Phenolic Compounds and Antioxidant Activities of Liriope muscari
Abstract
:1. Introduction

2. Results and Discussion
2.1. Isolation and Characterization of Compounds 1–5

2.2. In Vitro Antioxidant Activity
2.2.1. DPPH Scavenging Activity
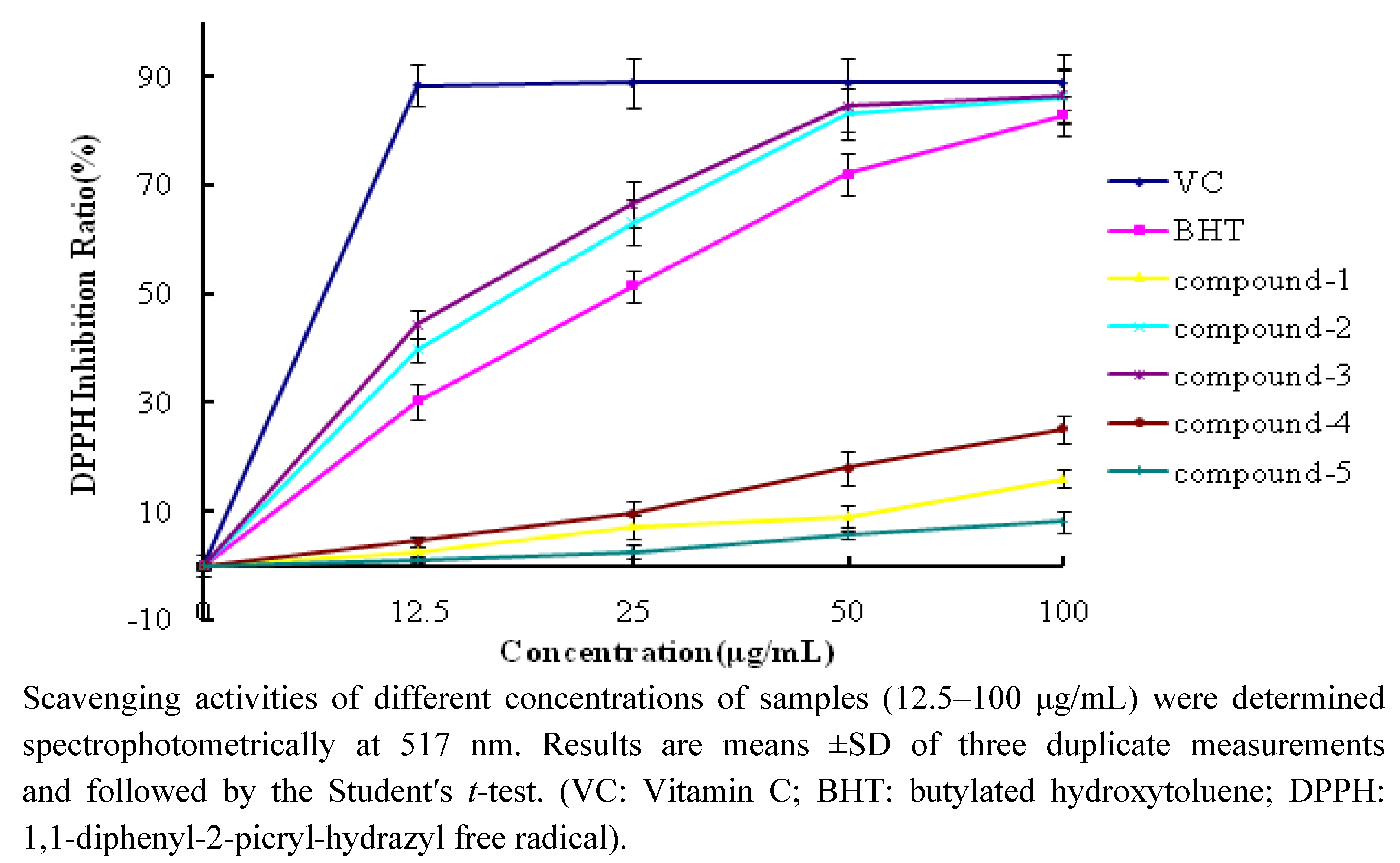
| DPPH Inhibition Ratio (%) | ABTS Inhibition Ratio (%) | |
|---|---|---|
| Compound 1 | 7.2 ± 0.6 | 32.7 ± 1.6 |
| Compound 2 | 63.2 ± 3.6 | 75.9 ± 2.0 |
| Compound 3 | 66.6 ± 2.3 | 70.0 ± 3.2 |
| Compound 4 | 9.6 ± 1.2 | 24.3 ± 1.9 |
| Compound 5 | 2.6 ± 0.5 | 16.8 ± 1.7 |
| VC | 88.9 ± 4.5 | 97.4 ± 5.0 |
| BHT | 51.5 ± 3.1 | 95.1 ± 5.3 |
2.2.2. ABTS Scavenging Activity


3. Experimental
3.1. General
3.2. Plant Material
3.3. Compound Isolation
| Position | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|
| 1 | 126.3 | 126.9 | 126.9 |
| 2 | 129.8 | 111.2 | 111.2 |
| 3 | 116.2 | 148.3 | 148.3 |
| 4 | 159.2 | 148.7 | 148.7 |
| 5 | 116.2 | 116.1 | 116.1 |
| 6 | 129.8 | 122.0 | 122.0 |
| 7 | 139.3 | 139.3 | 139.4 |
| 8 | 118.9 | 119.5 | 119.6 |
| 9 | 166.1 | 165.8 | 166.0 |
| 1′ | 129.1 | 130.0 | 134.5 |
| 2′ | 130.0 | 129.9 | 127.6 |
| 3′ | 115.6 | 115.6 | 115.2 |
| 4′ | 156.0 | 156.1 | 156.9 |
| 5′ | 115.6 | 115.6 | 115.2 |
| 6′ | 130.0 | 129.9 | 127.6 |
| 7′ | 34.7 | 34.9 | 71.6 |
| 8′ | 41.2 | 41.1 | 47.5 |
| -OCH3 | 56.0 | 55.9 |
3.4. Antioxidant Ability
3.4.1. DPPH Assay


3.4.2. ABTS Assay


4. Conclusions
Supplementary Materials
Acknowledgements
Conflict of Interest
- Sample Availability: Samples of the crude extracts and pure compounds are available from the authors.
References and Notes
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Alici, H.A.; Cesur, M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem. Pharm. Bull. 2005, 53, 281–285. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D. Natural antioxidants fromresidual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci. Technol. 2003, 36, 263–271. [Google Scholar]
- Celik, B.; Lee, J.H.; Min, D.B. Effects of light, oxygen and pH on the 2,2-diphenyl-1-picrylhydra-zyl (DPPH) method to evaluate antioxidants. J. Food Sci. 2003, 68, 487–490. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of l-adrenaline: A structure-activity insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar]
- Havsteen, B.H. The biochemistry and medicinal significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, A.G.; Han, Y. Anti-oxidation and anti-microorganism activities of purification polysaccharide from Lygodium japonicum in vitro. Carbohydr. Polym. 2006, 66, 34–42. [Google Scholar] [CrossRef]
- Yu, B.Y.; Xu, G.J.; Jin, R.L.; Xu, L.S. Drug resources and identification of commercial drugs on Radix Ophiopogonis (in Chinese with English abstract). J. Chin. Pharm. Univ. 1991, 22, 150–153. [Google Scholar]
- Tian, Y.Q.; Kou, J.P.; Li, L.Z.; Yu, B.Y. Anti-inflammatory effects of aqueous extract from Radix Liriope muscari and its major active fraction and component. Chin. J. Nat. Med. 2011, 9, 222–226. [Google Scholar]
- Yu, B.Y.; Hirai, Y.; Shoji, J.Z.; Xu, G.J. Comparative studies on the constituents of ophiopogonis tuber and its congeners. VI. Studies on the constituents of the subterranean part of Liriope spicata var. prolifera and L. muscari. Chem. Pharm. Bull. 1990, 38, 1931–1935. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Wu, T.; Yu, B.Y.; Xu, L.S. Studies on Chemical constituents of Liriope muscari (in Chinese). Zhong Cao Yao 2005, 36, 823–826. [Google Scholar]
- Cheng, Z.H.; Wu, T.; Guo, Y.L.; Yu, B.Y.; Xu, L.S. Two new steroidal glycosides from Liriope muscari. Chin. Chem. Lett. 2006, 17, 31–34. [Google Scholar]
- Watanabe, Y.; Sanada, S.; Ida, Y.; Shoji, J. Comparative studies on the constituents of ophiopogonis tuber and its congeners. IV. Studies on the homoisoflavonoids of the subterranean part of Ophiopogon ohwii Okuyama and O. jaburan (Kunth) Lodd. Chem. Pharm. Bull. 1985, 33, 5358–5363. [Google Scholar]
- Böhler, P.; Tamm, C. The homo-isoflavones, a new class of natural product. Isolation and structure of eucomin and eucomol. Tetrahedron Lett. 1967, 36, 3479–3483. [Google Scholar]
- Weber, H.P.; Heller, W.; Tamm, C. Homoisoflavanones. V. Crystal and molecular structure of (−)-7-O-(p-Bromophenacyl)-eucomol. The absolute configuration of (−)-eucomol). Helv. Chim. Acta 1977, 60, 1388–1392. [Google Scholar]
- Hernández, J.C.; León, F.; Estévez, F.; Quintana, J.; Bermejo, J. A homoisoflavonoid and a cytotoxic saponin from Dracaena draco. Chem. Biodivers. 2006, 3, 62. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cheng, M.J.; Wu, M.D.; Chen, I.S.; Yuan, G.F. A new sesquiterpene isolated from the extracts of the fungus Monascus pilosus-fermented rice. Nat. Prod. Res. 2010, 24, 750–758. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ryu, K.; Yoshida, J.; Ide, N.; Kodera, Y.; Sasaoka, T.; Rosen, R.T. Identification of six phenylpropanoids from garlic skin asmajor antioxidants. J. Agric. Food Chem. 2003, 51, 7313–7317. [Google Scholar] [CrossRef]
- Tomosaka, H.; Chin, Y.W.; Salim, A.A.; Keller, W.J.; Chai, H.; Kinghorn, A.D. Antioxidant and cytoprotective compounds from Berberis vulgaris (Barberry). Phytother. Res. 2008, 22, 979–981. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.L.; Wang, F.F.; Dong, H.L.; Guo, S.X.; Yang, J.S.; Xiao, P.G. Phenolic components and flavanones from Dendrobium candid (in Chinese with English abstract). Chin. Pharm. J. 2010, 45, 975–979. [Google Scholar]
- Sato, T.; Kawamoto, A.; Tawura, A.; Tatsumi, Y.; Fujii, T. Mechanism of antioxidant action of Pueraria Glycoside (PG-1) (an isoflavonoid) and Mangiferin (a xanthoniod). Chem. Pharm. Bull. 1992, 40, 721–724. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.P.; Zhu, D.N.; Yu, B.Y.; Yan, Y.Q. Study on the radical scavengers in the traditional Chinese medicine formula Shengmai San by HPLC-DAD coupled with chemiluminescence (CL) and ESI-MS/MS. J. Pharm. Biomed. Anal. 2010, 52, 438–445. [Google Scholar] [CrossRef]
- Gao, M.; Wang, X.L.; Gu, M. Separation of polyphenols using porous polyamide resin and assessment of mechanism of retention. J. Sep. Sci. 2011, 34, 1853–1858. [Google Scholar] [CrossRef]
- Holzbach, J.C.; Lopes, L.M.X. Aristolactams and alkamides of Aristolochia gigantean. Molecules 2010, 15, 9462–9472. [Google Scholar] [CrossRef]
- Yang, J.Q.; Wang, Y.; Yan, C.; Wang, N.N.; Hao, X.Y. Chemical constituents from Reineckia carnea Kunth (in Chinese with English abstract). Nat. Prod. Res. Dev. 2010, 22, 245–247. [Google Scholar]
- Munoz, O.; Piovano, M.; Garbarino, J.; Heuwing, V.; Breitmaier, E. Traopane alkaloids from Schizanthus Litoralis. Phytochemistry 1996, 43, 709–713. [Google Scholar]
- King, R.R.; Calhoun, L.A. Characterization of cross-linked hydroxycinnamic acid amides isolated from potato common scab lesions. Phytochemistry 2005, 66, 2468–2473. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, Y.; Kim, M.R.; Jung, H.J.; Seu, Y.B.; Hahm, K.S.; Woo, E.R. Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol. Lett. 2004, 26, 1125–1130. [Google Scholar]
- Leslie, J.H.; Guat-Lee, S.; Keng-Yeow, S. 5,7-Dihydroxy-8-methoxyflavone from Tetracera indica. Planta Med. 1994, 60, 493–494. [Google Scholar]
- Huang, W.; Tan, G.S.; Xu, K.P.; Li, F.S.; Li, Z.K.; Liu, Y.P. Cytotoxic constituents from the root of Ardisia crisp (in Chinese with English abstract). Nat. Prod. Res. Dev. 2010, 22, 949–951. [Google Scholar]
- Gülçin, İ. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthin on autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A.; Davies, M.J. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.J.; Cheng, X.L.; Liu, J.; Lin, R.C.; Wang, G.L.; Du, S.S.; Liu, Z.L. Phenolic Compounds and Antioxidant Activities of Liriope muscari. Molecules 2012, 17, 1797-1808. https://doi.org/10.3390/molecules17021797
Li WJ, Cheng XL, Liu J, Lin RC, Wang GL, Du SS, Liu ZL. Phenolic Compounds and Antioxidant Activities of Liriope muscari. Molecules. 2012; 17(2):1797-1808. https://doi.org/10.3390/molecules17021797
Chicago/Turabian StyleLi, Wen Jie, Xian Long Cheng, Jing Liu, Rui Chao Lin, Gang Li Wang, Shu Shan Du, and Zhi Long Liu. 2012. "Phenolic Compounds and Antioxidant Activities of Liriope muscari" Molecules 17, no. 2: 1797-1808. https://doi.org/10.3390/molecules17021797




