Overexpression of MADS-box Gene AGAMOUS-LIKE 12 Activates Root Development in Juglans sp. and Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
2.1. Walnut Tree Transformation
2.1.1. Walnut Tree Somatic Embryo Transformation
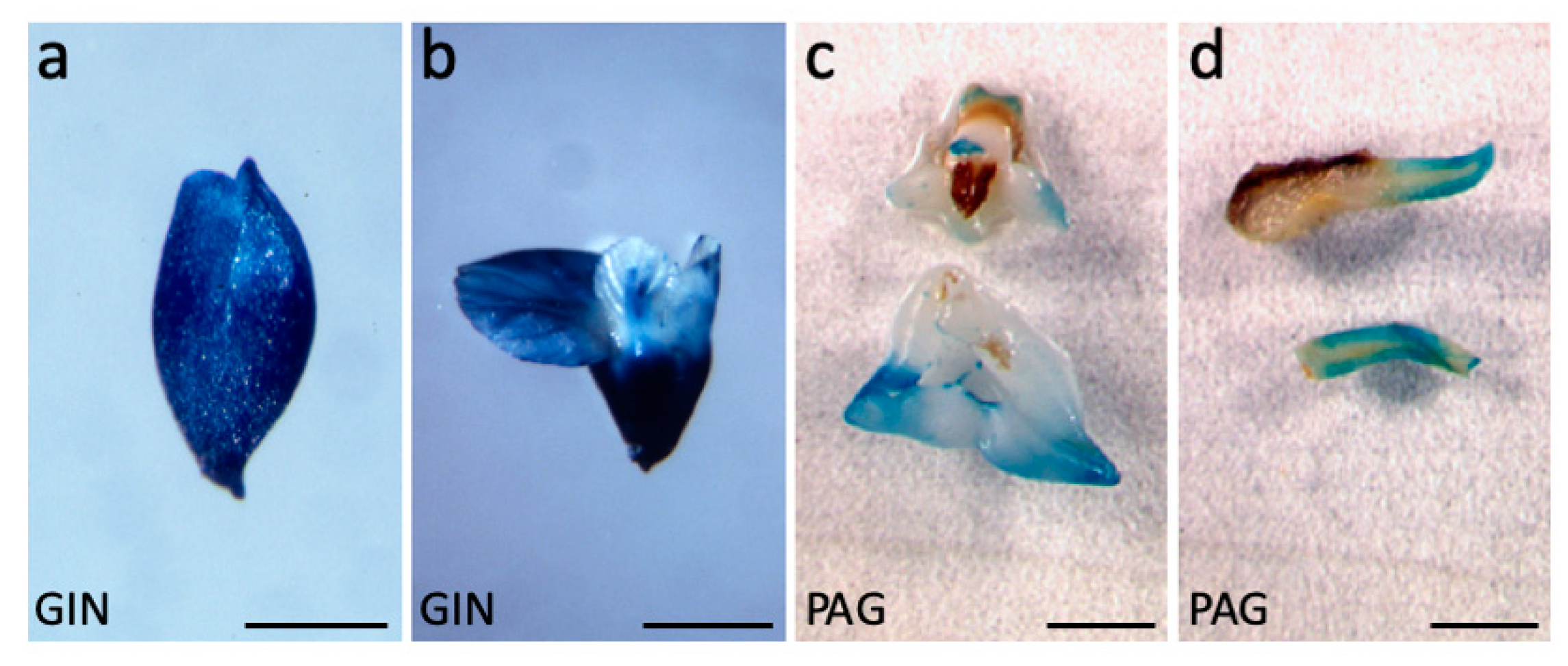
2.1.2. The Promoter of AtAGL12 is Functional in Walnut Tree Somatic Embryos
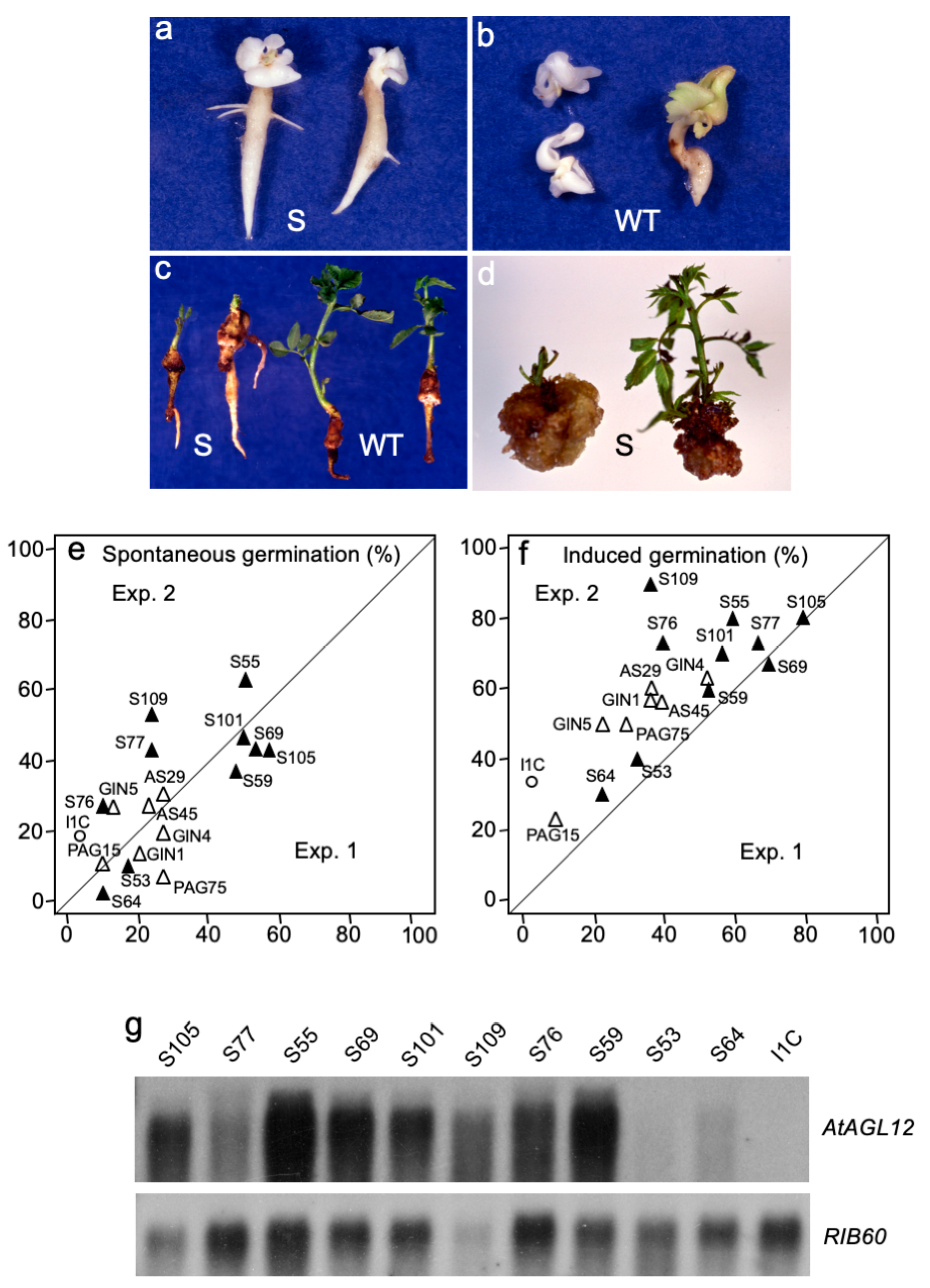
2.1.3. AtAGL12 Activates the Germination of Walnut Tree Somatic Embryos
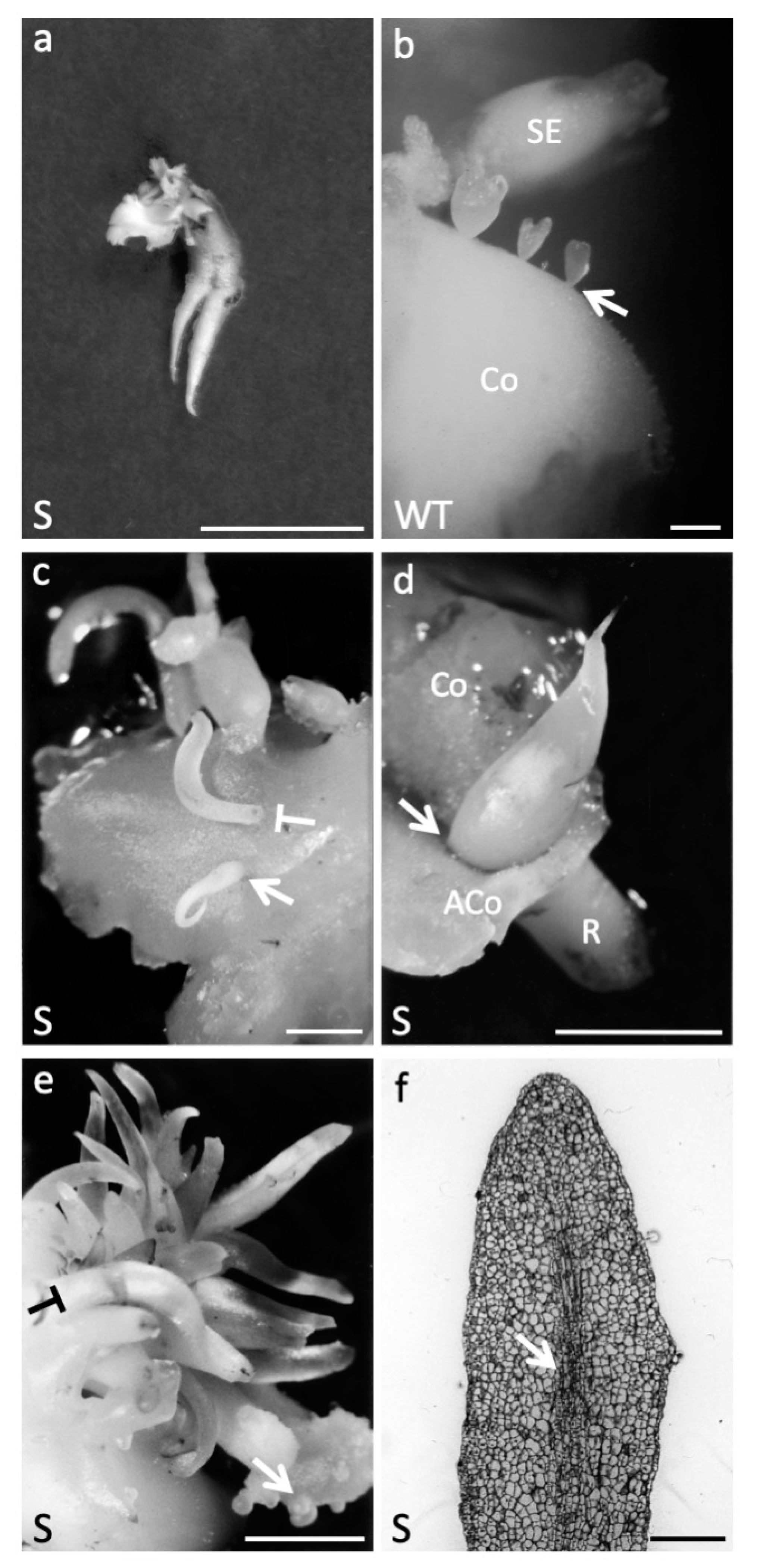
2.1.4. AtAGL12 Overexpression Causes Ectopic Root-like Structure at Different Stages of Walnut Tree Somatic Embryogenesis
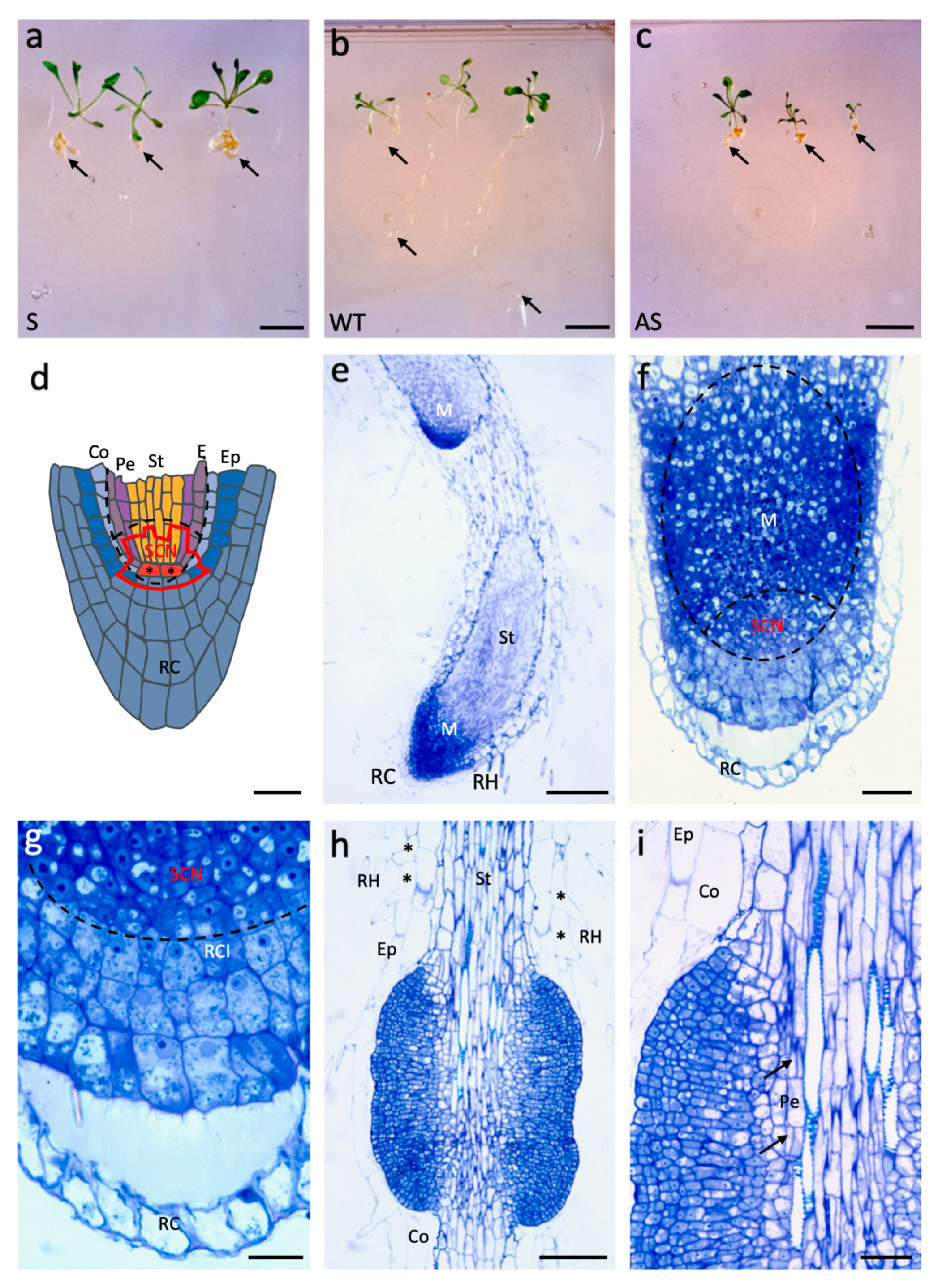
2.2. AtAGL12 Activates Cell Division and Differentiation in Arabidopsis thaliana Root Meristem
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Binary Vector Construction
4.3. Plant Transformation
4.4. Molecular Biology
4.5. Histochemical Revelation of B-glucuronidase Activity
4.6. Root Development Analysis
4.7. Microscopic Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yanofsky, M.F.; Ma, H.; Bowman, J.L.; Drews, G.N.; Feldmann, K.A.; Meyerowitz, E.M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 1990, 346, 35–39. [Google Scholar] [CrossRef]
- Soltis, D.E.; Soltis, P.S.; Albert, V.A.; Oppenheimer, D.G.; dePamphilis, C.W.; Ma, H.; Frohlich, M.W.; Theiβen, G. Missing links: The genetic architecture of flower and floral diversification. Trends Plant Sci. 2002, 7, 22–31. [Google Scholar] [CrossRef]
- O’Maoileidigh, D.S.; Graciet, E.; Wellmer, F. Gene networks controlling Arabidopsis thaliana flower development. New Phytol. 2014, 201, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Buylla, E.R.; Liljegren, S.J.; Pelaz, S.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; Vergara-Silva, F.; Yanofsky, M.F. MADS-box gene evolution beyond flowers: Expression in pollen, endosperm, guard cells, roots and trichomes. Plant J. 2000, 24, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Leseberg, C.H.; Li, A.; Kang, H.; Duvall, M.; Mao, L. Genome-wide analysis of the MADS-box gene family in Populus trichocarpa. Gene 2006, 378, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.Y.; Chen, W.J.; Peng, X.J.; Cheng, X.A.; Zhu, S.W.; Cheng, B.J. Whole-genome survey and characterization of MADS-box gene family in maize and sorghum. Plant Cell Tissue Organ Cult. 2011, 105, 159–173. [Google Scholar] [CrossRef]
- Smaczniak, C.; Immink, R.G.; Angenent, G.C.; Kaufmann, K. Developmental and evolutionary diversity of plant MADS-domain factors: Insights from recent studies. Development 2012, 139, 3081–3098. [Google Scholar] [CrossRef] [Green Version]
- Rounsley, S.D.; Ditta, G.S.; Yanofsky, M.F. Diverse roles for mads box genes in Arabidopsis development. Plant Cell 1995, 7, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.M.; Forde, B.G. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 1998, 279, 407–409. [Google Scholar] [CrossRef]
- Garay-Arroyo, A.; Ortiz-Moreno, E.; Sanchez, M.D.; Murphy, A.S.; Garcia-Ponce, B.; Marsch-Martinez, N.; de Folter, S.; Corvera-Poire, A.; Jaimes-Miranda, F.; Pacheco-Escobedo, M.A.; et al. The MADS transcription factor XAL2/AGL14 modulates auxin transport during Arabidopsis root development by regulating PIN expression. EMBO J. 2013, 32, 2884–2895. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.H.; Miao, Z.Q.; Qi, G.F.; Wu, J.; Cai, X.T.; Mao, J.L.; Xiang, C.B. MADS-Box transcription factor AGL21 regulates lateral root development and responds to multiple external and physiological signals. Mol. Plant 2014, 7, 1653–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, P.; Garcia-Ponce, B.; Fonseca-Salazar, G.; Alvarez-Buylla, E.R.; Yu, H. AGAMOUS-LIKE 17, a novel flowering promoter, acts in a FT-independent photoperiod pathway. Plant J. 2008, 55, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, R.V.; Garcia-Ponce, B.; Marsch-Martinez, N.; Ugartechea-Chirino, Y.; Villajuana-Bonequi, M.; de Folter, S.; Azpeitia, E.; Davila-Velderrain, J.; Cruz-Sanchez, D.; Garay-Arroyo, A.; et al. XAANTAL2 (AGL14) is an important component of the complex gene regulatory network that underlies Arabidopsis shoot apical meristem transitions. Mol. Plant 2015, 8, 796–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Buylla, E.R.; Garcia-Ponce, B.; Sanchez, M.D.; Espinosa-Soto, C.; Garcia-Gomez, M.L.; Pineyro-Nelson, A.; Garay-Arroyo, A. MADS-box genes underground becoming mainstream: Plant root developmental mechanisms. New Phytol. 2019, 223, 1143–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kassaby, Y.A.; Fashler, A.M.K.; Sziklai, O. Reproductive phenology and its impact on genetically improved seed production in Douglas-fir seed orchard. Silvae Genet. 1984, 33, 120–125. [Google Scholar]
- Mutke, S.; Gordo, J.; Gil, L. Variability of Mediterranean Stone pine cone production: Yield loss as response to climate change. Agric. For. Meteorol. 2005, 132, 263–272. [Google Scholar] [CrossRef]
- Theiβen, G.; Kim, J.T.; Saedler, H. Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J. Mol. Evol. 1996, 43, 484–516. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; Pelaz, S.; Liljegren, S.J.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; de Pouplana, L.R.; Martinez-Castilla, L.; Yanofsky, M.F. An ancestral MADS-box gene duplication occurred before the divergence of plants and animals. Proc. Natl. Acad. Sci. USA 2000, 97, 5328–5333. [Google Scholar] [CrossRef] [Green Version]
- Burgeff, C.; Liljegren, S.J.; Tapia-Lopez, R.; Yanofsky, M.F.; Alvarez-Buylla, E.R. MADS-box gene expression in lateral primordia, meristems and differentiated tissues of Arabidopsis thaliana roots. Planta 2002, 214, 365–372. [Google Scholar] [CrossRef]
- Tapia-Lopez, R.; Garcia-Ponce, B.; Dubrovsky, J.G.; Garay-Arroyo, A.; Pérez-Ruiz, R.V.; Kim, S.H.; Acevedo, F.; Pelaz, S.; Alvarez-Buylla, E.R. An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol. 2008, 146, 1182–1192. [Google Scholar] [CrossRef] [Green Version]
- Chavez Montes, R.A.; Coello, G.; Gonzalez-Aguilera, K.L.; Marsch-Martinez, N.; De Folter, S.; Alvarez-Buylla, E.R. ARACNe-based inference, using curated microarray data, of Arabidopsis thaliana root transcriptional regulatory networks. BMC Plant Biol. 2014, 14, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Cruz, K.V.; Garcia-Ponce, B.; Garay-Arroyo, A.; Sanchez, M.P.; Ugartechea-Chirino, Y.; Desvoyes, B.; Pacheco-Escobedo, M.A.; Tapia-Lopez, R.; Ransom-Rodriguez, I.; Gutierrez, C.; et al. The MADS-box XAANTAL1 increases proliferation at the Arabidopsis root stem-cell niche and participates in transition to differentiation by regulating cell-cycle components. Ann. Bot. 2016, 118, 787–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, R.; Chan, A.; Daly, M.; McPherson, J. Duplication of CaMV-35S promoter sequences creates a strong enhancer for plant genes. Science 1987, 236, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Leplé, J.C.; Brasileiro, A.C.M.; Michel, M.F.; Delmotte, F.; Jouanin, L. Transgenic poplars: Expression of chimeric genes using four different constructs. Plant Cell Rep. 1992, 11, 137–141. [Google Scholar] [CrossRef] [PubMed]
- El Euch, C.; Jay-Allemand, C.; Pastuglia, M.; Doumas, P.; Charpentier, J.P.; Capelli, P.; Jouanin, L. Expression of antisense chalcone synthase RNA in transgenic hybrid walnut microcuttings. Effect on flavonoid content and rooting ability. Plant Mol. Biol. 1998, 38, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Duroux, L.; Fontaine, F.; Breton, C.; Charpentier, J.P.; Doumas, P.; Jay-Allemand, C. Histological and biochemical characterization of adventitious root formation in walnut cotyledon fragments. In Biology of Root Formation and Development; Altmann, A., Waisel, Y., Eds.; Basic Life Sciences, Springer: Boston, MA, USA, 1997; Volume 65, pp. 75–84. [Google Scholar] [CrossRef]
- Ermel, F.F.; Vizoso, S.; Charpentier, J.P.; Jay-Allemand, C.; Catesson, A.M.; Couée, I. Mechanisms of primordium formation during adventitious root development from walnut cotyledon explants. Planta 2000, 211, 563–574. [Google Scholar] [CrossRef]
- Bouché, F. Arabidopsis—Root cell types. Figshare 2017. [Google Scholar] [CrossRef]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organization of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- Sozzani, R.; Iyer-Pascuzzi, A. Postembryonic control of root meristem growth and development. Curr. Opin. Plant Biol. 2014, 17, 7–12. [Google Scholar] [CrossRef]
- Moreno-Risueno, M.A.; Sozzani, R.; Yardimci, G.G.; Petricka, J.J.; Vernoux, T.; Blilou, I.; Alonso, J.; Winter, C.M.; Ohler, U.; Scheres, B.; et al. Transcriptional control of tissue formation throughout root development. Science 2015, 350, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.P.; Sozzani, R. Uncovering the networks involved in stem cell maintenance and asymmetric cell division in the Arabidopsis root. Curr. Opin. Plant Biol. 2016, 29, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Strabala, T.J.; Wagner, A. Potential transgenic routes to increase tree biomass. Plant Sci. 2013, 212, 72–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montiel, G.; Breton, C.; Thiersault, M.; Burlat, V.; Jay-Allemand, C.; Gantet, P. Transcription factor Agamous-like 12 from Arabidopsis promotes tissue-like organization and alkaloid biosynthesis in Catharanthus roseus suspension cells. Metab. Eng. 2007, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Chen, Q.; Tian, J. Studies on factors affecting the microshoot grafting survival of walnut. Acta Hort. 2010, 861, 327–331. [Google Scholar] [CrossRef]
- Polito, V.S.; McGranahan, G.; Pinney, K.; Leslie, C. Origin of somatic embryos from repetitively embryogenic cultures of walnut (Juglans regia L.): Implications for Agrobacterium-mediated transformation. Plant Cell Rep. 1989, 8, 219–221. [Google Scholar] [CrossRef]
- Smith, Z.R.; Long, J.A. Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature 2010, 464, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Nawy, T.; Lee, J.Y.; Colinas, J.; Wang, J.Y.; Thongrod, S.C.; Malamy, J.E.; Birnbaum, K.; Benfey, P.N. Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell 2005, 17, 1908–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinchee, M.A.W.; Rost, T.L. The control of lateral root development in cultured pea seedlings. II. Root fasciation induced by auxin inhibitors. Bot. Acta 1992, 105, 121–126. [Google Scholar] [CrossRef]
- Hinchee, M.A.W.; Rost, T.L. The control of lateral root development in cultured pea seedlings. III. Root fasciation induced by auxin inhibitors. Bot. Acta 1992, 105, 127–131. [Google Scholar] [CrossRef]
- Van Norman, J.M.; Xuan, W.; Beeckman, T.; Benfey, P.N. To branch or not to branch: The role of pre-patterning in lateral root formation. Development 2013, 140, 4301–4310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Risueno, M.A.; Van Norman, J.M.; Moreno, A.; Zhang, J.; Ahnert, S.E.; Benfey, P.N. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 2010, 329, 1306–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, T.; Joi, S.; Mimura, T.; Fukaki, H. The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 2012, 139, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Folter, S.; Immink, R.G.H.; Kieffer, M.; Parenicova, L.; Henz, S.R.; Weigel, D.; Busscher, M.; Kooiker, M.; Colombo, L.; Kater, M.M.; et al. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell. 2005, 17, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Soto, C.; Immink, R.G.H.; Angenent, G.C.; Alvarez-Buylla, E.R.; De Folter, S. Tetramer formation in Arabidopsis MADS domain proteins: Analysis of a protein-protein interaction network. BMC Syst. Biol. 2014, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Dastidar, M.G.; Jouannet, V.; Maizel, A. Root branching: Mechanisms, robustness, and plasticity. WIREs Dev Biol. 2012, 1, 329–343. [Google Scholar] [CrossRef]
- De Lucas, M.; Brady, S.M. Gene regulatory networks in the Arabidopsis root. Curr. Opin. Plant Biol. 2013, 16, 50–55. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, W.S.; Kim, S.H. Hormonal regulation of stem cell maintenance in roots. J. Exp. Bot. 2013, 64, 1153–1165. [Google Scholar] [CrossRef] [Green Version]
- Schiefelbein, J.; Huang, L.; Zheng, X.H. Regulation of epidermal cell fate in Arabidopsis roots: The importance of multiple feedback loops. Front. Plant Sci. 2014, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Cornu, D. Walnut somatic embryogenesis: Physiological and histological aspects. Ann. For. Sci. 1989, 46, 133s–135s. [Google Scholar] [CrossRef] [Green Version]
- Breton, C.; Cornu, D.; Chriqui, D.; Sauvanet, A.; Capelli, P.; Germain, E.; Jay-Allemand, C. Somatic embryogenesis, micropropagation and plant regeneration of “Early Mature” walnut trees (Juglans regia) that flower in vitro. Tree Physiol. 2004, 24, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Engelen, F.A.; Molthoff, J.W.; Conner, A.J.; Nap, J.P.; Pereira, A.; Stiekema, W.J. pBINPLUS: An improved plant transformation vector based on pBIN19. Trans. Res. 1995, 4, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, N.; Ellis, J.; Pelletier, G. In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Serie III Paris Life Sci. 1993, 316, 1194–1199. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
| Transformation Constructs | KanR Line Number | PCR+ Line Number | GUS+ Line Number | Transgenic Lines Selected for Phenotypic Analysis |
|---|---|---|---|---|
| d35S::GUS Intron | 60 | 27 (45%) | 26 (96%) | GIN: 1, 4, 5 |
| pAtAGL12::GUS | 70 | 27 (38%) | 15 (55%) | PAG: 1, 15, 16, 46, 48, 75 |
| d35S::AtAGL12S | 109 | 45 (41%) | NA 1 | S: 53, 55, 59, 64, 69, 76, 77, 101, 105, 109 |
| d35S::AtAGL12AS | 59 | 30 (51%) | NA 1 | AS: 20, 27, 29, 45 |
| Type of Line | n | Leaf Number | Stem Length (mm) | Root length (mm) | R/S (length) | Stem dw (mg) | Root dw (mg) | R/S (dw) |
|---|---|---|---|---|---|---|---|---|
| S | 19 | 1.6 ± 0.9 * | 9.5 ± 1.6 * | 53.8 ± 11.1* | 6.8 ± 1.9 * | 5.7 ± 1.4 * | 52.6 ± 12.7 * | 12.0 ± 4.1 * |
| WT | 20 | 3.0 ± 0.9 | 16.7 ± 5.1 | 30.6 ± 7.6 | 2.8 ± 1.1 | 11.4 ± 3.5 | 30.9 ± 8.9 | 3.6 ± 1.0 |
| AS | 23 | 1.9 ± 0.9 | 11.7 ± 4.4 | 39.0 ± 8.5 | 4.8 ± 1.4 * | 10.4 ± 4.2 | 45.0 ± 11.7 | 7.2 ± 3.6 |
| GIN | 8 | 1.0 ± 1.1 * | 8.6 ± 2.0 | 58.3 ± 18.0 * | 6.9 ± 1.7 * | 7.8 ± 2.5 | 54.4 ± 16.5 * | 9.4 ± 6.2 * |
| PAG | 13 | 2.2 ± 1.2 | 16.5 ± 6.2 | 65.2 ± 17.3 * | 8.6 ± 6.3 * | 12.2 ± 4.3 | 59.2 ± 15.2 * | 8.4 ± 6.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel, G.; Gaudet, M.; Laurans, F.; Rozenberg, P.; Simon, M.; Gantet, P.; Jay-Allemand, C.; Breton, C. Overexpression of MADS-box Gene AGAMOUS-LIKE 12 Activates Root Development in Juglans sp. and Arabidopsis thaliana. Plants 2020, 9, 444. https://doi.org/10.3390/plants9040444
Montiel G, Gaudet M, Laurans F, Rozenberg P, Simon M, Gantet P, Jay-Allemand C, Breton C. Overexpression of MADS-box Gene AGAMOUS-LIKE 12 Activates Root Development in Juglans sp. and Arabidopsis thaliana. Plants. 2020; 9(4):444. https://doi.org/10.3390/plants9040444
Chicago/Turabian StyleMontiel, Grégory, Muriel Gaudet, Françoise Laurans, Philippe Rozenberg, Matthieu Simon, Pascal Gantet, Christian Jay-Allemand, and Christian Breton. 2020. "Overexpression of MADS-box Gene AGAMOUS-LIKE 12 Activates Root Development in Juglans sp. and Arabidopsis thaliana" Plants 9, no. 4: 444. https://doi.org/10.3390/plants9040444






