Wild Argentinian Amaryllidaceae, a New Renewable Source of the Acetylcholinesterase Inhibitor Galanthamine and Other Alkaloids
Abstract
:1. Introduction
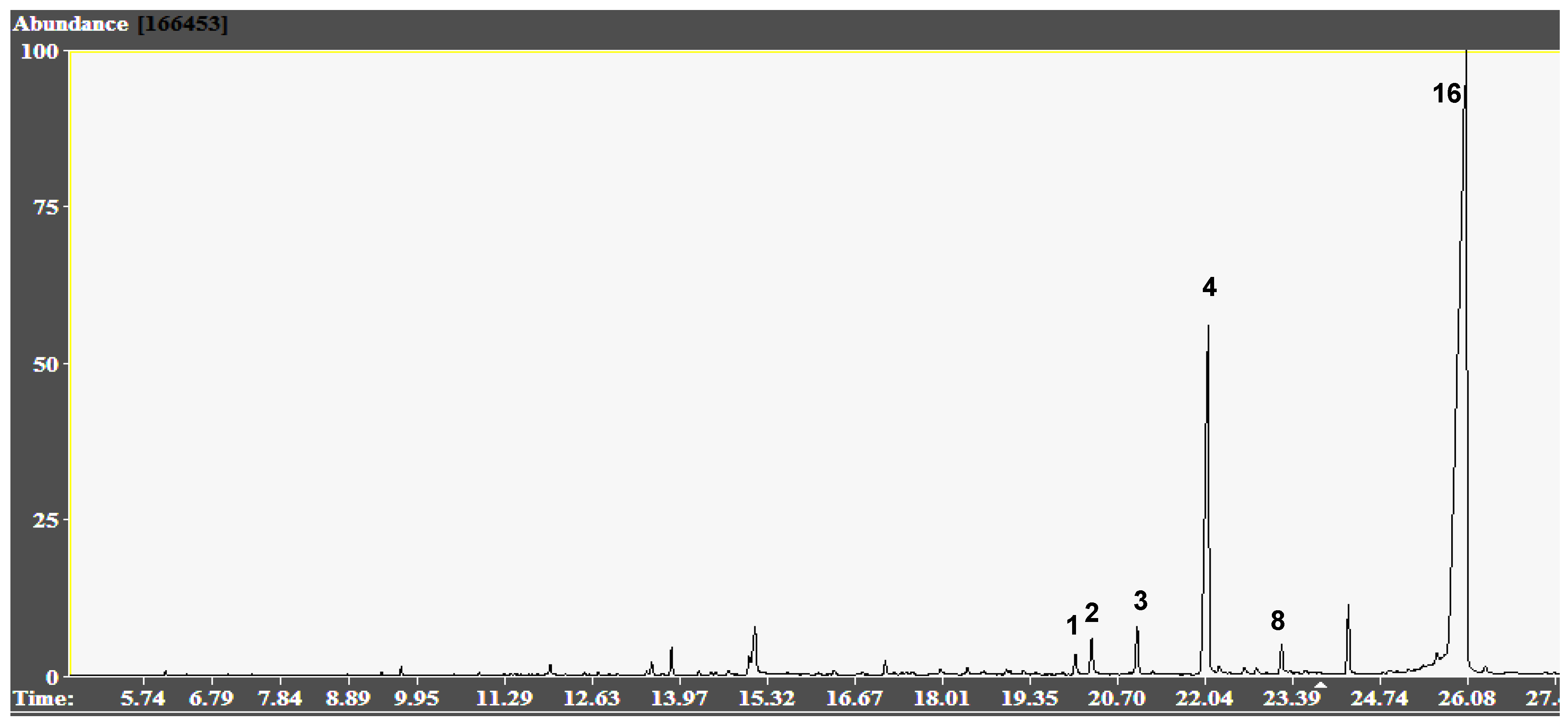
2. Results and Discussion
| Samples (voucher number) | BCE a | |
|---|---|---|
| Yield [%] b | IC50 [μg/mL] | |
| Phycella herbertiana SJ (IBT-Arg1) | 0.34 | 1.2 ± 0.12 |
| Habranthus jamesonii SJ (IBT-Arg2) | 0.25 | 2.0 ± 0.11 |
| Rhodophiala mendocina SJ (IBT-Arg3) | 0.38 | 2.0 ± 0.15 |
| Zephyranthes filifolia SJ (IBT-Arg4 ) | 0.21 | 1.0 ± 0.08 |
| Habranthus jamesonii MZA (IBT-Arg5) | 0.27 | 1.0 ± 0.01 |
| Rhodophiala mendocina NQN (IBT-Arg6) | 0.26 | 2.0 ± 0.20 |
| Galanthamine c | 0.29 ± 0.07 | |
| Compound | H. jamesonii a | P. herbertiana a | R. mendocina a | Z. filifolia a | ||
|---|---|---|---|---|---|---|
| SJ | MZA | SJ | SJ | NQN | SJ | |
| Trisphaeridine (1) | 0.7 | 0.1 | 1.2 | |||
| Ismine (2) | 0.7 | |||||
| 5,6-Dihydrobicolorine (3) | 1.7 | |||||
| Galanthamine (4) | 1.4 | 4.3 | 4.2 | 0.6 | 0.8 | 17.8 |
| Lycoramine (5) | 1.9 | 27.4 | 3.2 | |||
| Lycoraminone (6) | 0.5 | |||||
| Vittatine (7) | 13.2 | 0.1 | 1.2 | 0.2 | ||
| Narwedine (8) | 0.8 | 0.2 | 0.4 | 0.9 | ||
| Anhydrolycorine (9) | 2.5 | 1.5 | 0.4 | 0.9 | ||
| A-289 (10) | 1.1 | |||||
| A-315 (11) | 0.4 | |||||
| A-249 (12) | 1.3 | 0.8 | ||||
| A-319 (13) | 1.1 | |||||
| Montanine (14) | 5.7 | 1.8 | 9.1 | |||
| Haemanthamine/Crinamine(15) b | 2.9 | 2.5 | 31.2 | 6.8 | ||
| Tazettine (16) | 28.1 | 5.4 | 32.9 | 69.7 | ||
| A-301 (17) | 2.0 | |||||
| Pancracine (18) | 0.3 | |||||
| 11-Hydroxyvittatine (19) | 18.7 | 3.1 | 4.6 | |||
| Galanthine (20) | 4.9 | 17.2 | ||||
| Lycorine (21) | 8.2 | 43.6 | 33.2 | 13.3 | 20.4 | |
| Incartine (22) | 1.1 | |||||
| Methylpseudolycorine (23) | 0.2 | |||||
| Epimacronine (24) | 0.6 | |||||
| 8- O-Demethylhomolycorine (25) | 0.6 | |||||
| Homolycorine type (26) | 3.9 | |||||
| 2- O-Acetyllycorine (27) | 0.2 | 2.6 | ||||
| A-345 (28) | 2.7 | |||||
| Tazettamide (29) | 3.5 | |||||
| m/z 109 (Homolycorine type) (30) | 7.7 | |||||
| Sanguinine (31) | 0.5 | |||||
| m/z 83 (285) (32) | 1.1 | |||||
| m/z 297(33) | 11.8 | |||||
| m/z 281 (34) | 0.9 | |||||
| m/z 283 (35) | 2.3 | |||||
| N-Demethylgalanthamine (36) | 0.2 | |||||
| 2-O-Methylpancracine (37) | 3.8 | |||||
| N-Formylnorgalanthamine (38) | 0.1 | |||||
| Total Alkaloids identified | 40.0 | 84.6 | 99.1 | 99.9 | 32.7 | 92.0 |


3. Experimental
3.1. Plant Material
3.2. Alkaloid Extraction

3.3. Gas Chromatography-Mass Spectroscopy Analyses
3.4. Microplate Assay for Acetylcholinesterase Activity
3.5. TLC Analysis of BCE
4. Conclusions
Acknowledgements
References
- Lubbe, A.; Verpoorte, R. Review Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Houghton, P.J. Old yet new-pharmaceuticals from plants. J. Chem. Educ. 2001, 78, 175–184. [Google Scholar] [CrossRef]
- Bastida, J.; Lavilla, R.; Viladomat, F. Chemical and biological aspects of Narcissus alkaloids. In The Alkaloids; Cordell, G.A., Ed.; Elsevier Scientific: Amsterdam, The Netherlands, 2006; Volume 63, pp. 87–179. [Google Scholar]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop-the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar]
- Grutzendler, J.; Morris, J.C. Cholinesterase inhibitors for Alzheimer’s disease (Review). Drugs 2001, 61, 41–52. [Google Scholar] [CrossRef]
- Marco, L.; Carreiras, M.D. Galanthamine, a natural product for the treatment of Alzheimer’s disease. Recent Pat. CNS Drug Discov. 2006, 1, 105–111. [Google Scholar]
- Zhong, J. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2007, 24, 886–905. [Google Scholar] [CrossRef]
- Contelles, J.M.; Rodríguez, C.; Garcia, A.G. Chemical synthesis of galantamine, an acetylcholinesterase inhibitor for treatment of Alzheimer’s disease. Inform Healthc. 2005, 15, 575–587. [Google Scholar]
- Contelles, J.M.; do Carmo Carreiras, M.; Rodríguez, C.; Villarroya, M.; García, A.C. Synthesis and pharmacology of galantamine. Chem. Rev. 2006, 106, 116–133. [Google Scholar]
- Bulger, P.G.; Bagal, S.K.; Marquez, R. Recent advances in biomimetic natural product synthesis. Nat. Prod. Rep. 2008, 25, 254–297. [Google Scholar] [CrossRef]
- Arroyo-Leuenberger, S.C. Amaryllidaceae. In Catalogo de las Plantas Vasculares de la Republica Argentina I; Zuloaga, F.O., Morrone, O., Eds.; Missouri Botanical Garden: St. Louis, MO, USA, 1996; pp. 90–100. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar]
- Berkov, S.; Bastida, J.; Sidjimova, B.; Viladomat, F.; Codina, C. Alkaloid Diversity in Galanthus elwesii and Galanthus nivalis. Chem. Biodivers. 2011, 8, 115–130. [Google Scholar] [CrossRef]
- Bartolucci, C.; Perola, E.; Pilger, C.; Fels, G.; Lamba, D. Three-dimensional structure of a complex of galanthamine (Nivalin®) with acetylcholinesterase from Torpedo californica: Implications for the design of new anti-Alzheimer drugs. Proteins 2001, 42, 182–191. [Google Scholar] [CrossRef]
- Jitsuno, M.; Yokosuka, A.; Sakagami, H.; Mimaki, Y. Chemical constituents of the bulbs of Habranthus brachyandrus and their cytotoxic activities. Chem. Pharm. Bull. 2009, 57, 1153–1157. [Google Scholar]
- Wildman, W.C.; Brown, C.L.; Michel, K.H.; Bailey, D.T.; Heimer, N.E.; Shaffer, R.; Murphy, C.F. Alkaloids from Rhodophiala bifida, Crinum erubescens and Sprekelia formisissima. Pharmazie 1967, 22, 725. [Google Scholar]
- Pagliosa, L.B.; Monteiro, S.C.; Silva, K.B.; de Andrade, J.P.; Dutilh, J.; Bastida, J.; Cammarota, M.; Zuanazzi, J.A.S. Effect of isoquinoline alkaloids from two Hippeastrum species on in vitro acetylcholinesterase activity. Phytomedicine 2010, 17, 698–701. [Google Scholar]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- McNulty, J.; Nair, J.J.; Codina, C.; Bastida, J.; Pandey, S.; Gerasimoff, J.; Griffin, C. Selective apoptosis-inducing activity of crinum-type Amaryllidaceae alkaloids. Phytochemistry 2007, 68, 1068–1074. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef]
- McNulty, J.; Nair, J.J.; Bastida, J.; Pandey, S.; Griffin, C. Structure-activity studies on the lycorine pharmacophore: A potent inducer of apoptosis in human leukemia cells. Phytochemistry 2009, 70, 913–919. [Google Scholar] [CrossRef]
- Citoglu, G.; Tanker, M.; Gumusel, B. Antiinflammatory effects of lycorine and haemanthidine. Phytother. Res. 1998, 12, 205–206. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Jiao, X.; Wu, Y.; Lin, J.; Cai, Y. Lycorine induces apoptosis and down-regulation of Mcl-1 in human leukemia cells. Cancer Lett. 2009, 274, 16–24. [Google Scholar] [CrossRef]
- Weniger, B.; Italiano, L.; Beck, J.P.; Bastida, J.; Bergoñon, S.; Codina, C.; Lobstein, A.; Anton, R. Cytotoxic activity of Amaryllidaceae alkaloids. Planta Med. 1995, 61, 77–79. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ortiz, J.E.; Berkov, S.; Pigni, N.B.; Theoduloz, C.; Roitman, G.; Tapia, A.; Bastida, J.; Feresin, G.E. Wild Argentinian Amaryllidaceae, a New Renewable Source of the Acetylcholinesterase Inhibitor Galanthamine and Other Alkaloids. Molecules 2012, 17, 13473-13482. https://doi.org/10.3390/molecules171113473
Ortiz JE, Berkov S, Pigni NB, Theoduloz C, Roitman G, Tapia A, Bastida J, Feresin GE. Wild Argentinian Amaryllidaceae, a New Renewable Source of the Acetylcholinesterase Inhibitor Galanthamine and Other Alkaloids. Molecules. 2012; 17(11):13473-13482. https://doi.org/10.3390/molecules171113473
Chicago/Turabian StyleOrtiz, Javier E., Strahil Berkov, Natalia B. Pigni, Cristina Theoduloz, German Roitman, Alejandro Tapia, Jaume Bastida, and Gabriela E. Feresin. 2012. "Wild Argentinian Amaryllidaceae, a New Renewable Source of the Acetylcholinesterase Inhibitor Galanthamine and Other Alkaloids" Molecules 17, no. 11: 13473-13482. https://doi.org/10.3390/molecules171113473







