Enzymatic Synthesis of Fatty Hydroxamic Acid Derivatives Based on Palm Kernel Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis

2.2. Effect of Mol Ratio of Reactants

2.3. Characterization of MFHAs, IPFHAs and BFHAs
2.3.1. Elemental analysis
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)

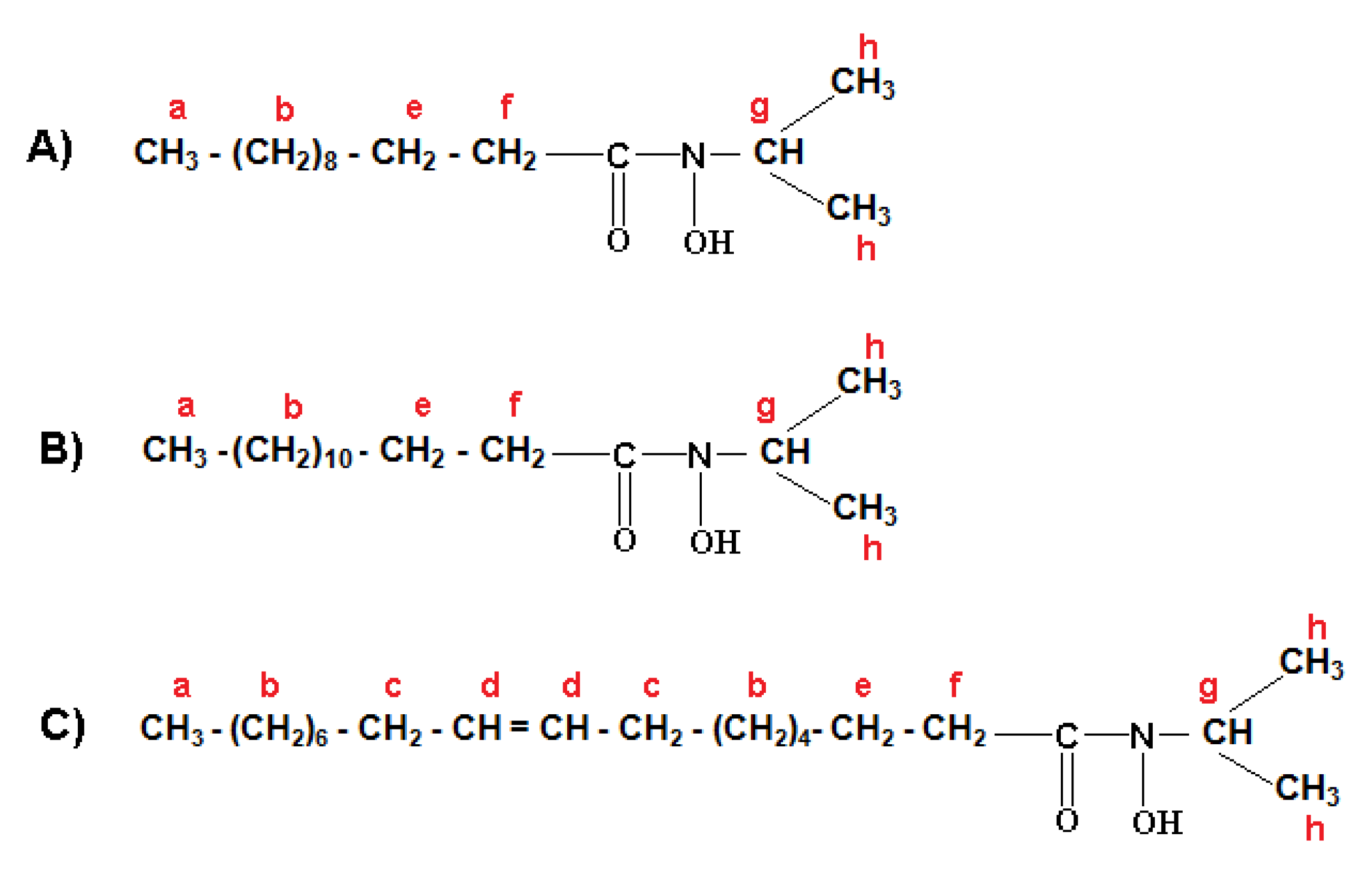
2.3.3. 1H Nuclear Magnetic Resonance (1H-NMR)


3. Experimental
3.1. Materials
3.2. Synthesis of MFHAs, IPFHAs and BFHAs
3.3. Characterization
4. Conclusions
References and Notes
- Coutts, R.T.; Hindmarsh, K.W.; Myers, G.E. Quinoline N-oxides and hydroxamic acids with antibacterial properties. Can. J. Chem. 1970, 48, 2393–2396. [Google Scholar] [CrossRef]
- Hector, R.B.; Lazo, W. Antialgal and antifungal activity of natural hydroxamic acids and related compounds. J. Agric. Food Chem. 1996, 44, 1569–1571. [Google Scholar] [CrossRef]
- Nakagawa, E.; Amano, T.; Hirai, N.; Iwamura, H. Non-induced cyclic hydroxamic acids in wheat during juvenile stage of growth. Phytochemistry 1995, 38, 1349–1354. [Google Scholar] [CrossRef]
- Lockhart, B.P.; Hamedi-Sangsari, F.; Vignon, J.; Privat, A.; Vila, J.A. N-methyl-kaspartate receptor-mediated neurotoxic effect of aspartate-based hydroxamate compounds in rat primary neuronal cultures. Brain Res. 1995, 670, 313–316. [Google Scholar]
- Tsafack, A.; Golenser, J.; Libman, J.; Shanzer, A.; Cabantchik, Z.I. Mode of action of iron(III) chelators as antimalarials. III. Overadditive effects in the combined action of hydroxamate-based agents on in vitro growth of plasmodium falciparum. Mol. Pharmacol. 1995, 47, 403–409. [Google Scholar]
- Dankwardt, S.M.; Martin, R.L.; Chan, C.S.; Van Wart, H.E.; Walker, K.A.M.; Delaet, N.G.; Robinson, L.A. Amino acid derived sulfonamide hydroxamates as inhibitors of procollagen c-proteinase: Solid-phase synthesis of ornithine analogues. Bioorg. Med. Chem. Lett. 2001, II, 1465–1468. [Google Scholar]
- Gijbels, K.; Galardy, R.E.; Steinman, L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J. Clin. Invest. 1994, 94, 2177–2182. [Google Scholar]
- Gao, W.Y.; Mitsuya, H.; Driscoll, J.S.; Johns, D.G. Enhancement by hydroxyurea of the anti-human immunodeficiency virus type 1 potency of 2’-β-fluor 2’,3’-dideoxyadenosine in peripheral blood mononuclear cells. Biochem. Pharmacol. 1995, 50, 274–276. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Kaur, H. Poly (β-styryl) hydroxamic acids: Synthesis and ion exchange separation of rear earths. React. Funct. Polym. 1999, 39, 155–164. [Google Scholar]
- Agrawal, Y.K.; Patel, S.A. Hydroxamic acids: Reagents for the solvent extraction and spectrophotometric determination of metals. Rev. Anal. Chem. 1980, 4, 237–276. [Google Scholar]
- Blatt, A.H. Organic Synthesis. Collective; John Wiley and Sons: New York, NY, USA, 1963; Volume 2, p. 67. [Google Scholar]
- Altenburger, J.M.; Mioskowski, C.; d'Orchymont, H.; Schirlin, D.; Schalk, C.; Tarnus, C. Useful hydroxylamine derivatives for the synthesis of hydroxamic acids. Tetrahedron Lett. 1992, 33, 5055–5058. [Google Scholar] [CrossRef]
- Staszak, M.A.; Doecke, C.W. The use of N,O-bis(tert-butoxycarbonyl)-hydroxylamine in the synthesis of N-hydroxylamines and hydroxamic acids. Tetrahedron Lett. 1994, 35, 6021–4024. [Google Scholar] [CrossRef]
- Servat, F.; Montet, D.; Pinao, M.; Galzy, P.; Arnaud, A.; Ledon, H.; Marcou, L.; Graillea, J. Synthesis of fatty hydroxamic acids catalyzed by the lipase of Mucor Miehei. JAOCS 1990, 67, 646–649. [Google Scholar] [CrossRef]
- Fournand, D.; Arnaud, A.; Galzy, P.; Bigey, F.; Ratomahenina, R. Biocatalyst improvement for the production of short-chain hydroxamic acids. Enzyme Microb. Tech. 1997, 20, 424. [Google Scholar] [CrossRef]
- Fournand, D.; Vaysse, L.; Dubreucq, E.; Arnaud, A.; Galzy, P. Monohydroxamic acid biosynthesis. J. Mol. Catal. B: Enzym. 1998, 5, 207–211. [Google Scholar] [CrossRef]
- Vaysse, L.; Dubreucq, E.; Pirat, J.L.; Galzy, P. Fatty hydroxamic acid biosynthesis in aqueous medium in the presence of the lipase-acyltransferase from Candida parapsilosis. J. Biotechnol. 1997, 53, 41–46. [Google Scholar]
- Jahangirian, H.; Haron, M.J.; Silong, S.; Yusof, N.A. Preparation of fatty hydroxamic acid from canola oil. Asian J. Chem. 2011, 23, 3371–3374. [Google Scholar]
- Jahangirian, H.; Haron, M.J.; Silong, S.; Yusof, N.A. Enzymatic synthesis of phenyl fatty hydroxamic acids from canola and palm oils. J. Oleo Sci. 2011, 60, 281–286. [Google Scholar]
- Suhendra, D.; Wan Yunus, W.M.Z.; Haron, M.J.; Basri, M.; Silong, S. Enzymatic synthesis of fatty hydroxamic acids from palm oil. J. Oleo Sci. 2005, 54, 33–38. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 3rd ed; Tomson Learning Inc.: Belmont, CA, USA, 2001; p. 71. [Google Scholar]
- Lie Ken Jie, M.S.F.; Pasha, M.K.; Alam, M.S. Synthesis and nuclear magnetic resonance properties of all geometric isomers of conjugated linoleic acids. Lipids 1997, 32, 1041–1044. [Google Scholar] [CrossRef]
- Lie Ken Jie, M.S.F. Analysis of conjugated linoleic acid esters by nuclear magnetic resonance spectroscopy. Eur. J. Lipid Sci. Technol. 2001, 103, 628–632. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jahangirian, H.; Haron, M.J.; Yusof, N.A.; Silong, S.; Kassim, A.; Rafiee-Moghaddam, R.; Peyda, M.; Gharayebi, Y. Enzymatic Synthesis of Fatty Hydroxamic Acid Derivatives Based on Palm Kernel Oil. Molecules 2011, 16, 6634-6644. https://doi.org/10.3390/molecules16086634
Jahangirian H, Haron MJ, Yusof NA, Silong S, Kassim A, Rafiee-Moghaddam R, Peyda M, Gharayebi Y. Enzymatic Synthesis of Fatty Hydroxamic Acid Derivatives Based on Palm Kernel Oil. Molecules. 2011; 16(8):6634-6644. https://doi.org/10.3390/molecules16086634
Chicago/Turabian StyleJahangirian, Hossein, Md Jelas Haron, Nor Azah Yusof, Sidik Silong, Anuar Kassim, Roshanak Rafiee-Moghaddam, Mazyar Peyda, and Yadollah Gharayebi. 2011. "Enzymatic Synthesis of Fatty Hydroxamic Acid Derivatives Based on Palm Kernel Oil" Molecules 16, no. 8: 6634-6644. https://doi.org/10.3390/molecules16086634
APA StyleJahangirian, H., Haron, M. J., Yusof, N. A., Silong, S., Kassim, A., Rafiee-Moghaddam, R., Peyda, M., & Gharayebi, Y. (2011). Enzymatic Synthesis of Fatty Hydroxamic Acid Derivatives Based on Palm Kernel Oil. Molecules, 16(8), 6634-6644. https://doi.org/10.3390/molecules16086634



