2.1. Composition of the Essential Oil of Schinus Molle L.
The essential oils of the ground fruits and leaves were obtained by stream stripping. The essential oil analysis were performed by GC and GC-MS, and
Table 1 shows the products quantified and identified using the NIST and Wiley databases.
Table 1.
Components and quantification of the essential oils of fruits and leaves of S. molle.
Table 1.
Components and quantification of the essential oils of fruits and leaves of S. molle.
| Number | Rt | M | Compounds | % |
|---|
| 1 | 8.51 | 136.2 | thujene | 1.48 |
| 2 | 9.14 | 136.2 | α-pinene | 5.32 |
| 3 | 11.47 | 136.2 | sabinene | 34.77 |
| 4 | 11.54 | 136.2 | β-pinene | 4.50 |
| 5 | 12.42 | 136.2 | myrcene | 1.72 |
| 6 | 14.17 | 136.2 | α-terpinene | 1.34 |
| 7 | 14.50 | 134.2 | p-cymene | 1.46 |
| 8 | 15.10 | 136.2 | L-limonene | 4.18 |
| 9 | 17.09 | 136.2 | γ-terpinene | 2.39 |
| 10 | 17.58 | 154.0 | sabinene hydrate | 0.16 |
| 11 | 18.54 | 136.2 | α-terpinolene | 0.51 |
| 12 | 20.06 | 154.0 | NI (alcohol) | 0.42 |
| 13 | 25.53 | 154.2 | terpinen-4-ol | 5.50 |
| 14 | 26.52 | 154.0 | α-terpineol | 0.62 |
| 15 | 38.59 | 204.4 | α-copaene | 0.37 |
| 16 | 40.06 | 204.0 | β-elemene | 1.97 |
| 17 | 41.53 | 204.3 | β-caryophyllene | 3.84 |
| 18 | 44.07 | 204.1 | α-humulene | 0.52 |
| 19 | 44.23 | 204.3 | alloaromadendrene | 0.85 |
| 20 | 46.00 | 204.3 | germacrene-D | 7.06 |
| 21 | 46.19 | 204.3 | β-selinene | 0.54 |
| 22 | 46.51 | 204.3 | germacrene-B | 3.87 |
| 23 | 47.07 | 204.3 | α-muurolene | 0.35 |
| 24 | 47.29 | 204.1 | germacrene-A | 0.60 |
| 25 | 47.59 | 204.2 | γ-cadinene | 1.22 |
| 26 | 48.23 | 204.3 | δ-cadinene | 1.14 |
| 27 | 51.57 | 220.1 | (+)-spathulenol | 3.91 |
| 28 | 52.05 | 220.1 | caryophyllene oxide | 1.02 |
| 29 | 55.55 | 222.0 | δ-cadinol | 2.11 |
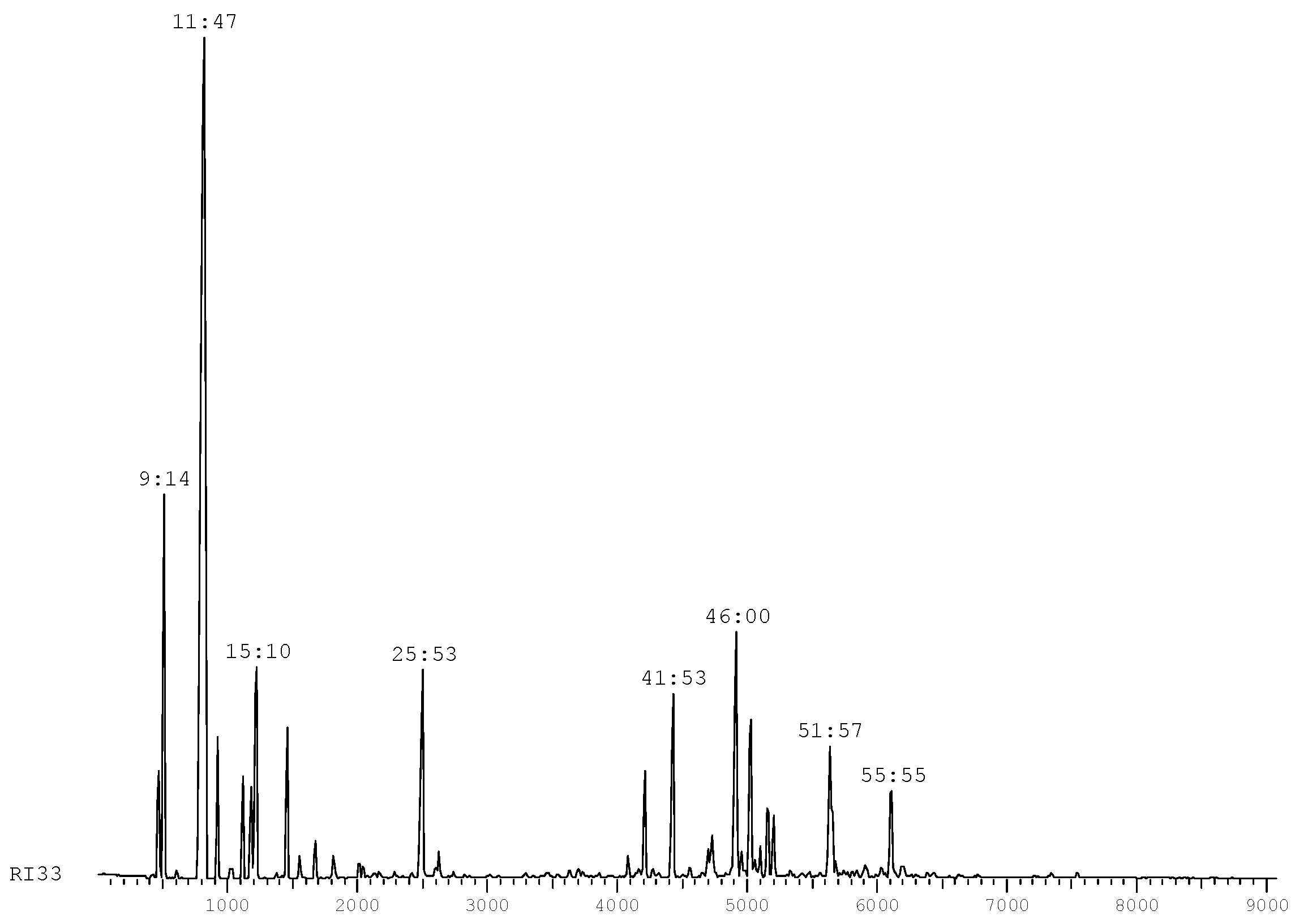
The chromatographic profile of this essential oil (
Figure 1) is consistent with the ranges defined by standard IRAM (Argentina) [
15] for the essential oil of the fruits and leaves of
Schinus molle L. (
Table 2).
The essential oil was chromatographed with a low-medium pressure pump system, using a silica-gel flash type column prepared in the laboratory. Fractions were collected with an automatic collector. The obtained fractions were analysed by
1H-,
13C-NMR, bidimensional 2D NMR and GC-MS to determine the components. In previous studies [
16], several components of
Schinus molle L. oil were identified, among which sabinene was the main component, followed by (α, β)-pinene and terpinen-4-ol, accounting for a total of 94.0% of the essential oil. In this work we isolated and identified more polar components of the essential oil, which correspond to terpinenediol derivatives and formyloxy derivatives of sabinene and terpinene. The fractions obtained in this work by column chromatography used for antibacterial testing (and their compositions) are summarized in
Table 3.
Figure 1.
Chromatographic profile of S. molle.
Figure 1.
Chromatographic profile of S. molle.
Table 2.
Composition of the IRAM standard (Argentina).
Table 2.
Composition of the IRAM standard (Argentina).
| Compounds | Minimum (%) | Maximum (%) |
|---|
| α-pinene | 1.5 | 12.5 |
| β-pinene | 2.5 | 20.0 |
| sabinene | 31.0 | 55.0 |
| terpinen-4-ol | 0.5 | 13.5 |
| germacrene-D | 5.2 | 10.0 |
Table 3.
Composition of S. molle oil fractions tested for antibacterial activity.
The most polar fraction F07 has as a major component a product with an M
+ to 198, which corresponds to the molecular formula C
11H
18O
3 (compound
1). HMRSNa was measured as 221.1146 (calc. for the formula C
11H
18O
3 221.1148). The
1H-NMR spectrum shows a one H singlet at 8.11 ppm that can be attributed to a formyloxy group (HCOO-); at 4.18 ppm and 4.12 ppm there are two doublets of 1H each,
J = 12.5 Hz, which can be attributed to the methylene group attached to the formyloxy group. At 0.96 ppm and 0.87 ppm two doublets of three H each (
J = 7.0 Hz) are observed, corresponding to two methyl groups that can be attributed to an isopropyl group. The signals at 0.43 ppm (dd,
J = 5.4 and 8.3 Hz) and 0.25 ppm (dd,
J = 3.6 and 5.4 Hz) 1H each, can be attributed to a cyclopropane methylene group in a bicyclic system [5.3] like in the skeleton of sabinene [
17]. The
13C-NMR spectrum confirms the skeleton carbons of sabinene, with the carbon of the methylene group of the cyclopropane ring at 13.0 ppm (
Table 4). Thus, compound
1 corresponds to a new derivative of the sabinene skeleton.
Table 4.
Attribution of the 13C-NMR data of compounds 1–3.
The major component of fraction F08 corresponds to a diol that shows in the mass spectrum a molecular ion at
m/z 170, corresponding to a molecular formula C
10H
18O
2. The
1H-NMR spectrum shows a multiplet integrating for 1H at 5.43 ppm, due to an olefinic hydrogen and at 3.80 ppm a 1H, m, signal of hydrogen atom geminal to an -OH group. Other signals are observed at 1.20 ppm (3H, s), corresponding to a methyl group geminal to a hydroxyl group (Me-7), at 1.03 ppm (3H, d,
J = 7.1 Hz), for a methyl group at position 9 and at 1.00 ppm (3H, d,
J = 7.1 Hz) corresponding to a methyl group at position 10. Taken together this data suggest a terpinenediol [
18]. The
13C-NMR data of this new derivative
2 is given in
Table 4 [
19,
20].
In fraction F09 another terpinene derivative was observed. The mass spectrum shows a molecular ion at 170 amu which corresponds to C
10H
18O
2. Its spectroscopic data was identical to that described in the literature for compound
3 [
21].
These formylated hydroxyl or hydroxylated derivatives are minor components that cannot be detected by GC or GC/MS. These products easily dehydrated at the high injector temperature giving rise to the corresponding hydrocarbons. No major influence of these derivatives has been observed in antibacterial activities studied.
2.4. Fractions of the Oil of S. molle L.—Cylindrical Cavities Agar Diffusion Method
To analyze of each of the fractions obtained by CC, three different concentrations (90 mg/mL, 45 mg/mL and 22.5 mg/mL) were prepared for each strain (
Table 7,
Table 8 and
Table 9). Analyzing these results, we conclude (as for crude extract) that the inhibitory effect of each fraction decreases with increasing dilution, demonstrating direct proportionality between the total concentrations of components and increased inhibition zone. All fractions have a greater inhibitory effect for the highest concentration (90 mg/mL), with the halo of inhibition (29.5 ± 0.3 mm) caused by fraction F01 (19% α-pinene, 2.5% β-pinene and 25% sabinene) against the Gram-positive strains standing out. Comparing this result with the halo of inhibition obtained by the effect of the vapor (14.6 ± 0.7 mm) it follows that there may be a synergy between these components (pinenes and sabinene) when they act against
S. aureus ATCC 25923. In future studies would be interesting to separate available fractions with pinenes and sabinene isolated in equal concentration, in order to reliably conclude which of the two components has higher antibacterial activity against
S. aureus ATCC 25923 and if indeed there is synergy between them. For the lowest concentration of 22.5 mg/mL the Gram-positive strain did not exceeded a value of 17.7 ± 0.3 mm for the halo of inhibition (moderately susceptible) while the remaining strains,
E. coli ATCC 25922 and
P. aeruginosa ATCC 27853, were resistant to oil at this concentration.
For S. aureus ATCC 25923 and comparing fractions F03 and F02, it was found that the inhibition zone is slightly higher than for F03, probably due to the increased concentration of sabinene and not the presence of (+)-spathulenol so there appears to be antagonism between these two components. Comparing F04, F05 and F06, it is notable that the fraction containing the highest percentage of (+)-spathulenol showed the lowest activity against the Gram-positive strain, possibly due to an antagonism between (+)-spathulenol and terpinen-4-ol. E. coli ATCC 25922 is more sensitive to terpinen-4-ol. F05 (14% (+)-spathulenol and 25% terpienen-4-ol) is the fraction with the highest inhibitory effect in this strain, followed by F06 (7% (+)-spathulenol and 19% terpinen-4-ol). Comparing the results, we highlight the possible effect of the antagonistic (+)-spathulenol on terpinen-4-ol and sabinene in both Gram-negative strains. Comparing the fractions F01, F02 and the absence of inhibition zone by the effect of vapor in Gram-negative strains, we conclude that this may also be associated with an antagonism between the pinenes and sabinene at this group. For P. aeruginosa ATCC 27853, the fraction that showed highest rate of inhibition was F02 (11% sabinene) followed by F03 (12% sabinene and 16% (+)-spathulenol). On the contrary, E. coli ATCC 25922 showed better inhibition halos for the terpinen-4-ol, possibly due to the greater symmetry of the biphospholipidic outer membrane characteristic of the genus Pseudomonas spp, compared to the family Enterobacteriaceae.
2.5. Method of Macrodilution—MIC and MBC
According to the methodology described in 2.9, we obtained the values summarized in
Table 8. Given the difficulty in visualizing the turbidity in the tubes due to the oil emulsion, the MIC results were evaluated according to the resazurin test. Predictably, and in accordance with the agar diffusion test, the oil has a stronger inhibitory effect on the Gram-positive strain with a MIC of 2.81 mg/mL corresponding to the dilution 1:32. The actual value for the MIC and MBC will be between 1.41 mg/mL and 2.81 mg/mL. For Gram-negative strains, the MIC and MBC observed were equal (1:8 dilution, 11.25 mg/mL) with the actual concentrations varying between 5.63 mg/mL and 11.25 mg/mL. Although a count of 440 CFU/mL was obtained for
P. aeruginosa ATCC 27853, this value is less than 0.1% (1,000 CFU/mL) of the original inoculum (10
6 CFU/mL), strengthening the lower bacterial effect (due to the proximity of MIC values and MBC) of oil of
S. molle on this strain. Comparing the macrodilution and cylindrical cavities agar diffusion methods, there appears to be a good correlation between MIC values and inhibition halos for the lower concentrations, possibly due to several factors, such as the fact of being an oil (lipophilic), in aqueous emulsion it may not occur an efficient contact between the oil and the bacterial cell and poor diffusion in the agar.
Despite the low antibacterial activity, sabinene showed the best inhibitory effect on
P. aeruginosa ATCC 27853, indicating that this strain is very insensitive/resistant to this oil, possibly due to low outer membrane permeability of strongly/moderately hydrophobic compounds and the multidrug efflux systems characteristic of this kind of organism. (+)-Spathulenol is the component that has a lower activity, or lack of it, with possible antagonism with the terpinen-4-ol level in all tested strains and the sabinene only in Gram-negative strains,
Table 9.