics and Effects on Experimentally Induced Gastric Ulcers in Rabbits" Metabolites 12, no. 12: 1158. https://doi.org/10.3390/metabo12121158
APA StyleJebahi, S., Ben Salah, G., Jarray, S., Naffati, M., Ahmad, M. A., Brahmi, F., Saeed, M., Siddiqui, A. J., Abdelmajid, K., & Badraoui, R. (2022). Chitosan-Based Gastric Dressing Materials Loaded with Pomegranate Peel as Bioactive Agents: Pharmacokinetics and Effects on Experimentally Induced Gastric Ulcers in Rabbits. Metabolites, 12(12), 1158. https://doi.org/10.3390/metabo12121158









