1. Introduction
Flavonoids are polyphenolic compounds based on a C
15 (C
6C
3C
6) framework. They contain a chroman ring (C-ring) with a second aromatic ring (B-ring) at the C-2, C-3, or C-4 position. The heterocyclic six-membered C-ring is sometimes replaced by a five-membered ring (e.g., aurones) or the acyclic form (chalcones). The oxidation state of the C-ring is used to classify flavonoids into different categories, of which typical examples are flavan-3-ols, flavanones, flavones and flavonols. The term
flavonoid can be ambiguous as it may refer either to the class of all C
6C
3C
6 compounds, or its meaning may be restricted to 2-arylchromans with a carbonyl group at C-4 (C-ring) [
1]. Flavonoids occur widely as glycosylated monomers or as flavan-3-ol oligomers (proanthocyanidins = condensed tannins).
Epidemiological studies suggest that the regular consumption of flavonoids protects humans against diseases associated with oxidative stress such as Alzheimer’s disease [
2], arteriosclerosis [
3], cancer [
4,
5], and ageing [
6]. The polyphenolic nature of flavonoids equates with ready oxidation and the formation of stable radicals and it is widely believed that flavonoids protect against free radical damage (caused by photolytically generated singlet oxygen and metabolic processes in living organisms) and act as antioxidants [
7]. Other biological effects include improved blood flow [
8], the inhibition of cholesterol absorption [
9] and protection from damage by ultraviolet B radiation [
10]. These have stimulated renewed interest in flavonoid synthesis and photochemical transformations that give access to molecules that are not available
via conventional chemistry. The increasing use of flavonoids as food additives for health purposes has also contributed towards the growing interest in flavonoid photostability and photochemistry. Flavonoids are commercially important constituents of red wine [
11], adhesives [
12], and black tea [
13].
The reason for the ubiquitous existence of flavonoid monomers and their oligomers as secondary metabolites in plants is controversial. Their polyphenolic nature allows complexation with proteins, as is evident in the widespread use of tannin extracts to tan leather, hence the name tannin [
14]. This property renders protein in food indigestible and supports their putative anti-feeding role that provides protection against insect predation. Certain flavonoids are toxic to insects and other organisms. Bark that contains rotenoids is used by tribal communities to poison and harvest fish from rivers [
15].
The light absorption properties of flavonoids and anthocyanidins in the visible ultraviolet light region are responsible for the colours associated with flowers and this plays an important role in pollination by insects and thus plant reproduction.
It has been shown that light is important in flavonoid biosynthesis [
16,
17], and that light is essential for anthocyanidin synthesis [
18,
19,
20,
21]. Flavonoids play important roles as development regulators and can regulate auxin transport
in vivo [
22]. Their role as antioxidants in plants [
23], in stress protection [
17] and in photoprotection [
24] has been discussed. The influence of light on plant defence against pest and pathogens has been reviewed [
25].
The postulate that flavonoids protect plants against ultraviolet light damage is supported not only by the fact that flavonoids absorb UV radiation and may act as sunscreens, but also by observations that exposure to UV radiation induces higher levels of flavonoids in plants. Caldwell [
26] demonstrated a correlation between flavonoid content in plants and ambient UV conditions. Alpine plants at high altitudes and tropical plants from regions exposed to intense UV radiation have higher flavonoid content than plants from other regions. Plants exposed to sunlight have short internodes and smaller thicker leaves than plants that grow in shade [
17].
It was observed that the biosynthesis of flavonoids with antioxidant properties (e.g., orthodihydroxy or catechol B-ring substitution) in plants is stimulated by UV light at the expense of flavonoids that are not considered as antioxidant (e.g., monohydroxy B-ring substitution) and flavonoids with good sunscreen properties (e.g., hydrocinnamic acid derivatives). This suggested that flavonoids’ photoprotection may also involve the removal of reactive oxygen species that form as a result of exposure to strong UV light.
Apart from the photochemical transformations that flavonoids may undergo due to their long daily exposure to sunlight, these compounds may also transfer or accept light energy to or from other molecules, i.e. act as sensitizers or quenchers.
A photochemical transformation requires excitation of an electron from a ground state orbital to an excited state orbital. This is usually achieved via the absorption of ultraviolet light (UV) by a chromophore. All flavonoids have aromatic chromophores, as indicated by UV absorptions in the 250 nm region of their UV spectra. These compounds may undergo π,π* excitation and react from π,π* excited states. Certain flavonoids contain carbonyl chromophores and absorb light in the 300 nm region. They may undergo n,π* excitation to react from n,π* excited states. Carbonyl chromophores that are conjugated with the aromatic ring (e.g., acetophenones and chalcones) absorb UV light in the 350 nm region. The n,π* and π,π* excited states of these compounds are almost degenerate and the state from which their reactions originates is sometimes controversial. Polyphenolic chalcones may absorb light in the visible region as is evident by their colours. Molecules that have no chromophores and cannot absorb light energy may be excited indirectly via sensitisation. This involves the transfer of mostly triplet energy.
The excited states may be in the triplet or singlet form. The triplet excited n,π* state [
3(n,π*) state] is associated with radical reaction type products and the singlet excited π,π* state [
1(π,π*) state] with ionic reaction type products. Solvent polarity is important and ionic type products are encouraged by polar solvents. Triplet excited states are formed indirectly from the initially formed singlet states
via intersystem crossing [
27]. Triplet excited states usually have much longer lifetimes than the corresponding singlet states, permitting photochemical transformations to compete more effectively with relaxation of the excited state to the photochemical inert ground state.
Much of the older photochemistry work in flavonoid chemistry was on compounds with unsubstituted aromatic rings and high yields were reported (see the review by Gupta
et al. [
28]). Polyphenolic flavonoids are more representative of naturally occurring molecules and have more interesting biological properties. These compounds generally afford lower yields and require special conditions to react due to deactivation by phenolic hydroxy and methoxy groups [
29].
2. Flavone and Flavonol Photochemistry
Flavones and flavonols are characterized by fully unsaturated C-rings that connect the A and B-rings in a single conjugated system. They are generally photochemically inert as indicated by their reported use as photosensitisers, photoquenchers and ultraviolet absorption filters [
30]. Their inertness prompted and allowed investigation into the potential of photochemically generated singlet oxygen to afford chemical transformations.
Waiss and Corse (1965) [
31] investigated per-
O-methylflavonols. Photoxidative cyclisation of quercetin penta-
O-methyl ether (
1) afforded the tetra-
O-methyl ether of β-photomethylquercetin (
2) in 32% yield in deoxygenated methanol under dry nitrogen (low pressure mercury lamps, 350 nm) (
Scheme 1). This result led the authors to speculate that photochemistry was involved in the biosynthesis of peltogynol (
3) and the rotenoids [e.g., dolichone (
4) and α-toxicarol (
5) (
Figure 1], which co-occur with 2-methoxyisoflavonoids.
Scheme 1.
Photo-oxidative cyclization of quercetin pentamethyl ether.
Scheme 1.
Photo-oxidative cyclization of quercetin pentamethyl ether.
Figure 1.
Structure of peltogynol and the naturally occurring rotenoids.
Figure 1.
Structure of peltogynol and the naturally occurring rotenoids.
In a subsequent paper Waiss and co-workers (1967) [
32] described the isolation under similar conditions of β-photomethylquercetin (
2, 31%), α-lumimethylquercetin (
6, 16%), α-photomethyl-quercetin (
7, 5%), and traces of methoxy β-photomethylquercetin (
8, 1%) (
Scheme 2). No β-lumi- methylquercetin (
9,
Figure 2) was detected. Repetition of the reaction in oxygen-free benzene yielded only photo-oxidised β-photomethylquercetin, and α-photomethylquercetin, at twice the rate of reaction. Rigorous efforts to exclude oxygen failed to yield α-and β-lumimethylquercetins. Yields in benzene were not reported. Failure to inhibit the reaction with triplet quenchers (anthracene or O
2) and low intensity of phosphorescence as compared to fluorescence led to postulation of a
1(π,π*) primary reactive intermediate.
Scheme 2.
Photochemistry of quercetin pentamethyl ether.
Scheme 2.
Photochemistry of quercetin pentamethyl ether.
Figure 2.
Structure of β-lumimethylquercetin.
Figure 2.
Structure of β-lumimethylquercetin.
Matsuura and Matsushima [
33] studied flavonols where the 3-hydroxy group had not been methylated. Photolysis of quercetin 5,7,3',4'-tetra-
O-methyl ether (
10b) in pyridine in the presence of rose bengal under bubbling oxygen (300 W tungsten lamp) followed by diazomethane methylation of the reaction mixture afforded depside (
11b, 77%), methyl 2-hydroxy-4,6-di-
O-methylbenzoate (
12b, 2%) and methyl 3,4-di-
O-methylveratrate (
13b, 11%) (
Scheme 3). Enzymatic oxygenation also yielded depside and there seemed to be a resemblance between enzymatic and photosensitised oxygenation. Liberated carbon monoxide (31%) and carbon dioxide (17%) were determined. Similar results were obtained with 3-hydroxyflavone (
10c). In a later publication Matsuura and co-workers [
34] irradiated (100 W high-pressure lamp) free phenolic quercetin (
10d) in methanol in the presence of rose bengal to get
12b. In the absence of an oxygen sensitiser no reaction took place. No reaction was observed with quercetin 3,7,3',4'-tetra-
O-methyl ether. It was suggested that the 3-hydroxy group was essential for photo-oxygenation. The mechanism for the reaction is given in
Scheme 4.
Scheme 3.
Photosensitized oxygenation of 3-hydroxyflavones.
Scheme 3.
Photosensitized oxygenation of 3-hydroxyflavones.
Scheme 4.
Suggested mechanism of photosensitized oxygenation of 3-hydroxyflavones.
Scheme 4.
Suggested mechanism of photosensitized oxygenation of 3-hydroxyflavones.
Matsuura and Matsushima [
35] postulated that, despite rigorous efforts by Waiss and co-workers [
31,
32] to work under oxygen-free conditions, oxygen must be involved in the transformation of intermediary lumimethylquercetin to photomethylquercetin. Photolysis of 3,7-dimethoxyflavone (
14) under oxygen in pyridine (high-pressure mercury lamp with a Pyrex filter) yielded at least seven compounds. Only the photomethyl analogue (
15, 4%) and the lactone (
16, 11%) were isolated. 3-Methoxyflavanone (
17) yielded only the lactone (
19, also 11%) (
Scheme 5).
Scheme 5.
Photo-oxidative cyclization of 3-methoxyflavones.
Scheme 5.
Photo-oxidative cyclization of 3-methoxyflavones.
The mechanism of the formation of the lactone is given in
Scheme 6. Quercetin 3,7,3',4'-tetra-
O-methyl ether (
20) and 5-hydro-3,7-dimethoxyflavone (
21) did not undergo photocyclisation and were recovered unchanged. This was attributed to hydrogen bonding between the 4-carbonyl and 5-hydroxyl group that interferes with the n,π* excitation of the carbonyl chromophore. This conclusion is supported by the fact that
o‑hydroxybenzophenone does not undergo photoreduction and is used as a photostabiliser (
Scheme 7).
Scheme 6.
Mechanism of the formation of the lactone.
Scheme 6.
Mechanism of the formation of the lactone.
Scheme 7.
Photostabilization of o-hydroxybenzophenone.
Scheme 7.
Photostabilization of o-hydroxybenzophenone.
Suginome and co-workers [
36] studied photo-oxygenation of dehydrorotenoids. Irradiation of dehydrorotenone (
22c) with a 150 W high-pressure mercury lamp in a dioxane-ethanol mixture containing sodium borohydride yielded rotenonone (
23c, 40%) (
Scheme 8). In the absence of sodium borohydride no isolable product was formed. It was postulated that sodium borohydride converted intermediary hydroperoxides (the product of singlet oxygen attack on the methylene moiety) into hemiacetals that converted to the lactone carbonyl in the product. Sodium borohydride may also destroy free radical initiators and prevent unwanted side reactions.
Table 1 gives yields obtained with analogues of dehydrorotenones
23a-d. Rotenone and isorotenone were inert under the reaction conditions.
Scheme 8.
Photoinduced oxygenation of dehydrorotenones.
Scheme 8.
Photoinduced oxygenation of dehydrorotenones.
Table 1.
Photoinduced oxygenation of dehydrorotenones.
Table 1.
Photoinduced oxygenation of dehydrorotenones.
| Irradiated compound 22 | Product 23 (time of irrad./h) | Yield (%)
with NaBH4 | Yield (%)
without NaBH4 |
|---|
| a | a (44) | 40 | trace |
| b | b (24) | 9 | trace |
| c | c (24) | 10 | trace |
| d | d (94)* | 16 | trace |
| Rotenone (
22c: 6a,12a-dihydro-) | (
23c: 6a,12a-dihydro-) | 0 | 0 |
| Isorotenone (
22a: 6a,12a-dihydro-) | (
23a: 6a,12a-dihydro-) | 0 | 0 |
Thakur and co-workers [
37] investigated the photochemistry of 3-
O-propargylflavonol (
24). Irradiation with Pyrex filtered light from a 125 W Hg lamp in dry benzene furnished cyclized products
25a (20%),
25b (16%),
26a (35%) and
26b (30%), as shown in
Scheme 9.
Scheme 9.
Phototransformations of 3-O-propargylflavonol.
Scheme 9.
Phototransformations of 3-O-propargylflavonol.
The mechanism was rationalised in terms of γ-hydrogen abstraction from the propargyl group by the excited carbonyl group followed by cyclisation of the 1,4-biradical (
Scheme 10).
Scheme 10.
1,4-Hydrogen abstraction in propargylethers.
Scheme 10.
1,4-Hydrogen abstraction in propargylethers.
Matsuura and co-workers [
38] irradiated flavonol
27b in isopropyl alcohol-benzene with a high pressure mercury lamp through Pyrex and obtained 3-aryl-3-hydroxy-1,2-inandione (
28b) in almost quantitative yield. Irradiation of 2'-methoxyflavonol
27a yielded
28a. A 2,3-epoxy-2-hydroxy-1-inandone intermediate
29, formed
via a formal [
σ2 +
π2] cycloaddition, was postulated (
Scheme 11).
Scheme 11.
Photorearrangement of 3-hydroxyflavones.
Scheme 11.
Photorearrangement of 3-hydroxyflavones.
Subsequently Matsuura and co-workers [
39] expanded the range of photolysed flavonols (
Scheme 12).
Table 2 includes previous results of Matsuura
et al. [
38] and the yields. Free phenolic quercetin (
30e) resisted photo-transformation. This could be due to tautomerisation at the 4-CO and 5-OH (cf. photo-enolisation of
o-hydroxyacetophenone [
40,
41]), internal quenching
via the hydrogen bond between 4-CO and 5-OH, or deprotonation from the excited state (cf. resistance of 3-hydroxy-benzophenone to photoreduction [
42]). Irradiation of the 3-
O-methoxyflavonol
32 yielded a tetracyclic product
33. The replacement of the 3-OH group (
i.e. 30) with a 3-OMe group (compound
32) prevents the rearrangement to an inandione (compound
31) (
Scheme 13).
Scheme 12.
Photorearrangement of 3-hydroxyflavones to 3-aryl-3-hydroxy-1,2-indandiones.
Scheme 12.
Photorearrangement of 3-hydroxyflavones to 3-aryl-3-hydroxy-1,2-indandiones.
Table 2.
Yields of indandiones obtained by photorearrangement of 3-hydroxyflavones.
Table 2.
Yields of indandiones obtained by photorearrangement of 3-hydroxyflavones.
| 30 | R1 | R2 | R3 | R4 | R5 | 31 | Yield (%) |
|---|
| a | H | H | OMe | H | J | a | Quant. |
| b | H | H | H | H | H | b | 84 |
| c | H | H | H | H | OMe | c | 71 |
| d | OMe | OMe | H | OMe | OMe | d | 69 |
| e | OH | OH | H | OH | OH | e | - |
Scheme 13.
3-OMe-group prevention of the rearrangement to an inandione.
Scheme 13.
3-OMe-group prevention of the rearrangement to an inandione.
Yokoe and co-workers [
43] irradiated flavonol
34a in methanol with a high-pressure mercury lamp and obtained, in addition to the 3-aryl-3-hydroxy-1,2-inandione (
35a, 8%) [
38], a phthalide
37a (0.7%) (
Scheme 14).
Scheme 14.
Photorearrangement of flavonols.
Scheme 14.
Photorearrangement of flavonols.
The formation of phthalide
37a was explained in terms of a photoinduced carbon monoxide loss from C-4
via a repeated Norrish type I process (α-fission) of the aliphatic carbonyl group (
Scheme 15). The reaction was repeated with a range of substituted flavonols (
Scheme 14 and
Table 3) and in some cases an aromatic α-ketoacid, of type
36 was isolated. It was concluded that the formation of phthalides was, despite the poor yields, a general photoreaction of flavonols. In some cases a benzoic acid degradation product was also isolated. The presence of metal ions such as Cu
2+, Ni
2+, Fe
3+, Co
2+, and Be
2+ prevented the photochemical rearrangement of flavonols. In contrast to these ions, Ca
2+, Mg
2+, and Hg
2+ had no effects on the photochemical reactivities of flavonols.
Table 3.
Products obtained by the irradiation of flavonol in methanol.
Table 3.
Products obtained by the irradiation of flavonol in methanol.
| | Yields (%) |
|---|
| 34 | 35 | 36 | 37 |
| a, R1 = H, R2 = H, R3 = H | 8 | | 0.7 |
| b, R1 = H, R2 = Me, R3 = H | 44 | | 2 |
| c, R1 = H, R2 = OMe, R3 = H | | 6 | 12 |
| d, R1 = H, R2 = O-CH2-O = R3 | | 14 | 6 |
| e, R1 = Me, R2 = H, R3 = H | 15 | | 2 |
| f, R1 = Me, R2 = Me, R3 = H | | | 3 |
| g, R1 = Me, R2 = OMe, R3 = H | | 1 | 0.2 |
| h, R1 = Me, R2 = O-CH2-O = R3 | | 4 | |
Scheme 15.
Mechanism of Norrish type I formation of phthalide.
Scheme 15.
Mechanism of Norrish type I formation of phthalide.
Ficarra and co-workers [
44] obtained only the indandione
35a upon irradiation of flavonol
34a in aerated or oxygen-free acetonitrile and dichloromehane solutions (high pressure mercury lamp with Bausch Lamb monochromator) (
Scheme 16). No indication of the formation of photo-oxygenated products was found. This contrasts with the results of Yokoe and co-workers [
43], where the oxygenated product dominated. They concluded that photo-rearrangement takes place from a singlet excited phototautomer (
1PT) and photo-oxygenation from a triplet phototautomer (
3PT). Alcoholic solvents (protic polar solvents) slow down rearrangements from (
1PT) and inhibit photo-rearrangement at the expense of photo-oxygenation from (
3PT). Aprotic polar solvents have the opposite effect and do not interfere with photo-rearrangement of (
1PT). Their formation of only the oxygenated product in heptane and other non-polar solvents was explained by a rapid conversion of (
1PT) to (
3PT) in non-polar solvents. They thus concluded that molecular oxygen affects photochemical reactions of flavones in alcoholic (protic-polar) and hydrocarbon (non-polar) solutions and does not participate in acetonitrile and dichloromethane (aprotic-polar) (
Figure 3).
Scheme 16.
Irradiation of flavonol oxygen-free acetonitrile and dichloromehane solutions.
Scheme 16.
Irradiation of flavonol oxygen-free acetonitrile and dichloromehane solutions.
Figure 3.
Proposed flavonol excited states: (GS ground state (singlet), 1ES excited state (singlet), PT ground state of phototautomer (singlet), its singlet excited state (1PT) and triplet excited state (3PT) a = light absorbtion; b = intersystem crossing; c and d = photochemical reaction; e = light energy emission).
Figure 3.
Proposed flavonol excited states: (GS ground state (singlet), 1ES excited state (singlet), PT ground state of phototautomer (singlet), its singlet excited state (1PT) and triplet excited state (3PT) a = light absorbtion; b = intersystem crossing; c and d = photochemical reaction; e = light energy emission).
Chen and co-workers [
45] investigated the photoinduced electron transfer reactions of flavones with amines. Irradiation of flavone
38 with 0.1 M triethylamine in acetonitrile under argon at >300 nm yielded
meso-2,2'-biflavanone
39, (±)-
meso-2,2'-biflavanone
40 and flavone [I-4,II-2]-flavanol
41 (
Scheme 17). Different amines were used (TEA, DMBA, DMAE, DMA) in acetonitrile and benzene to afford yields, based on consumed flavone
38, as shown in
Table 4. These products resulted from radical addition of 4-ketyl
43 and/or its isomeric 1,2-ketyl anion to flavones, respectively (
Scheme 19). Single electron transfer (SET) [
46] is a well-known photoreaction between amines and α,β-unsaturated carbonyl compounds. The amine donates an electron to form an exciplex or a contact ion radical pair [
47] (CIP) that undergoes hydrogen transfer to yield the radical responsible for dimerisation.
Scheme 17.
Photoinduced electron transfer reactions of flavone with amines.
Scheme 17.
Photoinduced electron transfer reactions of flavone with amines.
Table 4.
Photoinduced reactions of flavone with amines.
Table 4.
Photoinduced reactions of flavone with amines.
| Amine | Solvent | Irrad. Time/h | Conversion (%) | Products (yield, %) |
|---|
| TEA | ACN | 14 | 100 | 38 (38), 39 (14), 40 (25) |
| TEA | benzene | 25 | 100 | 38 (51), 40 (45) |
| DMBA | ACN | 18 | 100 | 38 (53), 40 (22) |
| DMAE | benzene | 12 | 89 | 38 (7), 39 (50), 40 (16) |
| DMA | ACN | 14 | 100 | 38 (41), 3 (35), 41 (18) |
| DMA | benzene | 12 | 68 | 38 (19), 39 (10), 40 (15), 41 (30) |
Chen and co-workers [
47] reinvestigated the photoinduced electron transfer reactions of flavone
38 with amines that was studied by his namesake in 1995. They used triethylamine or 2-(
N,
N-dimethylamino)ethanol at 250 and 300 nm in benzene, dichloromethane or acetonitrile, respectively, and isolated
meso-2,2'-biflavone
39, (+/-)-
meso-2,2'-biflavone
40 and flavanone
42 (
Scheme 18).
Scheme 18.
Photochemical synthesis of 2,2'-biflavanones from flavone.
Scheme 18.
Photochemical synthesis of 2,2'-biflavanones from flavone.
The mechanism of photolysis was proposed as shown in
Scheme 19. The yields and the ratio between
39 and
40 depended on the molar ratio of flavone to amine, the type of amine, and the solvent and the light source (Sankyo 254 nm Germicidal lamp or 306 UV-B lamp) (
Table 5). The combined yield of
meso-
39 and (+/-)-
meso-
40 varied beween 17.3 and 50.9%. They also isolated flavanone
42, resulting from photo-reduction, in yields of between 8.9 and 16.9%, depending on the reaction conditions (
Table 5). The absence of flavone [I-4,II-2]-flavan-4-ol
41 as described by Chen and co workers [
45] above, was attributed to the use of light with a wavelength longer than 300 nm.
Scheme 19.
Proposed mechanism of photoinduced biflavanone formation with amines.
Scheme 19.
Proposed mechanism of photoinduced biflavanone formation with amines.
Table 5.
Yields and conditions of photoinduced biflavanone formation with amines.
Table 5.
Yields and conditions of photoinduced biflavanone formation with amines.
| Reaction Conditions | Conversion (%) | Yields (%)*4 |
|---|
| Molar ratios*1, Amines*2, Solvents, Irradiation Time, Irradiation Source*3 | 39 | 40 | 41 |
|---|
| 1/2, TEA, benzene, 25 hrs, A | 75.0 | 22.0 | 22.3 | 13.0 |
| 1/4, TEA, ACN, 25 hrs, A | 78.4 | 15.9 | 26.1 | 10.2 |
| 1/1, TEA, ACN, 14 hrs, A | 88.5 | 12.2 | 9.7 | 11.6 |
| 1/2, TEA, ACN, 14 hrs, A | 78.0 | 20.9 | 30.0 | 15.8 |
| 1/4, TEA, ACN, 14 hrs, A | 85.4 | 16.7 | 14.6 | 14.4 |
| 1/10, TEA, ACN, 14 hrs, A | 82.6 | 29.7 | 13.5 | 15.8 |
| 1/2, DMAE, benzene, 12 hrs, A | 88.8 | 15.2 | 22.8 | 13.7 |
| 1/2, TEA, DCM, 16 hrs, A | 71.1 | 17.4 | 7.9 | 16.9 |
| 1/2, TEA, benzene, 25 hrs, B | 78.7 | 17.6 | 7.5 | 8.9 |
| 1/2, TEA, ACN, 14 hrs, B | 75.8 | 12.5 | 4.8 | 11.9 |
| 1/2, DMAE, benzene, 14 hrs, A | 79.1 | 9.6 | 19.3 | 11.2 |
Yokoe and co-workers [
48] obtained similar results. Photolysis (450 W high pressure mercury lamp, Pyrex filter) of flavone
44 in a methanol-water solution in the presence of sodium sulphite yielded
meso-2,2'-biflavanone
45 and (+/-)-
meso-2,2'-biflavanone
46.
Scheme 20.
Photoinduced formation of biflavanones with sodium sulphite.
Scheme 20.
Photoinduced formation of biflavanones with sodium sulphite.
Schönberg and Khandelwahl [
49] studied 1,2-cycloaddition to flavones. Irradiation of flavones
47a and
47b, respectively, -and diphenylacetylene in benzene with a Hanau quartz lamp in a quartz or Pyrex vessel yielded the corresponding cyclobutabenzopyrans
48a and
48b, respectively. Products
50,
52 and
54, respectively, were isolated upon irradiation of isoflavone
49, 7,8-benzoflavone
51 and 1-thioflavone
53, respectively. Yields were almost quantitative.
Scheme 21.
1,2-Cycloadition of diphenylacetylene and flavone.
Scheme 21.
1,2-Cycloadition of diphenylacetylene and flavone.
Gerard and co-workers [
50] described 1,3-dipolar cycloaddition between methyl cinnamate (
55) and flavonol
56 to obtain products
57 to
60 (rocaglamides)
via photochemical generation of an oxidopyrylium species in acetonitrile with a Hanovia UV lamp uranium filter (
Scheme 22).
Scheme 22.
Photochemical synthesis of rocaglamide.
Scheme 22.
Photochemical synthesis of rocaglamide.
Gerard and co-workers [
51] repeated the reaction in the presence of chiral Brønsted acids to obtain chiral rocaglamide derivatives. R
1 and R
2 are identical or different and selected from the group consisting of hydrogen, halogen, hydroxyl, alkoxy, aryloxy, heteoaryloxy, thioalkyl, thioaryl, and next variety of protecting groups.
Bhatacharyya and co-workers [
52] studied the photophysics of flavones. They concluded that flavone almost instantaneously forms a triplet state with a 90% intersystem crossing (ISC) yield after absorption of UV light. Polar solvents enhanced yields indicating a π,π*-character for the lowest triplet excited state. The flavone’s triplet is quenched by several typical triplet quenchers like hydrogen donors [
53], including amines [
54].
Christoff and co-workers [
55] investigated the photophysics of 3-methoxy- and 7-methoxyflavone. A 7-methoxy substituent increases the π,π*-character of the excited state further but does not change the energy level or known flavone deactivation pathways. In contrast, a 3-methoxy substituent leads to a strong geometric constraint which interferes with planar π-orbital conjugation between the carbonyl group and the aromatic ring. This reduces the π,π*-character and increases the n,π*-character and the triplet state energy. The methoxy group becomes an intramolecular hydrogen donor to the excited carbonyl n,π*-triplet. The spectroscopic properties of the transient species are compatible with a 1,4-biradical structure. Conventional photolysis indicates that the biradical is transformed into an ethereal ethylene group. The mechanism for the photocyclisation is given in
Scheme 23.
Scheme 23.
Mechanism of photocyclisation of the 3-methoxyflavone.
Scheme 23.
Mechanism of photocyclisation of the 3-methoxyflavone.
3. Chalcone and Flavanone Photochemistry
Chalcones and α-hydroxychalcones are key intermediates in flavonoid synthesis and biosynthesis and the photochemistry of these compounds has attracted much early interest. Of major interest is
cis-trans isomerisation of the olefinic bond and reversible interconversion of 2'-hydroxychalcones and flavanes. Lutz and Jordan [
56] obtained a photo-stationary mixture of 74%
cis- and 26%
trans-chalcone upon leaving the chalcone in benzene in sunlight. The
trans-isomer absorbs light more efficiently and is depleted compared to the
cis-isomer under the reversible reaction conditions. Volsteedt and co-workers (1973) [
57] isomerised
trans-α,2',4,4',6'-pentamethoxychalcone (
61) to the
cis form
62 in methanol at 350 nm (
Scheme 24).
Scheme 24.
Trans-cis – photoinduced isomerisation of α-methoxychalcone.
Scheme 24.
Trans-cis – photoinduced isomerisation of α-methoxychalcone.
Ferreira and Roux [
58] found that a
trans-chalcone with a free hydroxy group (e.g., 2'-hydroxy-4,4',6'-trimethoxy- and 4-hydroxy-2',4',6'-trimethoxy
-trans-chalcone
63a,
63b and
63c) did not undergo
cis-
trans isomerisation in methanol at 300 or 350 nm. Their
O-methoxymethyl derivatives
63d and
63e did undergo the desired isomerisations to a mixture of 60%
trans (compound
64d) and 40%
cis (compound
64e) (
Scheme 25). The products were, however, metastable and spontaneously reverted to the
trans isomer (half-life of about six months). Removal of the
O-methoxymethyl protecting group of the
cis chalcones
64d and
64e with acid, yielded the corresponding
trans isomers
63b and
63c. In contrast 2'-hydroxy-α-methoxy-
cis-chalcones
64f and
64g are relatively stable (
Figure 4).
Scheme 25.
Trans-cis – photoinduced isomerisation of α-methoxychalcones.
Scheme 25.
Trans-cis – photoinduced isomerisation of α-methoxychalcones.
Figure 4.
Structures of photochemical relatively stable 2'-hydroxy-α-methoxy-cis-chalcones
Figure 4.
Structures of photochemical relatively stable 2'-hydroxy-α-methoxy-cis-chalcones
Dewar and Sutherland [
59] transformed 2''-hydroxychalcone
65 into 2-ethoxy- or 2-methoxyflav-3-ene (compounds
66a or
b) (96%), and trace amounts of flavones (1%), upon irradiation at 350 nm in ethanol or methanol (
Scheme 26). The product (
67a or
b) was smoothly converted to a flavylium salt with acid.
Scheme 26.
The photochemistry of 2''-hydroxychalcone.
Scheme 26.
The photochemistry of 2''-hydroxychalcone.
Mack and Pinhey [
60] transformed flavanone
68,
via fragmentation of the heterocyclic benzylic O-C bond, into 2'-hydroxychalcone
70 (20%) in benzene at 250 nm. They also isolated salicylic acid (
73, 4%)
via the subsequent fragmentation of the C2-C3 bond to form a ketene
72 that can be trapped with water or methanol. The 4-phenyldihydrocoumarin (
74, 13%) was formed
via attack of the aromatic A-ring on the benzylic radical intermediate
69 to yield the intermediate dienone
71 (
Scheme 27). This reaction is related to the photo-Fries rearrangement of benzylic ethers.
Scheme 27.
Photochemistry of flavanone.
Scheme 27.
Photochemistry of flavanone.
Stermitz and co-workers [
61] transformed 2'-hydroxychalcone
70 to flavanone
68 (53%) upon irradiation with a Hanovia 450 W lamp with a Pyrex filter in benzene. It is known that the pKa values of phenols increase in the excited state [
62] and, thus, the reaction could have been acid self-catalyzed (
Scheme 28).
Scheme 28.
Photochemical transformation of 2'-hydroxychalcone to flavanone.
Scheme 28.
Photochemical transformation of 2'-hydroxychalcone to flavanone.
Matsushima and co-workers [
63] photolysed (100 W high pressure mercury lamp) flavanone
68 in benzene and obtained 2'-hydroxychalcone
70 (14%)
via cleavage of the pyrone ring. Repetition of the reaction in 2-propanol gave the pinacols
75 (39%) and solvent adducts
76 (8%) (
Scheme 29). Photolysis of 7,8-benzoflavanone
77 in 2-propanol gave no coupling products
79 and
80, but rather the ring-cleaved chalcone
78 (33%) (
Scheme 30). The lack of photo-reduction (coupling products) suggested that the lowest triplet state of 7,8-benzoflavanone was
3(π,π*) and that of the flavanones that had undergone photo-reduction to pinacols were
3(n,π*). Methoxylated flavanones are assumed to have considerable π,π* character in their lowest triplet state. This is supported by conversion of 4'-methoxyflavan to 2'-hydroxy-4-methoxyflavanone in yields of 69% in benzene (compared to the 14% conversion of flavanone), 66% in pyridine, 48% in acetonitrile and 31% in carbon tetrachloride.
Scheme 29.
Photolysis of flavanone in 2-propanol and benzene.
Scheme 29.
Photolysis of flavanone in 2-propanol and benzene.
Scheme 30.
Photolysis of 7,8-benzoflavanone.
Scheme 30.
Photolysis of 7,8-benzoflavanone.
Nakashima and co-workers [
64] investigated the photochemistry of 4′-methoxyflavanone (
81). They obtained chalcone
82 (14%), and a mixture of pinacols
83 (23%) upon irradiation of
81 with a high pressure Pyrex lamp in benzene or 2-propanol. 5,7,4'-Trimethoxyflavanone was inert in benzene and gave a complex mixture of at least six products that was not identified. 5,7-Dimethyl-4'-methoxyflavanone (
84) yielded bis-flavanone
85, probably
via intramolecular hydrogen abstraction by an n,π*-excited carbonyl from the 5-methyl group (
Scheme 31).
Scheme 31.
Photochemistry of 4'-methoxy- and 5,7-dimethyl-4'-methoxyflavanone.
Scheme 31.
Photochemistry of 4'-methoxy- and 5,7-dimethyl-4'-methoxyflavanone.
Matsushima and Kageyama [
65] studied the influence of the solvent and wavelength on the reactivity, reaction rate, and yield of photocyclisation of a series of 2'-hydroxychalcones
86a-e to flavanones
87a-e (
Scheme 32,
Table 6). They also investigated the effect of triplet quenchers and radical scavengers. All the chalcones were inert in chloroform and
t-butyl alcohol and all were reactive in benzene, ethyl acetate, and 1,4-dioxane (
Table 7). Light of a shorter wavelength gave slower reaction rates and more side reactions. The best results were obtained with wavelengths above 365 nm (
Table 8). Rates were highest in ethyl acetate and dioxane and low in benzene. The yields were the highest in ethyl acetate [e.g., 86% conversion of
86b] (
Table 9). Triplet quenchers and radical scavengers had no effect, suggesting that photocyclisation of 2'-hydroxychalcones is an ionic or polar reaction. Solvent effects are ambiguous, but it seems that polar aprotic (basic) solvents gave the best results, supporting the polar mechanism. Photo-enolization of 3-chromanones as described by Padwa
et al [
66], followed by
cis-
trans isomerisation that may require a second photon, seems to best explain the experimental results (
Scheme 33).
Scheme 32.
Photocyclization of 2'-hydroxychalcones to flavanones.
Scheme 32.
Photocyclization of 2'-hydroxychalcones to flavanones.
Table 6.
Photocyclization of 2'-hydroxychalcones to flavanones.
Table 6.
Photocyclization of 2'-hydroxychalcones to flavanones.
| 2'-Hydroxychalcone | R1 | R2 | Flavanone 87 | R1 | R2 |
|---|
| 86 |
|---|
| a | H | H | a | H | H |
| b | H | 2-OMe | b | H | 2'-OMe |
| c | 4'-OMe | H | c | 7-OMe | H |
| d | 6'-OMe | H | d | 5-OMe | H |
| e | 3',4'-Benzo | H | e | 7,8-Benzo | H |
Table 7.
Solvent effects on the photocyclization of 2'-hydroxychalcones to flavanones.
Table 7.
Solvent effects on the photocyclization of 2'-hydroxychalcones to flavanones.
| 2'-Hydroxychalcone | Benzene | CCl4 | CHCl3 | Et2O | EtOAc | 1,4-dioxane | ACN | EtOH | t-BuOH |
|---|
| 86 |
|---|
| a | 87a | 87a | - | 87a | 87a | 87a | 87a | 87a | - |
| b | 87b | - | - | 87b | 87b | 87b | 87b | 87b | - |
| c | 87c | - | - | 87c | 87c | 87c | - | - | - |
| d | 87d | - | - | 87d | 87d | 87d | - | - | - |
| e | 87e | - | - | | 87e | 87e | - | - | - |
Table 8.
Effect of the solvent on the relative rates of photochemical flavanone formation from 2'-hydroxychalcones.
Table 8.
Effect of the solvent on the relative rates of photochemical flavanone formation from 2'-hydroxychalcones.
| 2'-Hydroxychalcone 86 | Consumption of 2'-hydroxychalcone (%) in |
|---|
| Benzene | 1,4-dioxane | EtOAc |
|---|
| a | 8.2 | 94 | 95 |
| b | 10 | 97 | 91 |
| c | 14 | 35 | 63 |
| d | 30 | 30 | 84 |
| e | 7.4 | 1.4 | 1.3 |
Table 9.
Effect of irradiation time on the yield of photochemical flavanone 87b formation from 2'-hydroxychalcones.
Table 9.
Effect of irradiation time on the yield of photochemical flavanone 87b formation from 2'-hydroxychalcones.
| Solvent | Irradiation time/h | 86b (mM Recovered)a | 87b (mM Formed) | Yield of 87b (%)b |
|---|
| Benzene | 5 | 0.77 | 0.17 | 74 |
| | 10 | 0.66 | 0.24 | 71 |
| | 15 | 0.55 | 0.34 | 69 |
| | 20 | 0.52 | 0.40 | 83 |
| 1,4-dioxane | 1 | 0.79 | 0.17 | 82 |
| | 2 | 0.57 | 0.48 | 100 |
| | 3 | 0.31 | 0.55 | 79 |
| | 4 | 0.06 | 0.35 | 58 |
| EtOAc | 2 | 0.57 | 0.45 | 100 |
| | 3 | 0.32 | 0.62 | 91 |
| | 4.5 | 0.23 | 0.65 | 84 |
| | 6 | 0.14 | 0.86 | 100 |
Scheme 33.
Mechanism of photocyclization of 2'-hydroxychalcone to flavanone.
Scheme 33.
Mechanism of photocyclization of 2'-hydroxychalcone to flavanone.
Matsushima and Kageyama [
67] investigated the scope and mechanism of the photocyclisation of 2'-hydroxychalcones and photolysed a series of B-ring mono-substituted derivatives (
Scheme 34). They concluded that visible light (405 nm) gives the best results
via selective cyclisation, avoiding secondary reactions of the resulting flavanone such as hydrogen abstraction from the solvent or equilibration of the flavanone and chalcone. Methoxy and phenyl substituents on the B-ring enhanced the reaction rate while halogen atoms and a nitro group had little effect (
Table 10). Solvent effects on the consumption rate of
88a are given in
Table 11. Reaction rates are the highest in aprotic polar solvents, low in non-polar solvents and extremely low in hydroxylic solvents that interfere with intramolecular hydrogen bonding (except in
t-butyl alcohol). Ethyl acetate gave superior results compared to benzene and THF. Free radical inhibitors (2,6-di-
t-butylphenol, nitrosobenzene, or acrylonitrile) did not suppress the reaction, suggesting a non-radical mechanism as supported by increased reaction rates in polar solvents. Triplet quenchers ferrocene, cyclohexa-1,3-diene, anthracene, phenanthrene, or acenaphthylene had no effect, suggesting an excited singlet or very short-lived triplet state. Quantum yields were low. In π,π* excited states phenolic groups become more acidic and carbonyl groups more basic [
68,
69,
70] suggesting a charge transfer mechanism. 6-Methoxy-, 7-methoxy-, 4'-methoxy, 4'-carbomethoxy-, and 2-methyl-7-methoxyflavanones were also converted to the corresponding 2'-hydroxychalcones (no yields were reported).
Scheme 34.
Photochemical cyclization of 2'-hydroxychalcones.
Scheme 34.
Photochemical cyclization of 2'-hydroxychalcones.
Table 10.
Photocyclization of 2'-hydroxychalcones with visible light.
Table 10.
Photocyclization of 2'-hydroxychalcones with visible light.
| Compounda | Solvent | Irrad. time (h) | Consumption of 88 (mM) | Formation of 89 (mM) | (89)/-(88)b |
|---|
| 88 | ![Molecules 15 05196 i001]() |
|---|
| a | Phenyl | EtOAc | 10 | 0.79 | 0.72 | 0.91 |
| | | Benzene | 10 | 0.24 | 0.12 | 0.50 |
| | | THF | 5 | 0.30 | 0.25 | 0.83 |
| b | 4-Tolyl | EtOAc | 10 | 0.67 | 0.64 | 0.93 |
| | | Benzene | 10 | 0.18 | 0.14 | 0.77 |
| | | THF | 5 | 0.34 | 0.22 | 0.63 |
| c | 4-Methoxy-phenyl | EtOAc | 10 | 1.0 | 0.93 | 0.93 |
| | Benzene | 10 | 0.40 | 0.33 | 0.83 |
| | THF | 5 | 0..34 | 0.32 | 0.94 |
| d | 4-Fluorophenyl | EtOAc | 5 | 0.59 | c | |
| e | 4-Chlorophenyl | EtOAc | 10 | 0.93 | 0.82 | 0.88 |
| | | Benzene | 10 | 0.17 | 0.18 | 1.0 |
| | | THF | 5 | 0.49 | 0.37 | 0.75 |
| f | 4-Bromophenyl | EtOAc | 10 | 1.0 | 0.74 | 0.74 |
| | | Benzene | 10 | 0.17 | 0.16 | 0.94 |
| | | THF | 5 | 0.24 | 0.18 | 0.75 |
| g | 4-Nitrophenyl | EtOAc | 5 | 0.84 | c | |
| h | 2,4-Dimethoxy-phenyl | EtOAc | 10 | 1.0 | 0.96 | 0.96 |
| | Benzene | 10 | 0.62 | 0.50 | 0.81 |
| | THF | 10 | 0.77 | 0.70 | 0.91 |
| i | α-Naphthyl | EtOAc | 10 | 1.0 | 0.94 | 0.94 |
| | | Benzene | 10 | 0.45 | 0.32 | 0.71 |
| | | THF | 10 | 0.65 | 0.61 | 0.94 |
| j | Styryld | EtOAc | 10 | 0.45 | 0.31 | 0.69 |
| | | Benzene | 10 | 0.15 | 0.06 | 0.40 |
| | | THF | 10 | 0.15 | 0.09 | 0.60 |
Table 11.
Solvent effects on the consumption rate of 2'-hydroxychalcone 88a.
Table 11.
Solvent effects on the consumption rate of 2'-hydroxychalcone 88a.
| Solventa | Irrad. time (h) | Consumption (%) | Rate (% h-1b) |
|---|
| EtOAc | 0.5 | 7.0 | 14 |
| Dioxanec | 2 | 14 | 7.0 |
| Dioxane-H2O (95:5) | 20 | 12 | 0.6 |
| Dioxane-D2O (95:5) | 20 | 13 | 0.65 |
| THF
c | 2 | 11 | 5.4 |
| THF-H2O (90:10) | 5 | 6.4 | 1.3 |
| THF-H2O (90:10) | 5 | 7.0 | 1.4 |
| t-Butyl alcohol | 2 | 4.7 | 2.4 |
| DMF | 5 | 11 | 2.2 |
| Cyclohexane | 3 | 6.1 | 2.0 |
| ACN | 5 | 8.7 | 1.7 |
| ACN-H2O (95:5) | 50 | 0.0 | 0.0 |
| ACN-H2O (40:60) | 50 | 6.9 | 0.1 |
| Benzene | 14 | 8.1 | 0.6 |
| Tetrachloromethane | 40 | 11 | 0.3 |
| EtOH | 90 | 9.8 | 0.1 |
| MeOH | 90 | 7.7 | 0.1 |
| Chloroform | 40 | 0.0 | 0.0 |
Jain [
71] investigated the photochemistry of flavanones in alkaline medium. Flavanone
90a and 4-chloroflavanone
90b rearranged in alkaline medium (2 mL 10% NaOH in 2 mL ethanol) upon irradiation with UV of an unspecified source to the corresponding products
91a and
91b (
Scheme 35), but no yields were reported. A concerted mechanism was proposed (
Scheme 36).
Scheme 35.
Photochemistry of flavanone and 4'-chloroflavanone in alkaline medium.
Scheme 35.
Photochemistry of flavanone and 4'-chloroflavanone in alkaline medium.
Scheme 36.
Proposed mechanism of photochemical rearrangement of flavanone in alkaline medium.
Scheme 36.
Proposed mechanism of photochemical rearrangement of flavanone in alkaline medium.
Photochemical Fries rearrangement of phenyl cinnamates represents a convenient method to prepare 2'-hydroxychalcones. Obara and Takahashi [
72] irradiated phenyl cinnamate (
92) in benzene under nitrogen with a high-pressure 450 W mercury arc and obtained 2'-hydroxychalcone
70 (10%) and 4'-hydroxychalcone
93 (2%) (
Scheme 37).
Scheme 37.
The photochemical Fries rearrangement of phenyl cinnamate to 2'-hydroxy- and 4'-hydroxychalcone.
Scheme 37.
The photochemical Fries rearrangement of phenyl cinnamate to 2'-hydroxy- and 4'-hydroxychalcone.
Obara and co-workers [
73] repeated the reaction using a high-pressure 100 W mercury arc with 2-, 3-, and 4-hydroxyphenyl cinnamates
94a-c and obtained the corresponding 2',3'-, 2',4'-, and 2',5'-dihydroxychalcones
95a-c in yields of 20, 5 and 16%, respectively (
Scheme 38).
Scheme 38.
The photochemical Fries rearrangement of hydroxyphenyl cinnamate derivatives.
Scheme 38.
The photochemical Fries rearrangement of hydroxyphenyl cinnamate derivatives.
Onodera and Obara [
74] extended the work to
O-methoxymethyl-protected dihydroxyphenyl cinnamates
96a-c and obtained the corresponding hydroxy-bis(
O-methoxymethyl)-chalcones
97a-c (
Scheme 39).
Scheme 39.
The photochemical Fries rearrangement of O-methoxymethyl protected dihydroxyphenyl cinnamates to the corresponding hydroxychalcones.
Scheme 39.
The photochemical Fries rearrangement of O-methoxymethyl protected dihydroxyphenyl cinnamates to the corresponding hydroxychalcones.
Bhatia and Kagan [
75] transformed cinnamate
98 into 2',6'-dihydroxy-4'-methoxychalcone (
99a, 7%) and 2',4'-dihydroxy-6'-methoxychalcone (
99b, 12%) by irradiation in methanol at 250 nm (
Scheme 40).
Scheme 40.
The photochemical Fries rearrangement of 3'-hydroxy-5'-methoxycinnamate into 2',6'-dihydroxy-4'-methoxychalcone.
Scheme 40.
The photochemical Fries rearrangement of 3'-hydroxy-5'-methoxycinnamate into 2',6'-dihydroxy-4'-methoxychalcone.
Ramakrishnan and Kagan [
76] studied the photo-Fries reaction with the view of obtaining chalcones with the complex substitution patterns found in plants. A variety of chalcone derivatives were irradiated at 254 nm in different solvents, as given in
Scheme 41 and
Table 12.
Scheme 41.
The photochemical synthesis of 2'-hydroxychalcones from phenyl cinnamates.
Scheme 41.
The photochemical synthesis of 2'-hydroxychalcones from phenyl cinnamates.
Table 12.
R1 - R6 substituents on structure (100) and (101).
Table 12.
R1 - R6 substituents on structure (100) and (101).
| Substituent on structure 100 and 101 | Solvent | Irradiation time (h) |
|---|
| a, R1 = R2 = R3 = R4 = R5 = R6 = H | MeOH or chloroform | 20 |
| b, R1 = OMe, R2 = R3 = R4 = R5 = R6 = H | chloroform | 40 |
| c, R2 = OH, R1 = R3 = R4 = R5 = R6 = H | EtOH | 45 |
| d, R3 = OH, R1 = R2 = R4 = R5 = R6 = H )* | | |
| e, R2 = R3 = OH, R1 = R4 = R5 = R6 = H | MeOH | 22 |
| f, R2 = R3 = R4 = OH, R1 = R5 = R6 = H | MeOH | 16 |
| g, R2 = R3 = R6 = OH, R1 = R4 = R5 = H | MeOH | 22 |
| h, R2 = R3 = OH, R6 = OMe, R1 = R4 = R5 = H | MeOH | 22 |
| i, R2 = R3 = R5 = R6 = OH, R1 = R4 = H | MeOH | 36 |
| j, R2 = R3 = R6 = OH, R5 = OMe, R1 = R4 = H | MeOH | 17 |
| k, R2 = R3 = OH, R5 = R6 = OMe, R1 = R4 = H | MeOH | 36 |
Yields varied between 20 and 50% and it was postulated that the forming chalcones acted as internal filters and prevented complete conversion of the cinnamates. No cis-chalcones were isolated. After confirming that the photo-Fries reaction proceeded with the sample ester 100a, which yielded 101a as the major rearrangement product, different substituents were introduced on the A ring and it was found that the 2-methoxy- (compound 100b) and 3-hydroxy- (compound 100c) phenylcinnamates yielded the products of ortho migration, namely 101b from 100b and a mixture of 101c and 101d from 100c. Finally the set of compounds 100e-k was photolysed to get 101e-101k. The photo-Fries reaction of the phloroglucinol mono-ester is particularly simple since, for reasons of symmetry, the two products of ortho migration and that of para migration are identical.
Scheme 42.
Proposed mechanism of the photo-oxygenation of chalcones.
Scheme 42.
Proposed mechanism of the photo-oxygenation of chalcones.
Chawla and Chibber [
77] studied the photo-oxygenation of chalcones. Irradiation of 2',4',6'-trihydroxychalcone (
102) in methanol containing catalytic amounts of methylene blue under air with a 100 W tungsten lamp yielded the corresponding flavonol
103. The suggested mechanism is given in
Scheme 42. No yields were reported. No reaction took place in the absence of methylene blue, suggesting the involvement of singlet molecular oxygen. Replacement of methylene blue with rose bengal gave lower yields, probably due to a lower concentration of
1Δ
g oxygen and the formation of
1E
g oxygen which leads to the formation of side products [
78].
Subsequently Chawla and coworkers [
79] studied the conversion of chalcones into dihydroflavonols. Irradiation of 2'-hydroxy-4',6',3,4-tetramethoxychalcone (
104) in 4% aqueous methanol and a catalytic amount of methylene blue with a 100 W tungsten flood lamp yielded the corresponding flavanol
105 (compared to flavonol
103 in previous paper [
75]) (
Scheme 43), but no 2,3-relative stereochemistry was indicated. No reaction was observed in non-aqueous solvents such as benzene, benzene-methanol mixtures, or absolute methanol. Upon addition of small amounts of water (ca. 5%) the product was observed. This suggests that water is the source of the 3-OH in the flavanol product. Quinol, a well known radical quencher, inhibited the reaction, suggesting a radical mechanism. No yield was reported. The reaction was compared to the chlorophyll sensitised oxygenation in plants.
Scheme 43.
Photochemical conversion of chalcones to flavanols.
Scheme 43.
Photochemical conversion of chalcones to flavanols.
Climent and co-workers [
80] investigated the electron-transfer dehydrogenation of flavanones. They transformed flavanones
106a-c,e into the corresponding flavones
107a-c,e in yields ranging from 50 to 69% (
Scheme 44,
Table 13) using triphenylpyrilium tetrafluoroborate (TPT) in dichloromethane with a Pyrex immersion well and 125 W medium pressure lamp (potassium chromate solution as filter).
Scheme 44.
Photosensitized dehydrogenation of flavanones to flavones with 2,4,6-triphenylpyrylium tetrafluoroborate (TPT).
Scheme 44.
Photosensitized dehydrogenation of flavanones to flavones with 2,4,6-triphenylpyrylium tetrafluoroborate (TPT).
Table 13.
The effect of substituents on the yield of photosensitized dehydrogenation of flavanones to flavones with 2,4,6-triphenylpyrylium tetrafluoroborate (TPT) (R1 – R4 substituents on 106 and 107).
Table 13.
The effect of substituents on the yield of photosensitized dehydrogenation of flavanones to flavones with 2,4,6-triphenylpyrylium tetrafluoroborate (TPT) (R1 – R4 substituents on 106 and 107).
| Substituent | Yield (%) |
|---|
| a, R1 = R2 = R3 = R4 = H | 59 |
| b, R2 = OMe, R1 = R3 = R4 = H | 68 |
| c, R4 = OMe, R1 = R2 = R3 = H | 69 |
| d, R4 = NO2, R1 = R2 = R3 = H | 0 |
| e, R1 = R3 = Me, R2 = R4 = H | 52* |
4'-Nitroflavanone
106d was inert under the reaction conditions. The mechanism was rationalised in terms of an initial single electron transfer from the aromatic B-ring of the flavanone to the excited pyrilium salt to give the radical cation
108 or
110 which disproportionated to the flavones. Prevention of the reaction by an electron-withdrawing nitro group and acceleration by electron-donating methoxy substituents supports this mechanism (
Scheme 45).
Scheme 45.
Proposed single electron transfer mechanism of photosensitized dehydrogenation of flavanones to flavones with 2,4,6-triphenylpyrylium tetrafluoroborate (TPT).
Scheme 45.
Proposed single electron transfer mechanism of photosensitized dehydrogenation of flavanones to flavones with 2,4,6-triphenylpyrylium tetrafluoroborate (TPT).
5. Reactions Initiated via Hydrogen Abstraction by an Excited State Carbonyl
The excited state carbonyl group, generally assumed to be 3(n,π*), may abstract a suitably positioned hydrogen to form a biradical intermediate that may undergo further rearrangements to novel products. Photochemical keto-enol isomerisation and further transformations of the enol is included in hydrogen abstraction by carbonyls.
Scheme 50.
Photochemistry of 2,3-trans-flavanols.
Scheme 50.
Photochemistry of 2,3-trans-flavanols.
Fourie and co-workers [
86] studied the photochemistry of 2,3-
trans-flavanols
131a-c. Irradiation of free-phenolic (+/-)-2,3-
trans-fustin (
131a) at 300 nm in methanol yielded the thermodynamically less stable 2,3-
cis isomer
133 (27%) after complete methylation, and the isoflavone analogue
135 after final reduction and methylation. Both these products may form
via an enol intermediate
132. The tri-
O-methylether
131b gave none of the products obtained from free-phenolic fustin
131a, but instead underwent homolysis of both the heterocyclic O-C and α-carbonyl bonds (Norrish type I process) to yield methyl-2-hydroxy-4-methoxybenzoate
137via trapping of the intermediary ketene
136 (25%) (
Scheme 50). In contrast to the products obtained from free-phenolic and tri-
O-methyl fustin, the tetra-
O-methyl analogue
131c gave 2'-hydroxy-3,4,4'-trimethoxy-
trans-chalcone (
138, 58%) and tri-
O-methylflavanone
139 (34%), as shown in
Scheme 51. The mechanism requires abstraction of a hydrogen atom from the 3-methoxy group by the excited carbonyl chromophore, followed by formaldehyde loss. The absence of the
cis-chalcone suggested slow
trans-
cis isomerisation, probably due to inefficient light absorption at 300 nm.
Scheme 51.
Photochemistry of tetra-O-methyl fustin (permethylated flavanol).
Scheme 51.
Photochemistry of tetra-O-methyl fustin (permethylated flavanol).
Van der Westhuizen and co-workers [
87] described the photochemical deoxygenation of the series of flavanones depicted in
Figure 5.
Figure 5.
Flavanones and flavone obtained from flavanols.
Figure 5.
Flavanones and flavone obtained from flavanols.
Irradiation of (+)-fustin (140a) at 350 nm in dry ethyl acetate under nitrogen yielded the flavanone (-)-butin (141a) (2S) (37%). In the case of tri-O-methylfustin (140b) both the corresponding flavanone 141b (21%) and flavone 142b (15%) were isolated. (-)-Fustin gave the flavanone with the inverse configuration (2R). Addition of phloroglucinol as a hydrogen donor increased the yield of 141a to 47%, of 141b to 45%, and of 142b to 28%. Naphthalene, as triplet quencher, reduced the yield of 141a to 7% and increased the yield of the flavone 142b to 53%.
Scheme 52.
Photochemical deoxygenation of an α-ketol to a flavone.
Scheme 52.
Photochemical deoxygenation of an α-ketol to a flavone.
These results indicate that deoxygenation takes place from an n,π*-excited state
via hydrogen abstraction. Phloroglucinol probably enhances this process. Efforts to transform the flavanone
141b into the flavone photolytically led to only minor transformation to the flavone
142b (6%). It would appear that the flavanone
141b is not an intermediate in the formation of
142b from
140b. Nascent oxygen liberated during deoxygenation may be responsible for the oxidation of
141b to
142b (
Scheme 52). The low conversion of the flavanone
141b to the isomeric
trans-chalcone
143 in ethyl acetate (
Scheme 53) contrasts with the results in polar solvents (MeOH) where the equilibrium is towards the chalcone (
Scheme 51) [
86]. This allows isolation of optically pure flavanone
141b.
Scheme 53.
Photochemical conversion of flavanone to trans-chalcone.
Scheme 53.
Photochemical conversion of flavanone to trans-chalcone.
(+)-Tri-O-methyldihydroquercetin (140d) has a hydroxyl group at C-5 that may form a hydrogen bond with the carbonyl chromophore thus resisting photo-transformation, and hampering the general applicability of the method. Efforts to overcome the problem with triplet sensitizers gave poor results (2%), but addition of phloroglucinol increased the yields of the deoxygenated product 141d to 23%.
Van der Weshuizen and co-workers [
88] investigated the photochemical methoxy-hydroxymethyl isomerization of 4-methoxybenzo[b]furan-3(2
H)-ones
144a and
144b. Irradiation of 2-benzyl-2-hydroxybenzo[
b]furan-3(2
H)-one (
144a) at 350 nm in anhydrous ethyl acetate under nitrogen yielded
149 (21%) and
150 (16%). Irradiation of
144b yielded
147b. These products require methoxy-hydroxymethyl isomerisation of the 4-methoxy substituent (
Scheme 54).
Scheme 54.
Photochemical methoxy-hydroxymethyl isomerization of 4-methoxy-benzo[b]furan-3(2H)-ones.
Scheme 54.
Photochemical methoxy-hydroxymethyl isomerization of 4-methoxy-benzo[b]furan-3(2H)-ones.
In
144a the 2-hydroxy allows incorporation of the aromatic -CH
2OH group in the heterocyclic ring that changes the five-membered to a six-membered ring. In the case of the fully methylated 2-methoxybenzo[
b]furan-3(2
H)-one (
144b), the five-membered ring resisted ring-opening and the product with a free -CH
2OH (compound
147b) was isolated (11%). It was postulated that the benzylic acid rearrangement has ionic character and takes place from a π,π* excited state which has ionic character and is encouraged by polar solvents [
89]. Formation of
150 is described in
Scheme 60 [
90].
6. Reactions of Flavonoids with a Fully Saturated C-Ring (no Carbonyl Chromophore). Benzyl Ether Fission
Benzyl ethers typically undergo photolytic fission of the C-O bond. This is assisted by the stability of the benzylic radical or ionic intermediates. The heterocyclic C-ring of flavonoids contains an intramolecular benzylic ether bond.
Scheme 55.
Photochemistry of flavan-3-ols.
Scheme 55.
Photochemistry of flavan-3-ols.
Fourie and co-workers [
86] studied the photochemistry of flavan-3-ols. Irradiation of free phenolic flavan-3-ol
153c, fisetinidol (
153a) and its tetra-
O-methyl ether
153b at 300 nm in methanol yielded the corresponding optically active 1,3-diarylpropan-2-ols
156a and
156b (in 64 and 40% yield, respectively). The mechanism involves fission of the heterocyclic benzylic
O-C bond and trapping of the resulting intermediary benzylic carbocation
155 with methanol.
The methyl ether
153b also yielded 5-methoxy-2-methoxymethylphenol (
158),
via subsequent fission of the C3-C4 bond of radical
154 and trapping of the resulting
ortho quinone methide
157 with methanol. The absence of the free phenolic analogue of
157 probably indicates stabilization of the free-phenolic benzylic carbocation as a
para-quinone methide. Irradiation of the tetra-
O-methyl ether
153b in benzene did not give any product. Benzene is probably not sufficiently polar to stabilise the intermediate carbocation
155 (
Scheme 55). Under identical conditions free-phenolic catechin
153c gave the corresponding diarylpropan-2-ol
156c (20%). Irradiation of flavan-3-ols results in homolysis of the heterocyclic 1,2-(O-C) and 3,4-(C-C) bonds.
Van der Westhuizen and co-workers [
91] studied the photochemistry of tannin analogues. Benzophenone sensitized photolysis of free phenolic (2
R,3
S,4
S)-2,3-
trans-3,4-
trans-4-(2,4,6-tri-hydroxyphenyl)-flavan-3-ol (
159) at 350 nm in acetone yielded (2
S,3
S,4
S)-2,3-
cis-3,4-
cis-4-(2,4-dihydroxyphenyl)-flavan-3-ol (
160).
Scheme 56.
Synthesis of 2,3-cis-3,4-cis 4-arylflavan-3-ols by photolytic rearrangement.
Scheme 56.
Synthesis of 2,3-cis-3,4-cis 4-arylflavan-3-ols by photolytic rearrangement.
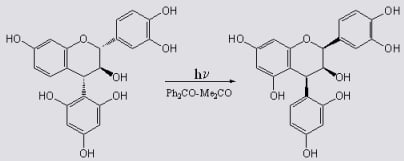
The mechanism involves formal cleavage of the heterocyclic ether bond followed by recyclisation via the quinone methide-stabilized benzylic carbocation 166 involving attack by the more nucleophilic phloroglucinol D-ring (isomerisation of the A-and D-ring) on the α-face of C-2 (anti to the 3-hydroxy substituent).
Inversion of configuration at C4 is thus a result of rotation about the C3-C4 bond and inversion of configuration at C2 is probably controlled by the orientation of the 3-OH substituent via a protonated oxirane intermediate 169. Traces of the (2R,3S,4R) 2,3-trans-3,4-cis-4-(2,4,6-trihydroxyphenyl)-flavan-3-ol (163), were also isolated.
Scheme 57.
Proposed mechanism for photochemical inversion of configuration at C-4 of 4-arylflavan-3-ols.
Scheme 57.
Proposed mechanism for photochemical inversion of configuration at C-4 of 4-arylflavan-3-ols.
Formation of
161 involves no ring-isomerisation but only inversion of configuration at C4, probably
via an A-ring quinone methide yielded the (2
R,3
R,4
R) 2,3-
cis-3,4-
cis product
163. The stereochemistry at C4 was determined by rotation about the C3-C4 bond and configuration at C2 by the orientation of the hydroxy group on C3. With a weak nucleophilic D-ring, isomerisation of the A- and D-ring is not observed. Photolysis of (2
R,3
S,4
R) 2,3-
trans-3,4-
trans-4-(2,4,-dihydroxyphenyl)-flavan-3-ol (
164) yielded the (2
R,3
S,4
S) 2,3-
trans-3,4-
cis isomer
165. Inversion of configuration at C4 probably takes place
via an A-ring quinone methide, similar to
170 described above. The reactions are given in
Scheme 56 and a suggested mechanism provided in
Scheme 57.
9. Diverse Reactions
Sundaryoano and co-workers [
29] investigated the photochemistry of 1,7-diphenyl-1,6-heptadiene-3,5-dione, a non-phenolic curcuminoid model. Upon irradiation of unsubstituted curcumin (
181) with a medium-pressure mercury lamp (400 W) in an ethanol-ethyl acetate mixture they obtained 2'-hydroxy-5',6'-benzochalcone
182 (4%), the corresponding flavanone
183 (22%), benzaldehyde, and cinnamaldehyde (
Scheme 64).
Formation of the chalcone
182 was postulated to take place
via photocyclisation of the excited triplet state of the enol form of
181 followed by a thermal oxidative step. Formation of the flavanone
183 was assumed to be
via photochemical cyclisation in agreement with the work by Matshushima and coworkers (1985) [
67]. This work represents a unique example of the photochemical conversion of a molecule from one important class of natural products (diarylheptanoids) into another important class (flavonoids).
Scheme 64.
Photochemistry of 1,7-diphenyl-1,6-heptadiene-3,5-dione.
Scheme 64.
Photochemistry of 1,7-diphenyl-1,6-heptadiene-3,5-dione.
Yokoe and co-workers [
96] studied the photochemistry of 2-steryl-4
H-chromen-4-ones
184. Irradiation of
184 in benzene with a high-pressure mercury lamp at room temperature under air yielded benzo[
a]xanthones
185 (
Scheme 65)
via cyclization in yields of between 43 and 88%, depending on the aromatic substituents (
Table 14). In the case of
184d and
184e, there are two possible directions of cyclization,
ortho or
para to the methoxy group on the benzene ring. However, the photocyclized products (
185d and
185e) each showed a single spot on TLC. This reaction provides a general route to polycyclic xanthene derivatives.
Scheme 65.
Photocyclization of 2-styryl-4H-chromen-4-ones.
Scheme 65.
Photocyclization of 2-styryl-4H-chromen-4-ones.
Table 14.
Effect of substituents on the yield of the photocyclization of 2-styryl-4H-chromen-4-ones.
Table 14.
Effect of substituents on the yield of the photocyclization of 2-styryl-4H-chromen-4-ones.
| 184 | R1 | R2 | R3 | R4 | Yield (%) of 185a-i |
|---|
| a | H | H | H | H | 22 |
| b | H | Me | H | H | 23 |
| c | H | OMe | H | H | 1 |
| d | H | H | OMe | H | 15 |
| e | H | OMe | OMe | H | 7 |
| f | Me | H | H | H | 11 |
| g | Me | Me | H | H | 13 |
| h | Me | OMe | H | H | 2 |
| i | H | H | H | Br | 31 |
Kamboj and co-workers [
97] observed similar results upon irradiation of 3-alkoxy-2-styryl-chromones
186a-d with a 125 W mercury vapour lamp in methanol under nitrogen to afford six different structures
187-
192 (
Scheme 66,
Table 15).
Scheme 66.
Photochemistry of some 3-alkoxy-2-sterylchromones.
Scheme 66.
Photochemistry of some 3-alkoxy-2-sterylchromones.
Table 15.
Effect of substituents on the photochemistry of 3-alkoxy-2-sterylchromones.
Table 15.
Effect of substituents on the photochemistry of 3-alkoxy-2-sterylchromones.
| | Yield (%) |
|---|
| 186 | 187 | 188 | 190 | 191 | 192 |
|---|
| a, R = H | 6 | 54 | 5 | 8 | |
| b, R = C6H5 | 7 | 55 | | 10 | 3.0 |
| c, R = CH=CH2 | 8.5 | 56 | | 14 | 10 |
| d, R = CH=C(CH3)2 | 9 | 58 | | 7 | 19 |
The suggested mechanism for the formation of the photoproduct through dealkylation and excited state intramolecular proton transfer is given in
Scheme 67.
Scheme 67.
Suggested mechanism for the formation of the photoproduct from 3-alkoxy-2-sterylchromones.
Scheme 67.
Suggested mechanism for the formation of the photoproduct from 3-alkoxy-2-sterylchromones.
Scheme 68 shows the mechanism
via isomerisation and cyclisation and
Scheme 69 represents formation of the photoproduct through dealkylation and excited state intramolecular proton transfer.
Scheme 68.
Suggested mechanism for the cyclisation of 3-alkoxy-2-styrylchromones.
Scheme 68.
Suggested mechanism for the cyclisation of 3-alkoxy-2-styrylchromones.
Scheme 69.
Suggested mechanism for the dealkylation and excited state intramolecular proton transfer of 3-alkoxy-2-styrylchromones.
Scheme 69.
Suggested mechanism for the dealkylation and excited state intramolecular proton transfer of 3-alkoxy-2-styrylchromones.
Dhande and co-workers [
98] investigated the photochemistry of
E-3-benzylideneflavanones
193. Aroylflavone
194 were obtained upon irradiation of compound
193 in dry benzene in the presence of air or oxygen (125 W high-pressure mercury lamp in quartz) as given in
Scheme 70. Yields varied between 27 and 90% depending on the substituent on the aromatic rings. Isomerisation of the double bond to
Z-3-benzylideneflavanones
195 was observed in all cases. Under inert atmosphere only the isomerisation product
195 was observed. As the
Z-isomer with the C-2 aryl in the equatorial position has the H-2 proton in a suitable axial position for a concerted ene reaction with singlet oxygen, it was suggested that isomerisation to the Z-isomer preceded oxidation to a hydroperoxide intermediate
196. Efforts to increase the reaction rate with rose bengal (a singlet oxygen source), however, failed. In dichloromethane with a phase transfer catalyst and rose bengal or methylene blue, no oxidation and only
E to
Z isomerisation was observed. Yields for each different derivative are given in
Table 16.
Halogen containing solvents (such as chloro-, bromo-, and iodobenzene) or addition of iodoform reduced the isomerisation time under nitrogen. Addition of iodoform to the benzene reaction mixture under air gave 3-α-hydroxybenzylflavones 197 upon photolysis using a Pyrex immersion well in yields of between 60 and 88% depending on the substituent. This represents a general route to these otherwise unavailable compounds. Arylideneflavanones 193 upon UV irradiation using quartz undergo auto-oxidation to 3-aroyl-flavones 194. Photolysis by using a Pyrex filter in the presence of iodoform furnishes 3-α-hydroxy-benzylflavones indicating the intermediacy of the hydroperoxide 196 and represents a new general method for the synthesis of these compounds not available by other routes. Under the inert conditions the irradiation furnishes the isomerised product 195.
Scheme 70.
Phototransformation of 3-arylideneflavanones to 3α-hydroxybenzylflavones.
Scheme 70.
Phototransformation of 3-arylideneflavanones to 3α-hydroxybenzylflavones.
Table 16.
Effect of substituents on the yields of phototransformation of 3-arylideneflavanones to 3α-hydroxybenzylflavones.
Table 16.
Effect of substituents on the yields of phototransformation of 3-arylideneflavanones to 3α-hydroxybenzylflavones.
| 193,194,197 | a | b | c | d | e | f | g | h | i |
|---|
| R1 | Me | Me | Me | H | H | H | H | H | H |
| R2 | OMe | Cl | OMe | H | H | H | H | H | H |
| R3 | Cl | H | OMe | H | Me | OMe | OCH2Ph | Cl | NO2 |
| 194, Yield (%) | 32 | 27 | 31 | 90 | | 81 | 86 | 81 | |
| 197, Yield (%) | | | | 65 | 66 | 61 | 60 | 70 | 88 |
Ishibe and co-workers (1975) [
99] irradiated 2-phenyl-7-methoxyisoflavone (
198a) in methanol with a medium-pressure mercury lamp and a Pyrex filter and obtained the corresponding 3,4-diphenyl-isocoumarin
199a (10%) and a pentacyclic structure
200a (42%), presumably
via intermediate
201. 2-Phenyl-7-hydroxyisoflavone was unreactive in air but gave (
200b) in the presence of iodine. Photo-isomerisation was not observed with 2-methyl-7-hydroxyisoflavone and 2-methylisoflavone in methanol, indicating that the presence of a 2-phenyl-substituent was a prerequisite (
Scheme 71).
Scheme 71.
Photochemistry of 2-phenylisoflavones.
Scheme 71.
Photochemistry of 2-phenylisoflavones.