Effects of Moringa oleifera Leaf Extracts on Xanthomonas campestris pv. campestris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Methods
- -
- Hydroalcoholic extract (HA-MOE): about 10 g of powder were mixed with 200 mL of hydroalcoholic solution (ethanol:water, 70:30) at room temperature for 1 h under magnetic stirring. The residue was filtered and concentrated in vacuum to provide the desired hydroalcoholic dry extract.
- -
- Methanolic extract (MeOH-MOE): about 5 g of powder were mixed with 100 mL of methanol and subjected to two sonication cycles (40 °C, 60 min, 80%) with subsequent centrifugation. The supernatant was concentrated under vacuum to obtain the desired product.
- -
- Decoction (In-MOE): 10 g of powder are left to infuse for 30 min with 150 mL of deionized water, previously brought to a boil. The decoction solution was filtered and lyophilized to obtain the dried water extracts.
- -
- Water extract with maltodextrins (WMD-MOE): dried M. oleifera Lam. leaves were extracted with water (raw material:solvent 1:10) for 45 min at 65 °C. After filtration, concentration and pasteurization, the extract was spray dried using maltodextrin, obtaining a fine powder.
- -
- Hydroalcoholic extract with maltodextrins (HAMD-MOE): dried M. oleifera Lam. leaves were extracted with 50% ethanol (raw material:solvent 1:10) for 45 min at 45 °C. After filtration, concentration and pasteurization, the extract was spray dried using maltodextrin, obtaining a fine powder.
2.2. Total Polyphenol Content
2.3. Determination of Total Flavonoid Content
2.4. Characterization of Polyphenols
2.4.1. Preparation of Standard Solutions and Sample
2.4.2. HPLC Apparatus and Chromatographic Conditions
2.5. X. campestris pv. campestris Strains and Culture Conditions
2.6. Determination of the Minimum Inhibitory Concentration (MIC) of Different M. oleifera Lam. Extracts
2.7. Membrane Permeability Assay
2.8. Swarming Motility Assay
2.9. Biofilm Formation
2.10. Effects of MOE on Radishes
2.11. Statistical Analysis
3. Results
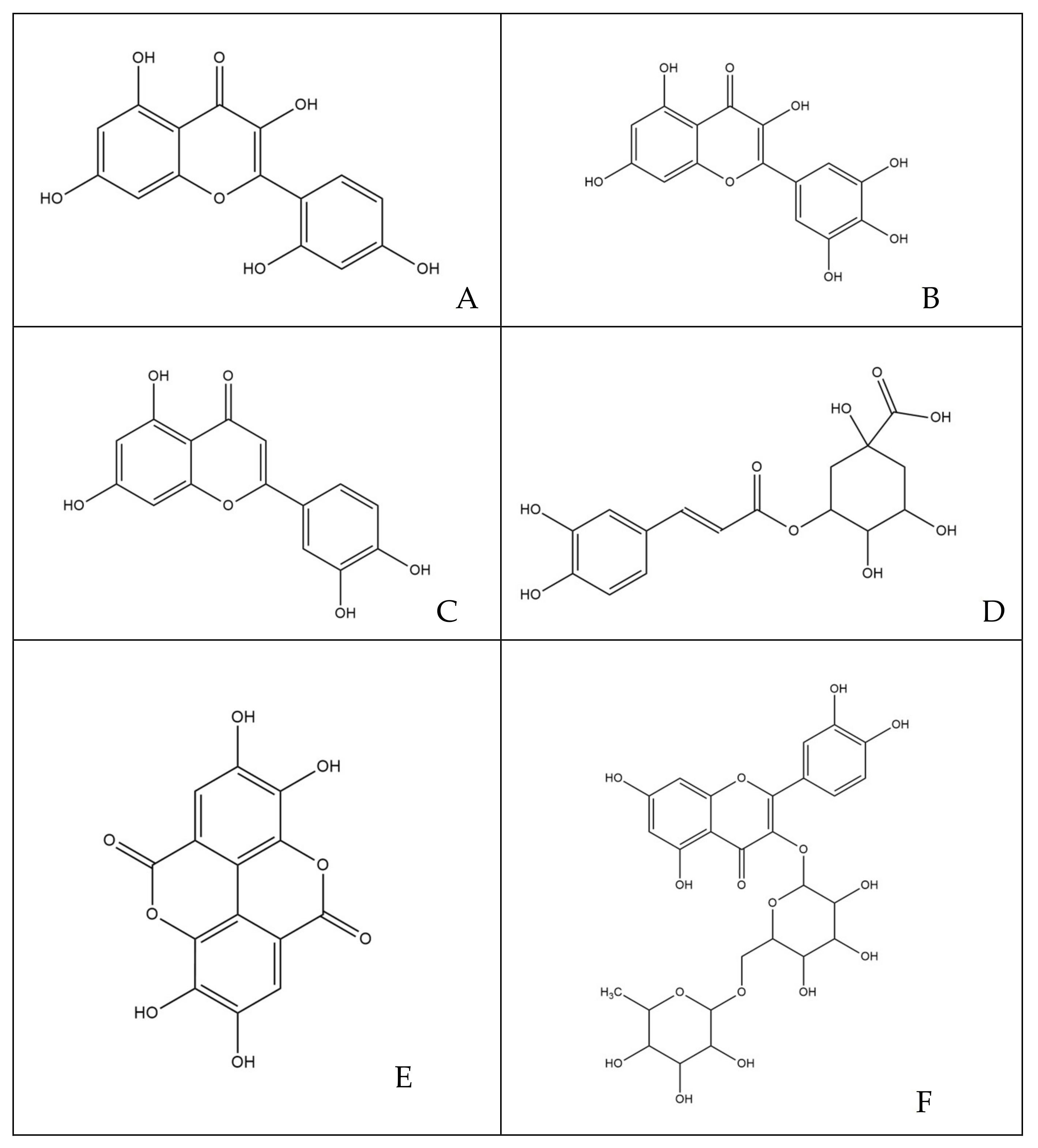
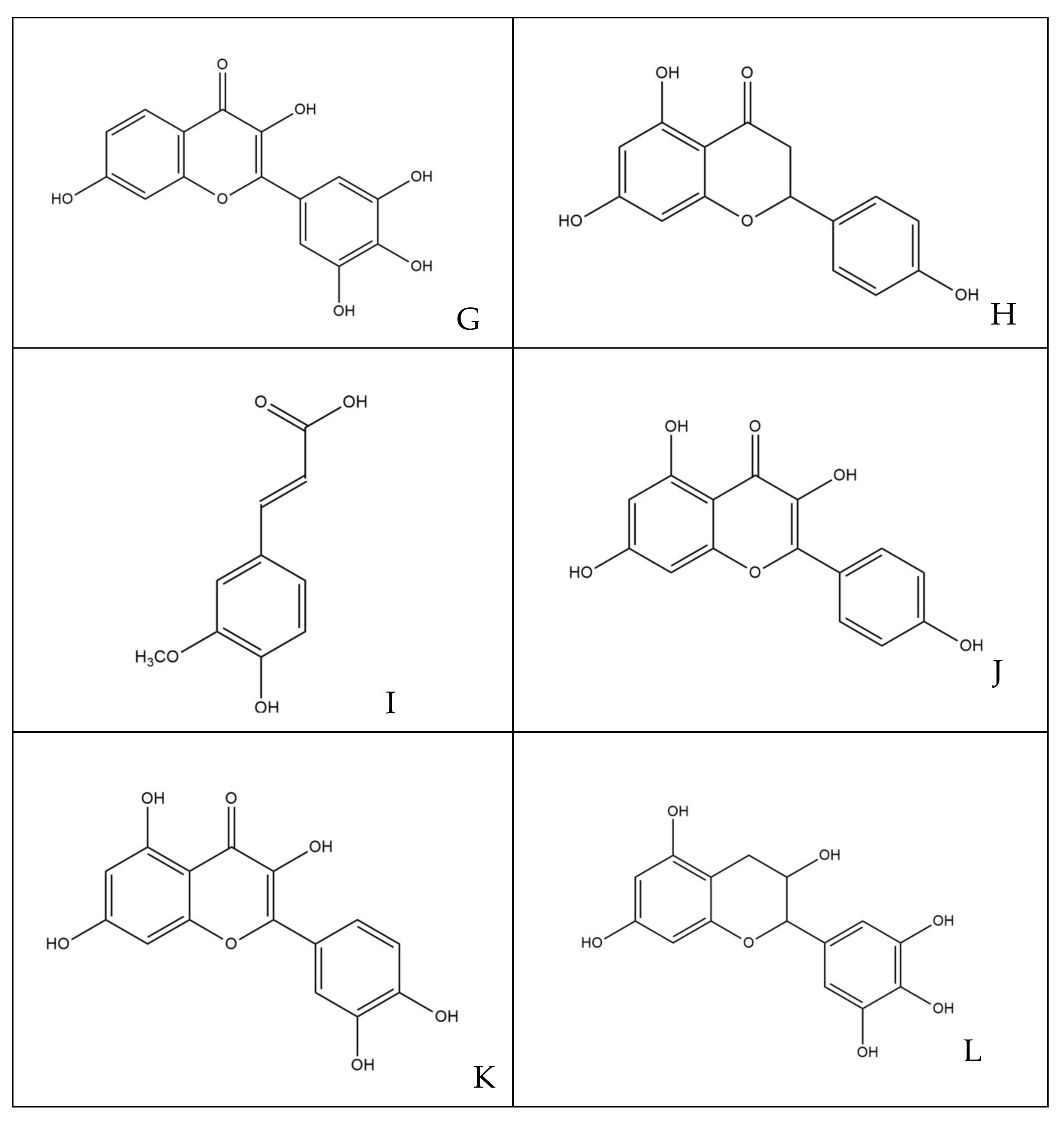
3.1. Quantitative and Qualitative Estimations of Polyphenols
3.2. Determination of MICs of MOEs
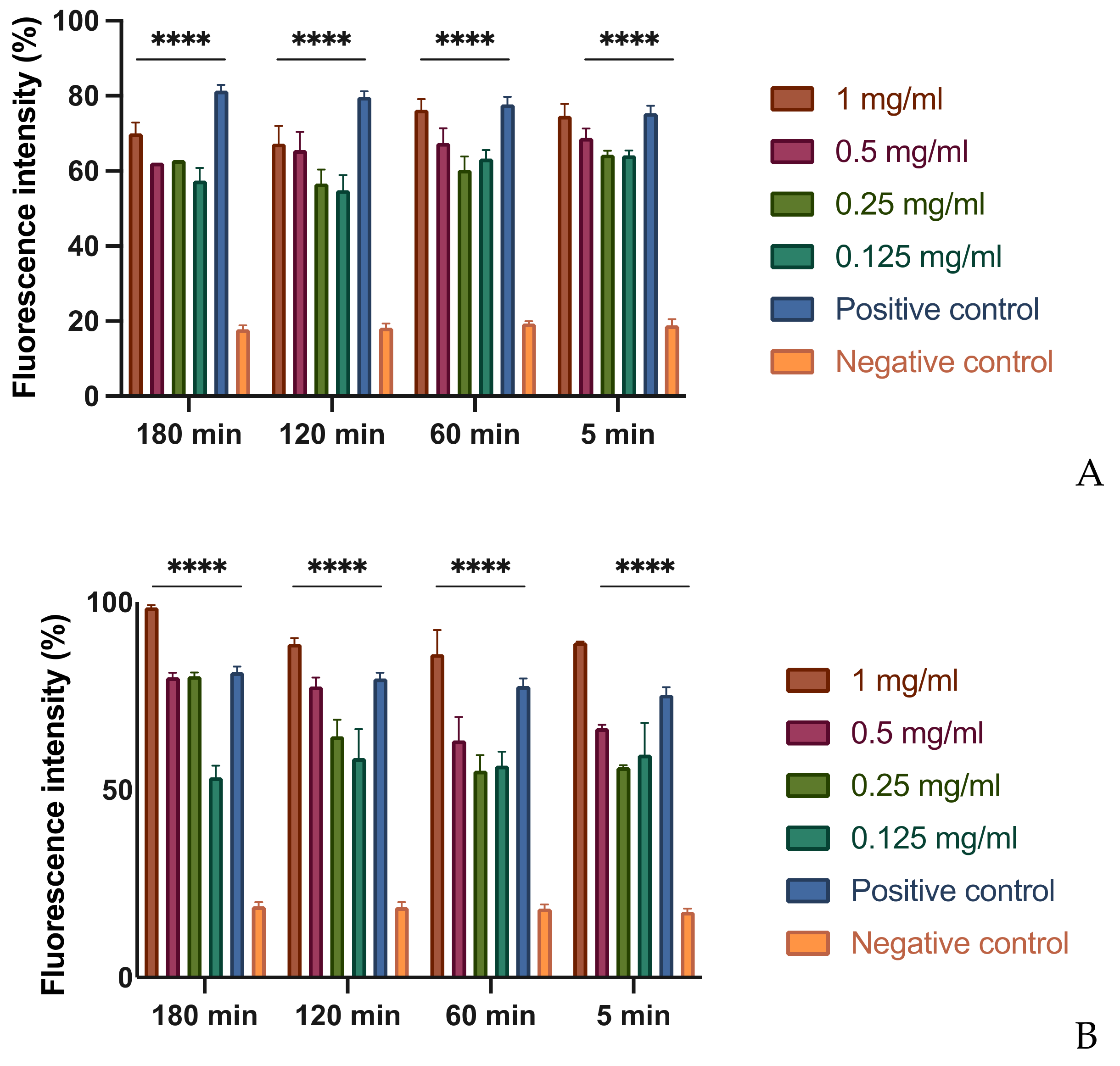
3.3. In Vitro Assessment of Membrane Permeability Alteration
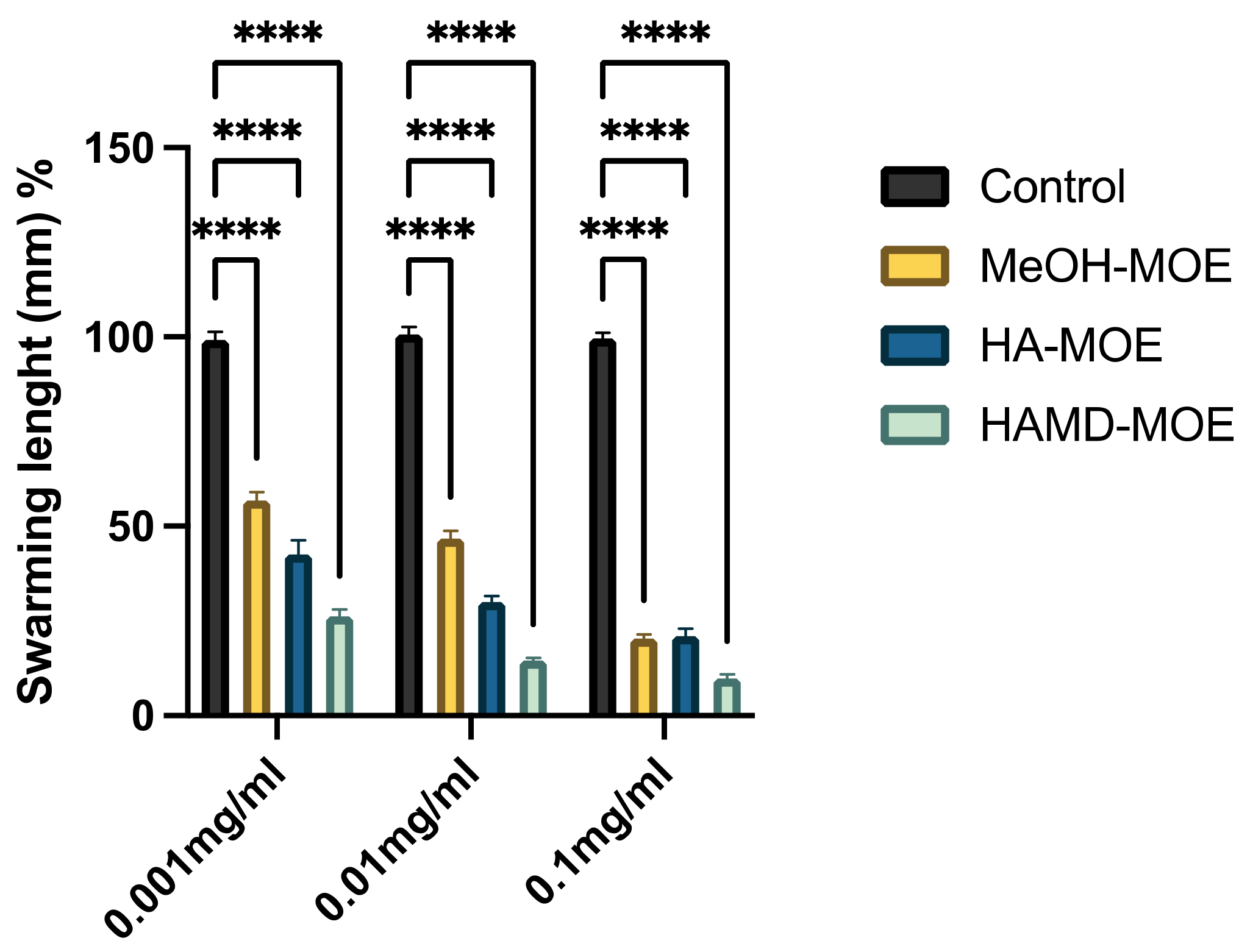
3.4. In Vitro Assessment of Bacterial Swarming
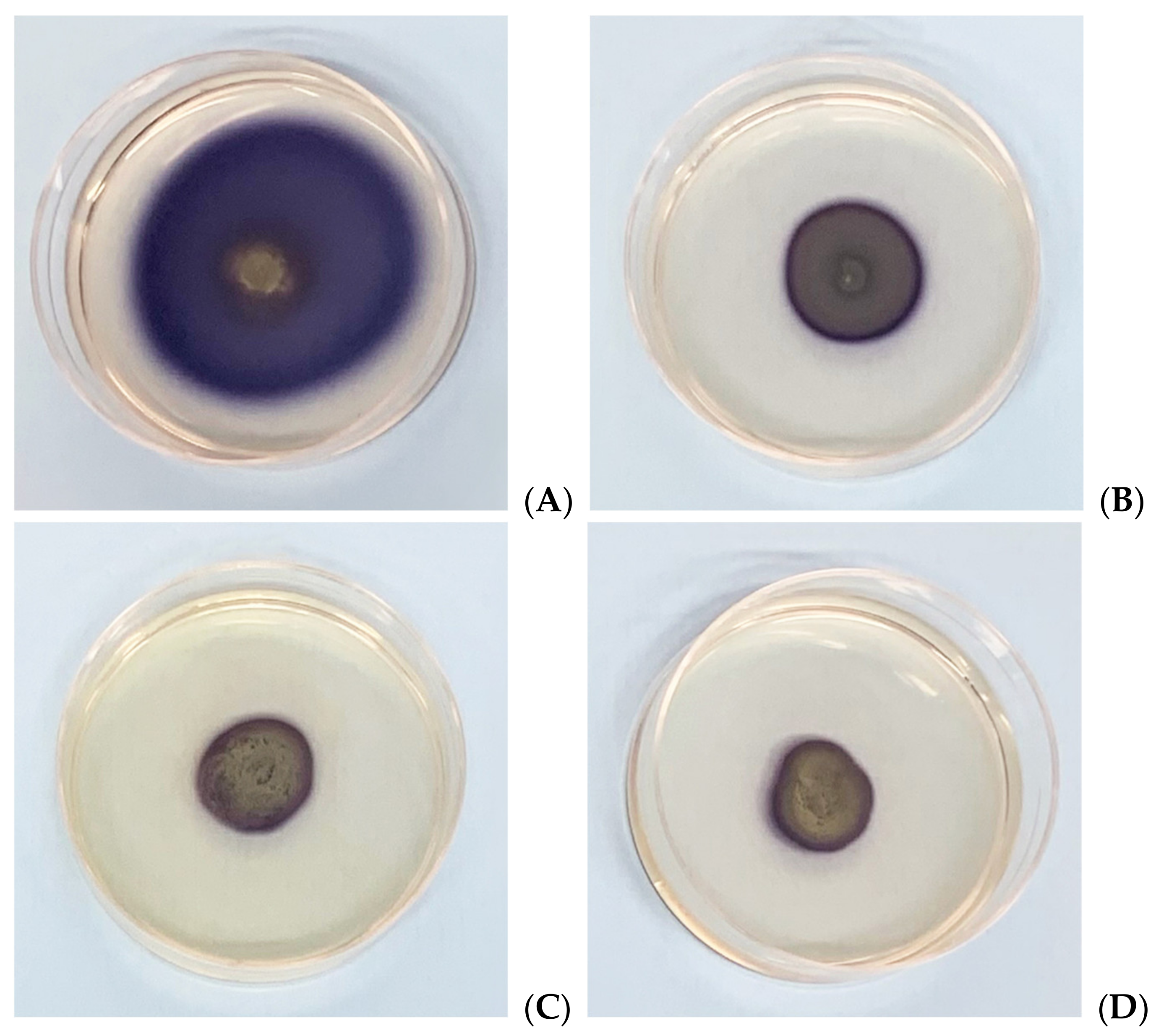
3.5. In Vitro Assessment of Biofilm Formation
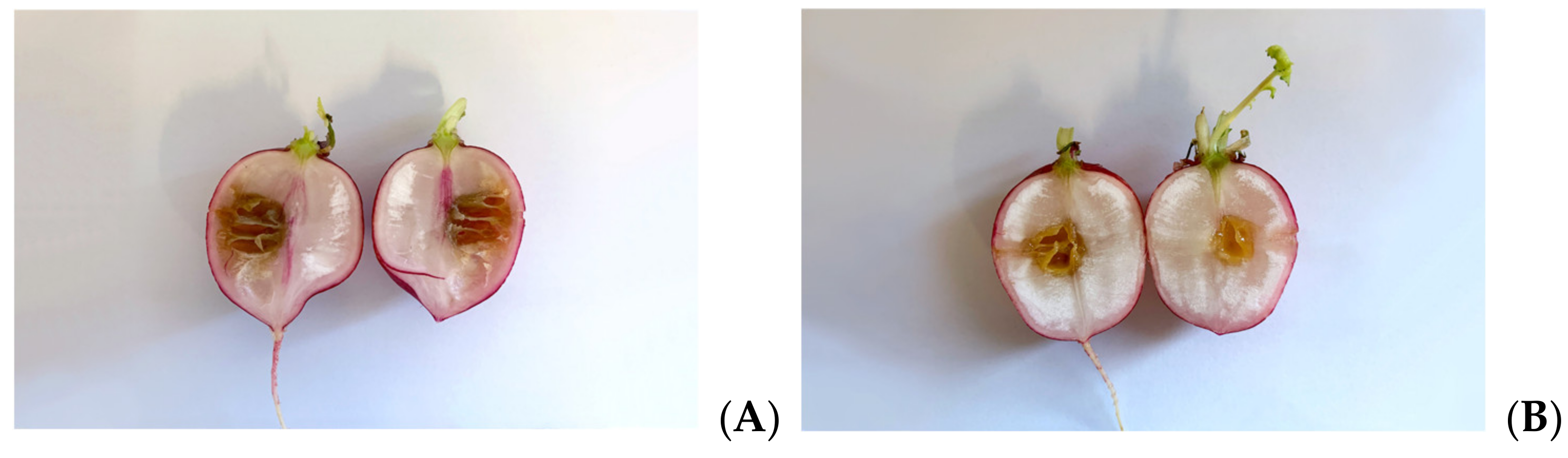
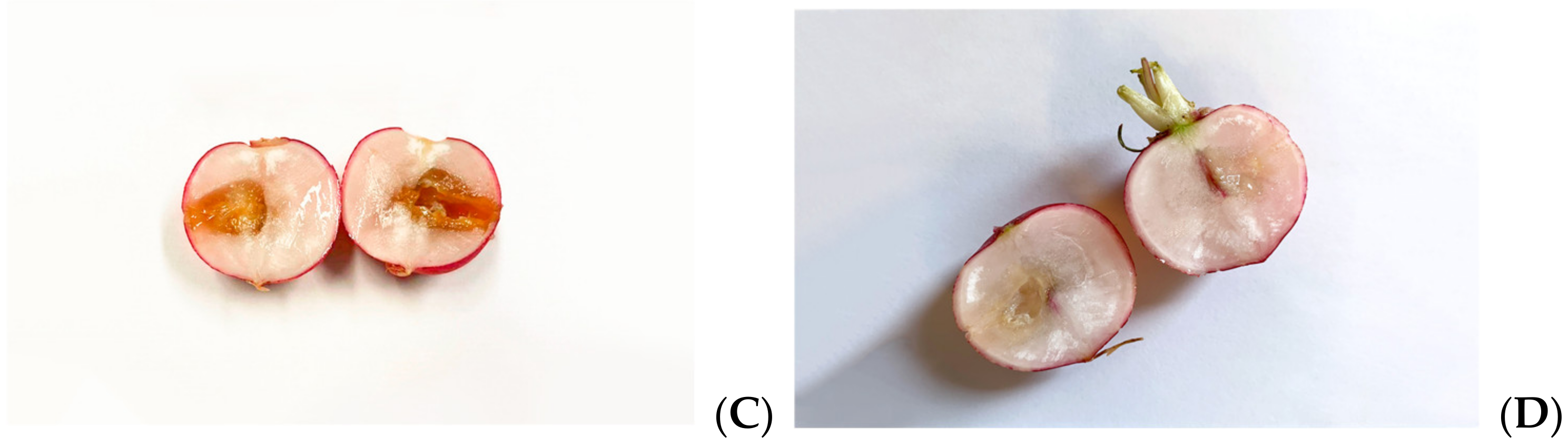
3.6. Antimicrobial Effects on Radishes
4. Discussion
4.1. Structure-Activity Considerations
4.2. Effects on Infected Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente, J.G.; Holub, E.B. Xanthomonas campestrispv.campestris(cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol. Plant Pathol. 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, C.; Wang, G.-H.; Wei, Z.-W.; Fu, Q.-X.; Hang, X.-H.; Yang, M.; Jiang, B.-L.; Tang, J.-L. Chemical Targeting and Manipulation of Type III Secretion in the Phytopathogen Xanthomonas campestris for Control of Disease. Appl. Environ. Microbiol. 2020, 86, e02349-19. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Dey, S.S.; Bhatia, R.; Batley, J.; Kumar, R. Molecular breeding for resistance to black rot [Xanthomonas campestris pv. campestris (Pammel) Dowson] in Brassicas: Recent advances. Euphytica 2018, 214, 196. [Google Scholar] [CrossRef]
- Qi, Y.-H.; Huang, L.; Liu, G.-F.; Leng, M.; Lu, G.-T. PilG and PilH antagonistically control flagellum-dependent and pili-dependent motility in the phytopathogen Xanthomonas campestris pv. campestris. BMC Microbiol. 2020, 20, 37. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-W.; Zhang, L.-H. Quorum sensing and virulence regulation inXanthomonas campestris. FEMS Microbiol. Rev. 2008, 32, 842–857. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-W.; Cao, X.-Q.; Poplawsky, A.R. Chemical Structure, Biological Roles, Biosynthesis and Regulation of the Yellow Xanthomonadin Pigments in the Phytopathogenic Genus Xanthomonas. Mol. Plant-Microbe Interact. 2020, 33, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Xanthomonas campestris Overcomes Arabidopsis Stomatal Innate Immunity through a DSF Cell-to-Cell Signal-Regulated Virulence Factor. Plant Physiol. 2009, 149, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the Polyphenol Content and Antioxidant Properties of Methanol Extracts of the Leaves, Stem, and Root Barks ofMoringa oleiferaLam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, nutritional, and therapeutic significance ofMoringa oleiferaLam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional Characterization and Phenolic Profiling of Moringa oleifera Leaves Grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [Green Version]
- Chhikara, N.; Kaur, A.; Mann, S.; Garg, M.; Sofi, S.A.; Panghal, A. Bioactive compounds, associated health benefits and safety considerations of Moringa oleifera L.: An updated review. Nutr. Food Sci. 2021, 51, 255–277. [Google Scholar] [CrossRef]
- Sahakitpichan, P.; Mahidol, C.; Disadee, W.; Ruchirawat, S.; Kanchanapoom, T. Unusual glycosides of pyrrole alkaloid and 4′-hydroxyphenylethanamide from leaves of Moringa oleifera. Phytochemistry 2011, 72, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.; Moussa, A.; Elboraey, A.; El-Sayed, H.; Al-Moghazy, M.; Abdalla, A. Microbiological Assessment of Moringa Oleifera Extracts and Its Incorporation in Novel Dental Remedies against Some Oral Pathogens. Open Access Maced. J. Med. Sci. 2016, 4, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera Leaf Extracts as Multifunctional Ingredients for “Natural and Organic” Sunscreens and Photoprotective Preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crop. Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Nouman, W.; Anwar, F.; Gull, T.; Newton, A.; Rosa, E.; Domínguez-Perles, R. Profiling of polyphenolics, nutrients and antioxidant potential of germplasm’s leaves from seven cultivars of Moringa oleifera Lam. Ind. Crop. Prod. 2016, 83, 166–176. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. Available online: http://www.ajevonline.org/content/16/3/144.abstract (accessed on 30 October 2020).
- Singh, V.; Guizani, N.; Musthafa, M.E.; Hakkim, L. Comparative analysis of total phenolics, flavonoid content and antioxidant profile of different date varieties (Phoenix dactylifera L.) From Sultanate of Oman. Int. Food Res. J. 2012, 19, 1063. Available online: https://www.researchgate.net/publication/277309552 (accessed on 30 October 2020).
- Azieana, J.; Zainon, M.N.; Noriham, A.; Rohana, M.N. Total Phenolic and Flavonoid Content and Antioxidant Activities of Ten Malaysian Wild Mushrooms. Open Access Libr. J. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Tarighi, S.; Taheri, P. Effects of plant essential oils on growth and virulence factors of Erwinia amylovora. J. Plant Pathol. 2019, 102, 409–419. [Google Scholar] [CrossRef]
- Crowley, L.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tang, J.; Mani, S. An Inexpensive Way to Record and Quantify Bacterial Swarming. Res. Sq. 2019. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review HHS Public Access. J. Eng. Technol. 2017, 6, 4. [Google Scholar]
- Zhao, Y.; Wang, D.; Nakka, S.; Sundin, G.W.; Korban, S.S. Systems level analysis of two-component signal transduction systems in Erwinia amylovora: Role in virulence, regulation of amylovoran biosynthesis and swarming motility. BMC Genom. 2009, 10, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, F. Farmacognosia Botanica, Chimica E Farmacologia Delle Piante Medicinali, 2nd ed.; Springer-Verlag: Milan, Italy, 2011. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Cypionka, H. Propidium ion enters viable cells with high membrane potential during live-dead staining. J. Microbiol. Methods 2017, 142, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Ponzone, A. Il Test Del Nitroblu Tetrazolio. La Ric. Clin. Lab. 1974, 4, 638–643. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.; Donnell, C.P.O.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Pettinato, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P. Encapsulation of antioxidants from spent coffee ground extracts by spray drying. Chem. Eng. Trans. 2017, 57, 1219–1224. [Google Scholar]
- Harsha, P.S.S.; Lavelli, V. Effects of Maltodextrins on the Kinetics of Lycopene and Chlorogenic Acid Degradation in Dried Tomato. Molecules 2019, 24, 1042. [Google Scholar] [CrossRef] [Green Version]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Et Biophys. Acta BBA Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Griep, M.A.; Blood, S.; Larson, M.; Koepsell, S.A.; Hinrichs, S.H. Myricetin inhibits Escherichia coli DnaB helicase but not primase. Bioorganic Med. Chem. 2007, 15, 7203–7208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Marrufo, T.; Nazzaro, F.; Mancini, E.; Fratianni, F.; Coppola, R.; De Martino, L.; Agostinho, A.B.; De Feo, V. Chemical Composition and Biological Activity of the Essential Oil from Leaves of Moringa oleifera Lam. Cultivated in Mozambique. Molecules 2013, 18, 10989–11000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Muñoz-Cazares, N.; García-Contreras, R.; Pérez-López, M.; Castillo-Juárez, I. Phenolic compounds with anti-virulence properties. In Phenolic Compounds—Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia-Mateos, M., Eds.; InTechOpen: Rijeka, Croatia, 2017; Volume 8, pp. 139–167. [Google Scholar] [CrossRef] [Green Version]
- Dehò, G.; Galli, E.; Bernardini, M.L. Biologia dei Microrganismi; Casa Editrice Ambrosiana: Rozzano, Italy, 2019; Available online: https://www.zanichelli.it/chi-siamo/fotocopie- (accessed on 30 October 2020).
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Fan, S.; Tian, F.; Li, J.; Hutchins, W.; Chen, H.; Yang, F.; Yuan, X.; Cui, Z.; Yang, C.-H.; He, C. Identification of phenolic compounds that suppress the virulence of Xanthomonas oryzae on rice via the type III secretion system. Mol. Plant Pathol. 2016, 18, 555–568. [Google Scholar] [CrossRef] [PubMed]



| BACTERIA | Sample ID | Host | Province of Isolation | Year of Isolation |
|---|---|---|---|---|
| Xanthomonas campestris pv. campestris | 10863 | BRASSICA seeds | Bologna | 2011 |
| Xanthomonas campestris pv. campestris | 11043 | BRASSICA seeds | Bologna | 2011 |
| Xanthomonas campestris pv. campestris | 15616 | BRASSICA seeds | Forlì-Cesena | 2011 |
| Xanthomonas campestris pv. campestris | 15619 | BRASSICA seeds | Forlì-Cesena | 2012 |
| Xanthomonas campestris pv. campestris | 15622 | BRASSICA seeds | Forlì-Cesena | 2012 |
| Xanthomonas campestris pv. campestris | 30788 | BRASSICA seeds | Ravenna | 2014 |
| Xanthomonas campestris pv. campestris | 3586 | BRASSICA | DSMZ | 1995 |
| Total Phenol Content (µg GAE/mg) | Total Flavonoid Content (µg QE/mg) | |
|---|---|---|
| HA-MOE | 45.63 ± 3.41 | 601.25 ± 44.00 |
| MeOH-MOE | 36.37 ± 2.33 | 1611.98 ± 70.44 |
| In-MOE | 37.56 ± 2.77 | 193.88 ± 1.74 |
| WMD-MOE | 25.65 ± 1.20 | 176.67 ± 9.56 |
| HAMD-MOE | 42.49 ± 1.39 | 291.07 ± 14.91 |
| Percentage of Detected Compounds (w/w) | |||||
|---|---|---|---|---|---|
| Chlorogenic Acid | Rutin | Ellagic Acid | Ferulic Acid | Quercetin | |
| HA-MOE | 0.50 ± 0.04 | 1.05 ± 0.04 | 0.20 ± 0.01 | 2.22 ± 0.03 | -1 |
| MeOH-MOE | 0.34 ± 0.02 | 0.84 ± 0.04 | 0.20 ± 0.01 | 1.12 ± 0.11 | -1 |
| In-MOE | 0.29 ± 0.03 | 0.49 ± 0.01 | 0.06 ± 0.00 | 1.17 ± 0.03 | -1 |
| WMD-MOE | 0.43 ± 0.02 | 0.30 ± 0.02 | 0.10 ± 0.00 | 0.35 ± 0.01 | -1 |
| HAMD-MOE | 0.90 ± 0.04 | 0.63 ± 0.034 | 0.21 ± 0.01 | 0.91 ± 0.00 | -1 |
| M. oleifera Lam. Leaves Extract | MIC (mg/mL) |
|---|---|
| Infusion (In-MOE) | >2 |
| Hydroalcoholic extract (HA-MOE) | 0.5 |
| Methanolic extract (MeOH-MOE) | 0.5 |
| Water extract with maltodextrins (WMD-MOE) | >2 |
| Hydroalcoholic extract with maltodextrins (HAMD-MOE) | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, R.; Caproni, A.; Buzzi, R.; Sicurella, M.; Buratto, M.; Salvatori, F.; Pappadà, M.; Manfredini, S.; Baldisserotto, A.; Marconi, P. Effects of Moringa oleifera Leaf Extracts on Xanthomonas campestris pv. campestris. Microorganisms 2021, 9, 2244. https://doi.org/10.3390/microorganisms9112244
Fontana R, Caproni A, Buzzi R, Sicurella M, Buratto M, Salvatori F, Pappadà M, Manfredini S, Baldisserotto A, Marconi P. Effects of Moringa oleifera Leaf Extracts on Xanthomonas campestris pv. campestris. Microorganisms. 2021; 9(11):2244. https://doi.org/10.3390/microorganisms9112244
Chicago/Turabian StyleFontana, Riccardo, Anna Caproni, Raissa Buzzi, Mariaconcetta Sicurella, Mattia Buratto, Francesca Salvatori, Mariangela Pappadà, Stefano Manfredini, Anna Baldisserotto, and Peggy Marconi. 2021. "Effects of Moringa oleifera Leaf Extracts on Xanthomonas campestris pv. campestris" Microorganisms 9, no. 11: 2244. https://doi.org/10.3390/microorganisms9112244









