Efficient Microwave-Assisted Synthesis of 5-Deazaflavine Derivatives
Abstract
:1. Introduction

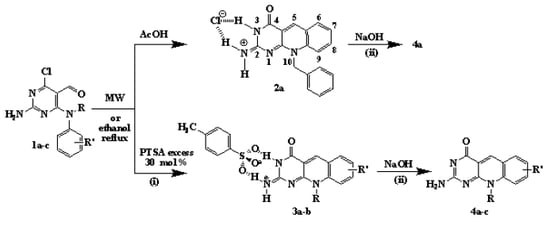
2. Results and Discussion



| Entry | Compound 1 | Reaction conditions | Compound yield % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | R’ | MW (10 min) | Δ/Ethanol (60 min) | ||||||
| 2a | 3a-b | 4a-c | 2a | 3a-b | 4a-c | ||||
| a | CH2C6H5 | H | AcOH | 80 | - | - | 85 | - | - |
| PTSA (1.3 mmol) | - | 70 | - | - | 72 | - | |||
| (i) PTSA (1.3 mmol) (ii) NaOH | - | - | 70 | - | - | 68 | |||
| b | CH3 | p-H3C | PTSA (1.3 mmol) | - | 70 | - | - | 70 | - |
| (i) PTSA (1.3 mmol) (ii) NaOH | - | - | 70 | - | - | 68 | |||
| c | CH3 | o-H3C | PTSA (0.2 mmol) | - | - | 60 | - | - | 62 |
3. Experimental
3.1. General
3.2. General procedure for the synthesis of pyrimido[4,5-b]quinolin-2(3H)-iminium-4-toluene-sulfonates 3
4. Conclusions
Acknowledgements
References
- Penzkofer, A.; Bansal, A.K.; Song, S.-H.; Dick, B. Fluorescence quenching of flavins by reductive agents. Chem. Phys. 2007, 336, 14–21. [Google Scholar] [CrossRef]
- Ohno, A.; Kunitomo, J.; Kawai, Y.; Kawamoto, T.; Tomishima, M.; Yoneda, F. Atropisomeric Flavoenzyme Models with a Modified Pyrimidine Ring: Syntheses, Physical Properties and Stereochemistry in the Reactions with NAD(P)H Analogs. J. Org. Chem. 1996, 61, 9344–9355. [Google Scholar] [CrossRef]
- El-Gazzar, A.B.A.; Hafez, H.N.; Nawwar, G.A.M. New acyclic nucleosides analogues as potential analgesic, anti-inflammatory, anti-oxidant and anti-microbial derived from pyrimido[4,5-b]quinoline. Eur. J. Med. Chem. 2009, 44, 1427–1436. [Google Scholar] [CrossRef]
- Ali, H.I.; Ashidab, N.; Nagamatsu, T. Antitumor studies. Part 4: Design, synthesis, antitumor activity, and molecular docking study of novel 2-substituted 2-deoxoflavin-5-oxides, 2-deoxoalloxazine-5-oxides, and their 5-deaza analogs. Bioorg. Med. Chem. 2008, 16, 922–940. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.I.; Tomita, K.; Akaho, E.; Kunishima, M.; Kawashima, Y.; Yamagishi, T.; Ikeya, H.; Nagamatsu, T. Antitumor studies – Part 2: Structure–activity relationship study for flavin analogs including investigations on their in vitro antitumor assay and docking simulation into protein tyrosine kinase. Eur. J. Med. Chem. 2008, 43, 1376–1389. [Google Scholar] [CrossRef]
- Ali, H.I.; Tomita, K.; Akaho, E.; Kambara, H.; Miura, S.; Hayakawa, H.; Ashida, N.; Kawashima, Y.; Yamagishi, T.; Ikeya, H.; Yoneda, F.; Nagamatsu, T. Antitumor studies. Part 1: Design, synthesis, antitumor activity, and AutoDock study of 2-deoxo-2-phenyl-5-deazaflavins and 2-deoxo-2-phenylflavin-5-oxides as a new class of antitumor agents. Bioorg. Med. Chem. 2007, 15, 242–256. [Google Scholar] [Green Version]
- Joshi, A.A.; Viswanathan, C.L. Docking studies and development of novel 5-heteroarylamino-2,4-diamino-8-chloropyrimido-[4,5-b]quinolines as potential antimalarials. Bioorg. Med. Chem. Lett. 2006, 16, 2613–2617. [Google Scholar] [CrossRef]
- Chen, Y.L; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Rechthaler, K.; Schamschule, R.; Parusel, A.B.J.; Rotkiewicz, K.; Piorun, D.; Kohler, G. Picosecond laser photolysis studies of DMA–DMPP in solution. Acta Phys. Pol. A 1999, 95, 321–334. [Google Scholar]
- He, Z.; Milburn, G.; Danel, A.; Puchala, A; Tomasik, P.; Rasala, D. Synthesis of new quinoline fused heterocycles such as benzo[h]-1,6-naphthyridines and pyrazolo[4,3-c]quinolines. J. Mater. Chem. 1997, 7, 2323–2325. [Google Scholar] [CrossRef]
- Brack, A. Dipirazolopyridine. Belg. Patent 616472, 16 October 1962. [Google Scholar]
- Parusel, A.B.J.; Schamschule, R.; Piorun, D.; Rechthaler, K.; Puchala, A.; Rasala, D.; Rotkiewicz, K.; Kohler, G. Semiempirical characterization of substituted bis-pyrazolopyridines as new bulky electron donor-acceptor systems in their electronic ground state. J. Mol. Struct. (TEOCHEM) 1997, 419, 63–75. [Google Scholar] [CrossRef]
- Tao, Y.T.; Chuen, C.H.; Ko, C.W.; Peng, J.W. Efficient Blue Light-Emitting Diodes Based on Triarylamine-Substituted Dipyrazolopyridine Derivatives. Chem. Mater. 2002, 14, 4256–4261. [Google Scholar] [CrossRef]
- Chuen, C.H.; Tao, Y.T. Highly-bright white organic light-emitting diodes based on a single emission layer. Appl. Phys. Lett. 2002, 81, 4499–4502. [Google Scholar] [CrossRef]
- Ko, C.W.; Tao, Y.T. Bright white organic light-emitting diode. Appl. Phys. Lett. 2001, 79, 4234–4235. [Google Scholar] [CrossRef]
- Chena, Y.; Wub, S.; Tub, S.; Shib, F.; Lib, C. An efficient synthesis of new benzo[1′,2′:6,7]quinolino[2,3-d]-pyrimidine derivatives via three-component microwave-assisted reaction. J. Heterocycl. Chem. 2008, 45, 1243–1246. [Google Scholar] [CrossRef]
- Shi, D.-Q.; Niu, L.-H.; Yao, H.; Jiang, H. An efficient synthesis of pyrimido[4,5-b]quinoline derivatives via three-component reaction in aqueous media. J. Heterocycl. Chem. 2009, 46, 237–242. [Google Scholar] [CrossRef]
- Quiroga, J.; Hormaza, A.; Insuasty, B.; Ortíz, A.J.; Sánchez, A.; Nogueras, M. Synthesis of pyrimido[4,5-b]quinolines in the reaction of 6-aminopyrimidines with dimedone and benzaldehydes. J. Heterocycl. Chem. 1998, 35, 231–233. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J. Regioselective formylation of pyrazolo[3,4-b]pyridine and pyrazolo[1,5-a]pyrimidine systems using Vilsmeier–Haack conditions. Tetrahedron Lett. 2008, 49, 2689–2691. [Google Scholar] [CrossRef]
- Trilleras, J.; Quiroga, J.; Low, J.N.; Cobo, J.; Glidewell, C. 10-Ethyl-4-oxo-2,3,4,10-tetrahydropyrimido[4,5-b]quinolin-2-iminium 4-toluenesulfonate: a polarized electronic structure in the cation anda hydrogen-bonded sheet structure. Acta Cryst. 2008, C64, 382–384. [Google Scholar]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Marchal, A.; Cobo, J. A straightforward synthesis of pyrimido[4,5-b]quinoline derivatives assisted by microwave irradiation. Tetrahedron Lett. 2010, 51, 1107–1109. [Google Scholar]
- Bradsher, C.K. Aromatic Cyclodehydration. Chem. Rev. 1946, 38, 447–449. [Google Scholar] [CrossRef]
- Trilleras, J.; López, L.G.; Cobo, J.; Glidewell, C. 10-Benzyl-4-oxo-2,3,4,10-tetrahydropyrimido[4,5-b]quinolin-2-iminium chloride sesquihydrate: a polarized electronic structure within a complex hydrogen-bonded sheet structure. Acta Cryst. 2010, C66, 469–472. [Google Scholar]
- Sample Availability: Samples of the compounds are available from authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Trilleras, J.; López, L.G.; Pacheco, D.J.; Quiroga, J.; Nogueras, M.; Torre, J.M.d.l.; Cobo, J. Efficient Microwave-Assisted Synthesis of 5-Deazaflavine Derivatives. Molecules 2010, 15, 7227-7234. https://doi.org/10.3390/molecules15107227
Trilleras J, López LG, Pacheco DJ, Quiroga J, Nogueras M, Torre JMdl, Cobo J. Efficient Microwave-Assisted Synthesis of 5-Deazaflavine Derivatives. Molecules. 2010; 15(10):7227-7234. https://doi.org/10.3390/molecules15107227
Chicago/Turabian StyleTrilleras, Jorge, Luis Gabriel López, Dency José Pacheco, Jairo Quiroga, Manuel Nogueras, José M. de la Torre, and Justo Cobo. 2010. "Efficient Microwave-Assisted Synthesis of 5-Deazaflavine Derivatives" Molecules 15, no. 10: 7227-7234. https://doi.org/10.3390/molecules15107227