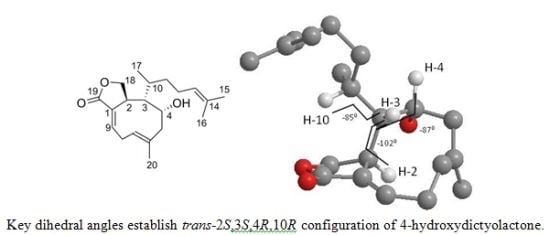
Update of Spectroscopic Data for 4-Hydroxydictyolactone and Dictyol E Isolated from a Halimeda stuposa — Dictyota sp. Assemblage †
Abstract
:1. Introduction
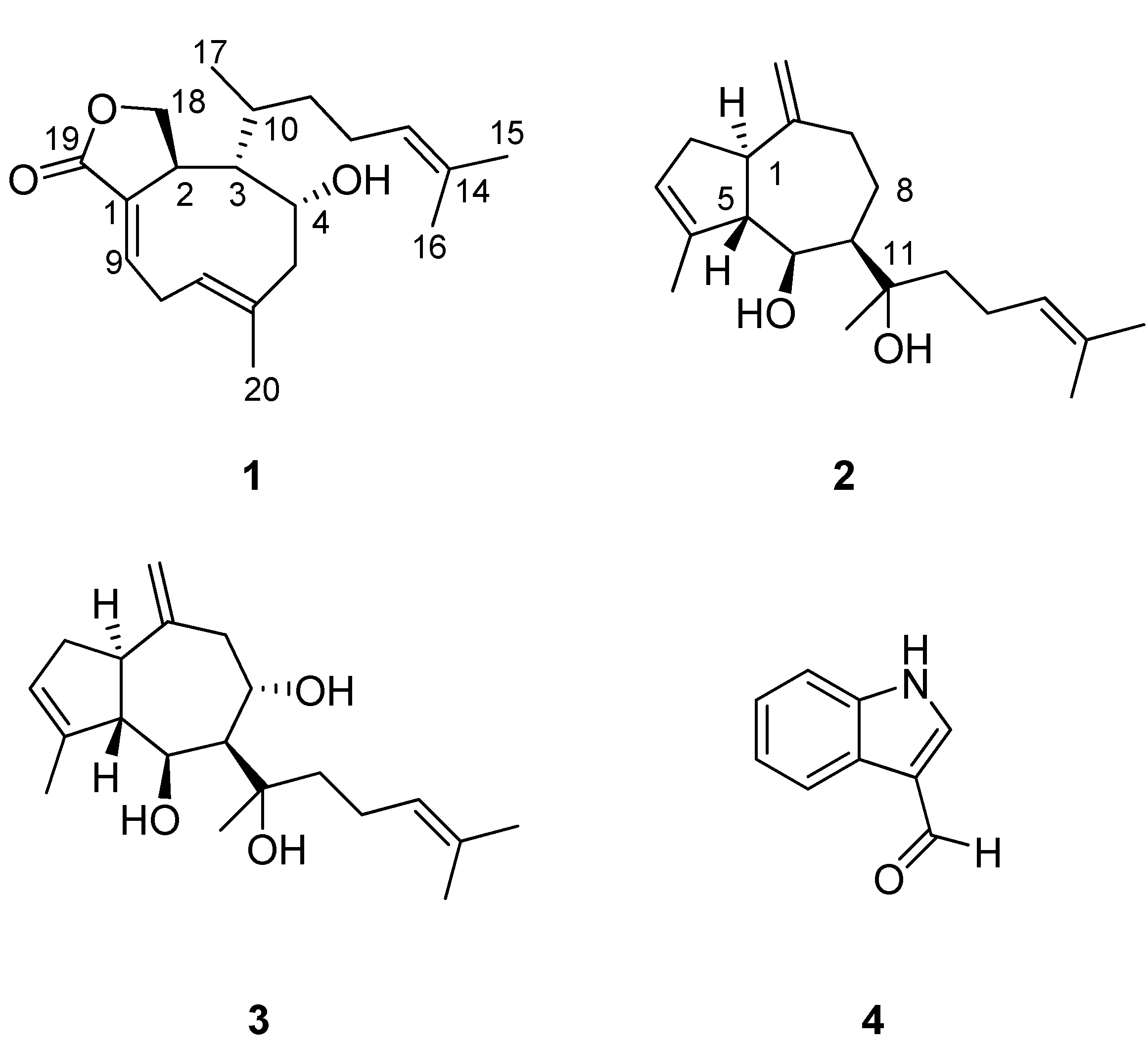
2. Results and Discussion

| No. | 13Cδ (m) | 1Hδ (m, J Hz) | COSY | gHMBC |
|---|---|---|---|---|
| 1 | 46.0 (d) | 2.60 (1H, q, 9.1) | H2-2, H-5, H2-18 | C-2, C-5, C-6, C-10, C-18 |
| 2 | 33.7 (t) | 2.51 (1H, m ) | H-1, Hb-2 | C-1, C-3, C-4, C-5 |
| 2.22 (1H, dd, 14.8, 7.8) | H-1, Ha-2, H-3 | C-1, C-3, C-4, C-5 | ||
| 3 | 124.2 (d) | 5.34(1H, br s) | Hb-2, H3-17, H-5 | C-1, C-2, C-4, C-5, C-17 |
| 4 | 141.0 (s) | |||
| 5 | 60.4 (d) | 2.37 (1H, m) | H-1, H3-17, H-3, H-6 | C-1, C-3, C-4, C-6, C-10 |
| 6 | 74.7 (d) | 4.20 (1H, dd, 7.8, 2.0) | H-5, H-7 | C-4, C-5, C-7, C-8, |
| 7 | 48.7 (d) | 1.67 (1H, m) | H-6 | C-9, C-11, C-12 |
| 8 | 21.6 (t) | 1.81 (1H, m) | Hb-8, Ha-9 | C-6, C-10, C-19 |
| 1.73 (1H, m,) | Ha-8 | C-7, C-11 | ||
| 9 | 40.6 (t) | 2.69 (1H, ddd, 14.8, 4.6, 2.4) | Hb-9, H2-8 | C-1, C-7, C-8, C-10, C-18 |
| 2.13 (1H, m ) | Ha-9, H2-18 | C-8, C-10, C-18 | ||
| 10 | 152.0 (s) | |||
| 11 | 76.3 (s) | |||
| 12 | 40.9 (t) | 1.74 (2H, t, 8.6) | H2-13 | C-7, C-11, C-13, C-14, C-19 |
| 13 | 23.2 (t) | 2.12 (1H, dd, 14.8, 8.6) | H2-12, Hb-13, H-14 | C-11, C-12, C-14, C-15 |
| 2.02 (1H, dq, 14.8, 6.9) | H2-12, Hb-13, H-14 | C-11, C-12, C-14, C-15 | ||
| 14 | 124.2 (d) | 5.16 (1H, br t, 6.9) | H2-13, H3-20, | C-12, C-13, C-16, C-20 |
| 15 | 132.0 (s) | |||
| 16 | 25.7 (q) | 1.69 (3H, s) | H-14 | C-14, C-15, C-20 |
| 17 | 15.9 (q) | 1.82 (3H, br s) | H-3, H-5 | C-3, C-4, C-5 |
| 18 | 107.4 (t) | 4.78 (1H, br s) | H-1 | C-1, C-5, C-9, C-10 |
| 4.76 (1H, br s) | Hb-9 | C-1, C-5, C-9, C-10 | ||
| 19 | 25.3 (q) | 1.24 (3H, s) | C-7, C-11, C-12 | |
| 20 | 17.5 (q) | 1.62 (3H, br s) | C-14, C-15, C-16 |
| Compound | SF-268 a | MCF-7 b | H460 c | HT-29 d | CHO-K1 e |
|---|---|---|---|---|---|
| 1 | 25 | 27 | 20 | 61 | 72 |
| 2 | 16 | 22 | 17 | 46 | 48 |
| 3 | 20 | 38 | 20 | 88 | 103 |
| 4 | NAf | NA | NA | NA | NA |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Bioassay
3.4. Extraction and Isolation
3.4.1. 4-Hydroxydictyolactone (1)
3.4.2. Dictyol E (2)
3.4.3. 8α,11-Dihydroxypachydictyol A (3)
3.4.4. Indole-3-carboxaldehyde (4)
4. Conclusions
Supplementary Materials
Acknowledgements
- Samples Availability: Not available.
References nand Notes
- Fenical, W.; Paul, V.J. Antimicrobial and cytotoxic terpenoids from tropical green algae of the family Udoteaceae. Hydrobiologia 1984, 116, 135–140. [Google Scholar] [CrossRef]
- Tillekeratne, L.M.V.; Schmitz, F.J. 4,9-Diacetoxyudoteal: A linear diterpene aldehyde from the green alga Halimeda opuntia. Phytochemistry 1984, 23, 1331–1333. [Google Scholar]
- Koehn, F.E.; Gunasekera, S.P.; Niel, N.; Cross, S. Halitunal, an unusual diterpene aldehyde from the marine alga Halimeda tuna. Tetrahedron Lett. 1991, 32, 169–172. [Google Scholar]
- Su, J.-Y.; Xu, X.-H.; Zeng, L.-M.; Wang, M.-Y.; Lu, N.; Lu, Y.; Zhang, Q.-T. Sym-triazinederivative from Halimeda xishaensis. Phytochemistry 1998, 48, 583–584. [Google Scholar]
- Sun, H.H.; McConnell, O.J.; Fenical, W.; Hirotsu, K.; Clardy, J. Tricyclic dieterpenoids of the dolastane ring system from the marine alga Dictyota divaricata. Tetrahedron 1981, 37, 1237–1242. [Google Scholar] [CrossRef]
- Bourne, D.J.; Pilchowski, S.E.; Murphy, P.T. A novel sulfonoglycolipid from the brown alga Dictyota ciliolata. Aust. J. Chem. 1999, 52, 69–70. [Google Scholar] [CrossRef]
- Hay, M.E.; Fenical, W.; Gustafson, K. Chemical defense against diverse coral-reef herbivores. Ecology 1987, 68, 1581–1591. [Google Scholar] [CrossRef]
- Segawa, M.; Enoki, N.; Ikura, M.; Hikichi, K.; Ishida, R.; Shirahama, H.; Matsumoto, T. Dictymal, a new seco-fusicoccin type diterpene from the brown alga Dictyota dichotoma. Tetrahedron Lett. 1987, 28, 3703–3704. [Google Scholar] [CrossRef]
- Siamopoulou, P.; Bimplakis, A.; Iliopoulou, D.; Vagias, C.; Cos, P.; Vanden Berghe, D.; Roussis, V. Diterpenes from the brown algae Dictyota dichotoma and Dictyota linearis. Phytochemistry 2004, 65, 2025–2030. [Google Scholar]
- Viano, Y.; Bonhomme, D.; Camps, M.; Briand, J.-F.; Ortalo-Magne, A.; Blache, Y.; Piovetti, L.; Culioli, G. Diterpenoids from the Mediterranean Brown Alga Dictyota sp. Evaluated as antifouling substances against a marine bacterial biofilm. J. Nat. Prod. 2009, 72, 1299–1304. [Google Scholar] [CrossRef]
- Moreau, J.; Pesando, D.; Caram, B. Antifungal and antibacterial screening of dictyotales from the French Mediterranean coast. Hydrobiologia 1984, 116, 521–524. [Google Scholar] [CrossRef]
- Ishitsuka, M.O.; Kusumi, T.; Kakisawa, H. Antitumor xenicane and norxenicane lactones from the brown alga Dictyota dichotoma. J. Org. Chem. 1988, 43, 5010–5013. [Google Scholar]
- Pereira, H.S.; Leao-Ferreira, L.R.; Moussatche, N.; Teixeira, V.L.; Cavalcanti, D.N.; Costa, L.J.; Diaz, R.; Frugulhetti, I.C.P.P. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1). Antiviral Res. 2004, 64, 69–76. [Google Scholar]
- Boyd, M.R. The NCI in Vitro Anticancer Drug Discovery Screen: Concept, Implementation and Operation, 1985-1995. In Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval; Teicher, B.A., Ed.; Humana Press: Totowa, NJ, USA,, 1997; pp. 23–42. [Google Scholar]
- Ochi, M.; Masui, N.; Kotsuki, H.; Miura, I.; Tokoroyama, T. The structures of fukurinolal and fukurinal, two new dieterpenoids from the brown seaweed Dilophus okamurai Dawson. Chem. Lett. 1982, 1927–1930. [Google Scholar]
- Danise, B.; Minale, L.; Riccio, R.; Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C.; Fattorusso, S.; Magno, S.; Mayol, L. Further perhydroazulene diterpenes from marine organisms. Experientia 1977, 33, 413–415. [Google Scholar] [CrossRef]
- Jongaramruong, J.; Kongkam, N. Novel diterpenes with cytotoxic, anti-malarial and anti-tuberculosis activities from a brown alga Dictyota sp. J. Asian Nat. Prod. 2007, 9, 743–751. [Google Scholar] [CrossRef]
- Cardellina, J.H., II; Nigh, D.; VanWagenen, B.C. Plant growth regulatory indoles from the sponges Dysidea etheria and Ulosa ruetzleri. J. Nat. Prod. 1986, 49, 1065–1067. [Google Scholar] [CrossRef]
- Williams, D.R.; Walsh, M.J.; Miller, N.A. Studies for the synthesis of xenicane diterpenes. A stereocontrolled total synthesis of 4-hydroxydictyolactone. J. Am. Chem. Soc. 2009, 131, 9038–9045. [Google Scholar]
- Guella, G.; Chiasera, G.; N’Diaye, I.; Pietra, F. Xenicane diterpenes revisited: Thermal (E)→(Z) isomerization and conformational motions. A unifying picture. Helv. Chim. Acta 1994, 77, 1203–1221. [Google Scholar] [CrossRef]
- Kotsos, M.P.; Aligiannis, N.; Myrianthopoulos, V.; Mitaku, S.; Skaltsounis, L. Sesquiterpene lactones from Staehelina fruticosa. J. Nat. Prod. 2008, 71, 847–851. [Google Scholar] [CrossRef]
- Lange, G.L.; Lee, M. 13C NMR determination of the configuration of methyl-substituted double bonds in medium- and large-ring terpenoids. 1986, 24, 656–658. [Google Scholar]
- Lightner, D.A. The Octant Rule. In Circular Dichroism, Principles and Applications, 2nd; Berova, N., Nakanisi, K., Woody, R.W., Eds.; Wiley-VCH: Weinheim, Germany, 1994; pp. 10–261. [Google Scholar]
- Finer, J.; Clardy, J.; Fenical, W.; Minale, L.; Riccico, R.; Battaile, J.; Kirkup, M.; Moore, R.E. Structures of the dictyodial and dictyolactone, unusual marine diterpenoids. J. Org. Chem. 1979, 44, 2044–2047. [Google Scholar] [CrossRef]
- ChemBio3D Ultra, version 11.0.1, ChemBioOffice. 2008. Available online: http://www.cambridgesoft.com accessed on 6 February 2012.
- de Nys, R.; Wright, A.D.; König, G.M.; Sticher, O. A diterpene from the alga Glossophora kunthii. Phytochemistry 1993, 32, 463–465. [Google Scholar]
- Kim, J.Y.; Alamsjah, M.A.; Hamada, A.; Fujita, Y.; Ishibashi, F. Algicidal diterpenes from the brown alga Dictyota dichotoma. Biosci. Biotechnol. Biochem. 2006, 70, 2571–2574. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Wright, A.D.; Motti, C.A.; Tapiolas, D.M. Comosusols A-D and Comosone A: Cytotoxic compounds from the brown alga Sporochnus comosus. J. Nat. Prod. 2011, 74, 739–743. [Google Scholar] [CrossRef]
- Enoki, N.; Ishida, R.; Urano, S.; Ochi, M.; Tokoroyama, T.; Matsumoto, T. Structures and conformations of new nine-membered ring diterpenes from the marine alga Dictyota dichotoma. Chem. Lett. 1982, 1749–1752. [Google Scholar]
- Kirkup, M.P.; Moore, R.E. Two minor diterpenes related to dictyodial A from the brown alga Dictyota crenulata. Phytochemistry 1983, 22, 2539–2541. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Sticher, O. Diterpenes from the brown alga Dictyota divaricata. Phytochemistry 1991, 30, 3679–3682. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Sticher, O. New xenicane and hydroazulenoid diterpenes from an Australian collection of Dictyota divaricata. Tetrahedron 1991, 47, 1399–1410. [Google Scholar] [CrossRef]
- Ravi, B.N.; Wells, R.J. New nine-membered diterpenes from the brown alga Dictyota prolificans. Aust. J. Chem. 1982, 35, 121–128. [Google Scholar] [CrossRef]
- Tanaka, J.; Higa, T. Hydroxydictyodial, a new antifeedant diterpene from the brown alga Dictyota spinulosa. Chem. Lett. 1984, 231–232. [Google Scholar]
- Ishitsuka, M.; Kusumi, T.; Tanaka, J.; Kakisawa, H. New diterpenoids from Pachydictyon coriaceum. Chem. Lett. 1982, 1517–1518. [Google Scholar]
- Ishitsuka, M.; Kusumi, T.; Kakisawa, H.; Nagai, Y.; Kawakami, Y.; Sato, T. Structure of pachyaldehyde, a novel norditerpene from the brown alga Pachydictyon coriaceum. J. Chem. Soc. Chem. Comm. 1984, 906–908. [Google Scholar]
- Ishitsuka, M.; Kusumi, T.; Tanaka, J.; Chihara, M.; Kakisawa, H. New diterpenes from the brown alga Pachydictyon coriaceum. Chem. Lett. 1984, 151–154. [Google Scholar]
- Norte, M.; Gonzalez, A.G.; Arroyo, P.; Zarraga, M.; Perez, C.; Rodriguez, M.L.; Ruiz-Perez, C.; Dorta, L. New xenicane diterpenes from the brown algae of Dictyotaceae. Tetrahedron 1990, 46, 6125–6132. [Google Scholar] [CrossRef]
- Schlenk, D.; Gerwick, W.H. Dilophic acid, a diterpenoid from the tropical brown seaweed Dilophus guineensis. Phytochemistry 1987, 26, 1081–1084. [Google Scholar]
- algaeBASE. Available online: http://www.algaebase.org/ accessed on 6 February 2012.
- Wright, A.D.; König, G.M.; Sticher, O. New and highly oxidized hydroazulenoid diterpenes from the tropical marine brown alga Dictyota volubilis. Tetrahedron 1993, 49, 571–580. [Google Scholar] [CrossRef]
- Ayyad, S.E.; Slama, M.O.; MoKhtar, A.H.; Anter, A.F. Cytotoxic bicyclic diterpene from the brown alga Sargassum crispum. Boll. Chim. Farm. 2001, 140, 155–159. [Google Scholar]
- Ayyad, S.N.; Abdel-Halim, O.B.; Shier, W.T.; Hoye, T.R. Cytotoxic hydroazulene diterpenes from the brown alga Cystoseira myrica. Z. Naturforsch. C. Biosci. 2003, 58, 33–38. [Google Scholar]
- Alvarado, A.B.; Gerwick, W.H. Dictyol, H. a new tricyclic diterpenoid from brown seaweed Dictyota dentate. J. Nat. Prod. 1985, 48, 132–134. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Update of Spectroscopic Data for 4-Hydroxydictyolactone and Dictyol E Isolated from a Halimeda stuposa — Dictyota sp. Assemblage. Molecules 2012, 17, 2929-2938. https://doi.org/10.3390/molecules17032929
Ovenden SPB, Nielson JL, Liptrot CH, Willis RH, Tapiolas DM, Wright AD, Motti CA. Update of Spectroscopic Data for 4-Hydroxydictyolactone and Dictyol E Isolated from a Halimeda stuposa — Dictyota sp. Assemblage. Molecules. 2012; 17(3):2929-2938. https://doi.org/10.3390/molecules17032929
Chicago/Turabian StyleOvenden, Simon P. B., Jonathan L. Nielson, Catherine H. Liptrot, Richard H. Willis, Dianne M. Tapiolas, Anthony D. Wright, and Cherie A. Motti. 2012. "Update of Spectroscopic Data for 4-Hydroxydictyolactone and Dictyol E Isolated from a Halimeda stuposa — Dictyota sp. Assemblage" Molecules 17, no. 3: 2929-2938. https://doi.org/10.3390/molecules17032929
APA StyleOvenden, S. P. B., Nielson, J. L., Liptrot, C. H., Willis, R. H., Tapiolas, D. M., Wright, A. D., & Motti, C. A. (2012). Update of Spectroscopic Data for 4-Hydroxydictyolactone and Dictyol E Isolated from a Halimeda stuposa — Dictyota sp. Assemblage. Molecules, 17(3), 2929-2938. https://doi.org/10.3390/molecules17032929