Behaviors in Ethylene Polymerization of MgCl2-SiO2/TiCl4/THF Ziegler-Natta Catalysts with Differently Treated SiO2
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Polymerization Reaction
3.4. Polymer and Catalyst Characterization
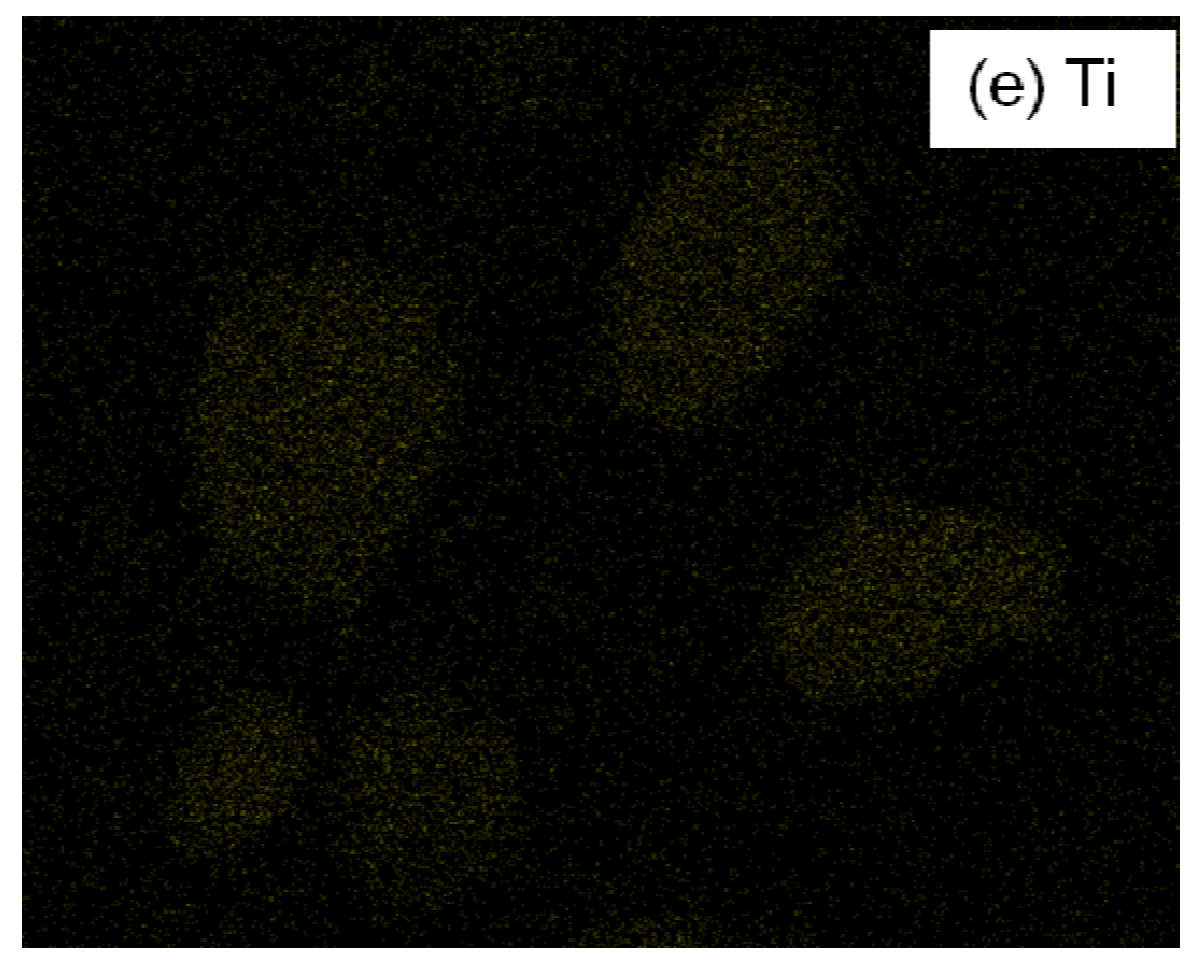
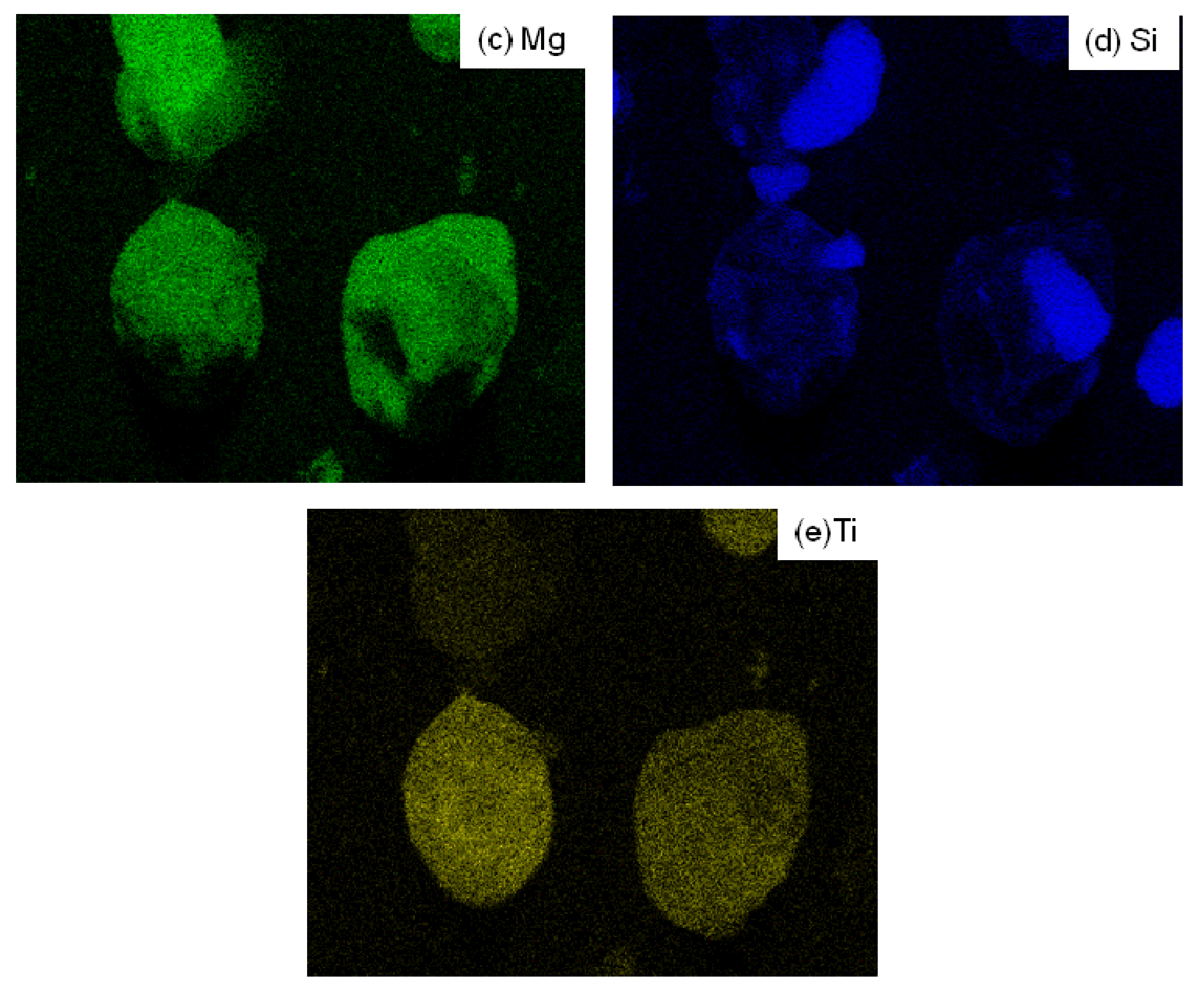
3.4.1. Scanning electron microscopy and energy dispersive X-ray spectroscopy (SEM/EDX)
3.4.2. Inductively coupled plasma (ICP)
3.4.3. X-ray photoelectron spectroscopy (XPS)
3.4.4. Gel permeation chromatography (GPC)
4. Conclusions
Acknowledgements
References and Notes
- Moore, E.P., Jr. Polypropylene Handbook, 1st ed.; Hanser Publishers: New York, NY, USA, 1996; pp. 86–87. [Google Scholar]
- Ray, W.H. Transition Metal Catalyzed Polymerizations, 1st ed.; Quirk, R.P., Ed.; Cambridge University Press: New York, NY, USA, 1988; pp. 563–567. [Google Scholar]
- Simonazzi, T.; Cecchin, G.; Mazzaulo, S. An outlook on progress in polypropylene-based polymer technology. Prog. Polym. Sci. 1991, 16, 303–329. [Google Scholar] [CrossRef]
- Senso, N.; Khaubunsongserm, S.; Jongsomjit, B.; Praserthdam, P. The influence of mixed activators on ethylene polymerization and ethylene/1-hexene copolymerization with silica-supported ziegler-natta catalyst. Molecules 2010, 15, 9323–9339. [Google Scholar] [CrossRef] [PubMed]
- Hock, C.W. How titanium chloride catalysts control the texture of as-polymerized polypropylene. J. Polym. Sci. A-Polym. Chem. 1966, 4, 3055–3064. [Google Scholar] [CrossRef]
- Kakugo, M.; Sadatoshi, H.; Sakai, J.; Yokoyama, M. Growth of polypropylene particles in heterogeneous Ziegler-Natta polymerization. Macromolecules 1989, 22, 3172–3177. [Google Scholar] [CrossRef]
- Kashiwa, N. The discovery and progress of MgCl2-supported TiCl4 catalysts. J. Polym. Sci. A-Polym. Chem. 2004, 42, 1–8. [Google Scholar]
- Nejad, M.H.; Ferrari, P.; Pennini, G.; Cecchin, G. Ethylene homo- and copolymerization over MgCl2-TiCl4 catalysts: Polymerization kinetics and polymer particle morphology. J. Appl. Polym. Sci. 2008, 108, 3388–3402. [Google Scholar] [CrossRef]
- Lu, H.; Xiae, S. Highly isospecific silica/magnesium chloride bisupported catalyst for propene polymerization. Makromol. Chem. 1993, 194, 2095–2102. [Google Scholar] [CrossRef]
- Kim, I.I.; Woo, S.I. Morphological study of HDPE prepared with the highly active silica-supported titanium tetrachloride/magnesium chloride catalyst. Polym. J. 1989, 21, 697–707. [Google Scholar] [CrossRef]
- Pasquet, V.; Spitz, R. Preparation of bisupported catalysts for ethylene polymerization. Makromol. Chem. 1990, 191, 3084–3096. [Google Scholar] [CrossRef]
- Robert, J.J.; Belle Mead, N.J.; Elton, D.F.; Victoria, T.; George, L.G.; Belle Mead, N.J. Process for producing ethylene polymers having reduced hexane extractable content. US Patent No. 5,290,745, 1994. [Google Scholar]
- Isaac, J.L.; Frederick, J.K.; Belle Mead, N.J. Processes for preparing polyethylene catalysts by heating catalyst precursors. US Patent No. 4,719,193, 1988. [Google Scholar]
- Lakomaa, E.-L.; Haukka, S.; Suntola, T. Atomic layer growth of titania on silica. Appl. Surf. Sci. 1992, 742, 60–61. [Google Scholar]
- Haukka, S.; Lakomaa, E.-L.; Suntola, T. Analytical and chemical techniques in the study of surface species in atomic layer epitaxy. Thin Solid Films 1993, 280, 225. [Google Scholar] [CrossRef]
- Haukka, S.; Lakomaa, E.-L.; Jylha, O.; Vilhunen, J.; Hornytzkyj, S. Dispersion and distribution of titanium species bound to silica from TiCl4. Langmuir 1993, 9, 3497–3506. [Google Scholar] [CrossRef]
- Legrand, A.P. The Surface Properties of Silicas, 1st ed.; Wiley: New York, NY, USA, 1998; pp. 415–464. [Google Scholar]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surface, 6th ed.; Wiley: New York, NY, USA, 1997; pp. 365–369. [Google Scholar]
- Gregg, S.J.; Sing, K. S.W. Adsorption Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Barthel, H.; Rosch, L.; Weis, J. Organosilicon Chemistry II. From Molecules to Materials; Auner, N., Weis, J., Eds.; VCH: Weinheim, Germany, 1996; pp. 761–778. [Google Scholar]
- Degussa, A.G. Basic Characteristics of Aerosil. Tech. Bull. Pigment. 1997, 997, 11–12. [Google Scholar]
- Hertl, W.; Hair, M.L. Reaction of hexamethyldisilazane with silica. J. Phys. Chem. 1971, 75, 2181–2185. [Google Scholar]
- Haukka, S.; Lakomaa, E.L. An IR and NMR study of the chemisorption of titanium tetrachloride on silica. J. Phys. Chem. 1993, 97, 5085–5094. [Google Scholar] [CrossRef]
- Blitz, J.P. Reactions of titanium tetrachloride with modified silica gel surfaces studied by diffuse reflectance FTIR spectroscopy. Colloids Surf. 1990, 63, 11–19. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Kaewkrajang, P.; Wanke, S.E.; Praserthdam, P. A comparative study of ethylene/α-olefin copolymerization with silane-modified silica-supported MAO using zirconocene catalysts. Catal. Lett. 2004, 94, 205–208. [Google Scholar] [CrossRef]
- Tangjituabun, K.; Jongsomjit, B.; Praserthdam, P. Catalytic behaviors of SiO2-supported various aluminoxanes as coactivator in MgCl2/DEP/TiCl4-TEA catalysts for propylene polymerization. Catal. Commun. 2009, 10, 1319–1323. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Ngamposri, S.; Praserthdam, P. Catalytic activity during copolymerization of ethylene and 1-hexene via mixed TiO2/SiO2-supported MAO with rac-Et[Ind]2ZrCl2 metallocene catalyst. Molecules 2005, 10, 672–678. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the polyethylene compounds are available from the authors. |


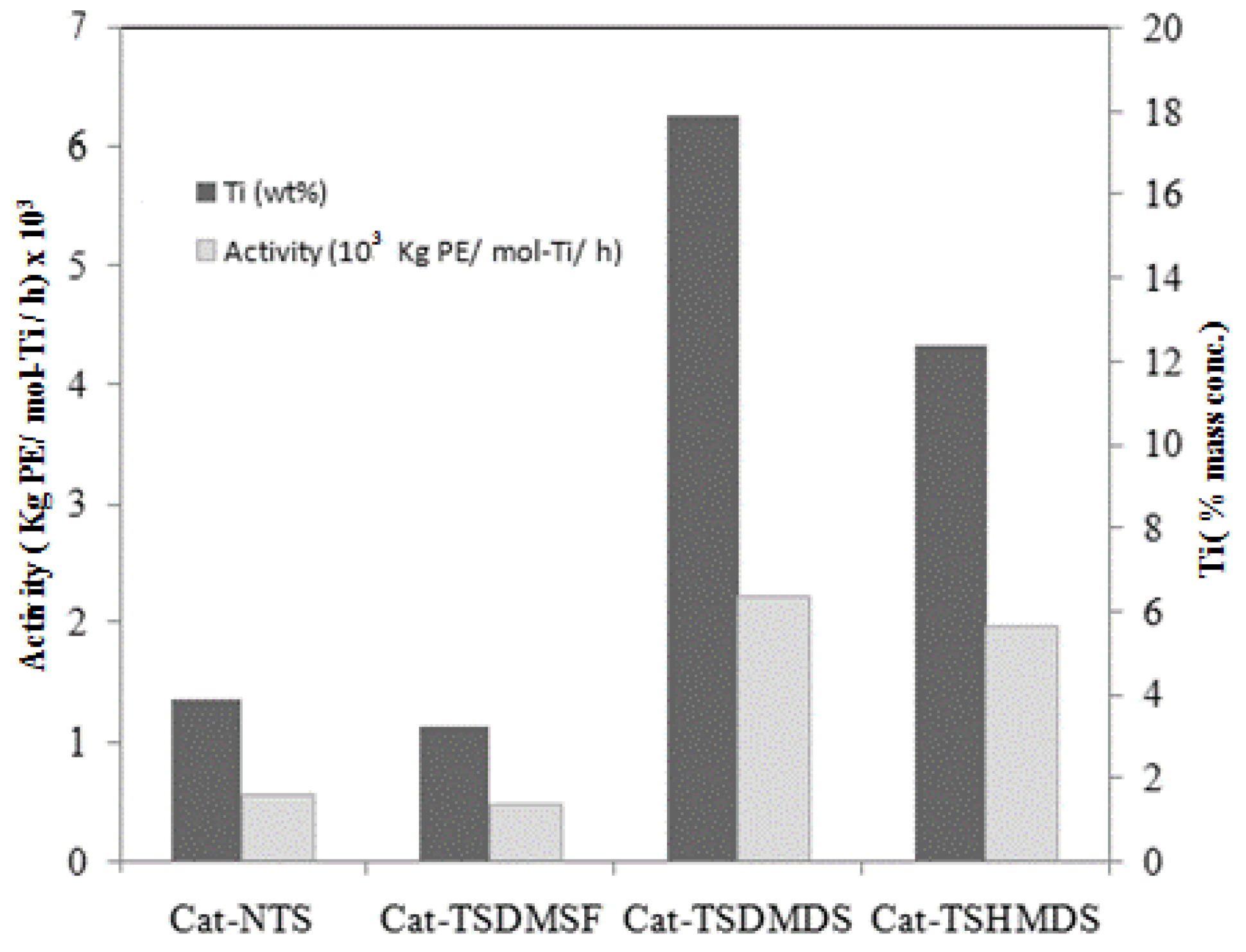




| Sample | Treatment | Ti in bulk of catalysts (wt %) a | Activity b (kg PE/mol-Ti/h) |
|---|---|---|---|
| Cat-NTS | Untreated silica | 2.27 | 1,570 |
| Cat-TSDMSF | Dimethylsilicone Fluid | 2.32 | 1,370 |
| Cat-TSDMDCS | Dimethyldichlorosilane | 2.34 | 6,370 |
| Cat-TSHMDS | Hexamethyldisilazane | 2.11 | 5,620 |
| Peak | Cat-NTS | Cat-TSDMSF | Cat-TSDMDCS | Cat-TSHMDS |
|---|---|---|---|---|
| Ti2p | 3.10 | 3.22 | 17.90 | 12.40 |
| Si2p | 64.05 | 88.55 | 37.81 | 56.13 |
| Mg2s | 32.85 | 8.23 | 44.30 | 31.47 |
| Sample | Mn (kg/mol) | Mw (kg/mol) | Mz (kg/mol) | Mv (kg/mol) | MWD (Mw/Mn) |
|---|---|---|---|---|---|
| Cat-NTS | 40 | 1,028 | 4,574 | 739 | 25.7 |
| Cat-TSDMSF | 23 | 359 | 4,073 | 339 | 15.6 |
| Cat-TSDMDCS | 55 | 787 | 3,086 | 595 | 14.3 |
| Cat-TSHMDS | 24 | 437 | 4,064 | 405 | 18.2 |
© 2011 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Senso, N.; Jongsomjit, B.; Praserthdam, P. Behaviors in Ethylene Polymerization of MgCl2-SiO2/TiCl4/THF Ziegler-Natta Catalysts with Differently Treated SiO2. Molecules 2011, 16, 1323-1335. https://doi.org/10.3390/molecules16021323
Senso N, Jongsomjit B, Praserthdam P. Behaviors in Ethylene Polymerization of MgCl2-SiO2/TiCl4/THF Ziegler-Natta Catalysts with Differently Treated SiO2. Molecules. 2011; 16(2):1323-1335. https://doi.org/10.3390/molecules16021323
Chicago/Turabian StyleSenso, Nichapat, Bunjerd Jongsomjit, and Piyasan Praserthdam. 2011. "Behaviors in Ethylene Polymerization of MgCl2-SiO2/TiCl4/THF Ziegler-Natta Catalysts with Differently Treated SiO2" Molecules 16, no. 2: 1323-1335. https://doi.org/10.3390/molecules16021323




