Influence of Chemical Extraction Conditions on the Physicochemical and Functional Properties of Polysaccharide Gum from Durian (Durio zibethinus) Seed
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volumes-Weighted Mean (D [4,3])
| Regression coefficient | Volume mean (μm) | Span | Solubility (80 °C, %) | WHC (g water/100 g gum) | OHC (g oil/100 g gum) |
|---|---|---|---|---|---|
| Constant | −51.76 | 4.2821 | 20.4917 | 279.50 | 185.18 |
| b1 | 0.82 | −0.0021 | 0.2796 | - | 0.25 |
| b2 | 12.68 | −0.7422 | - | −16.72 | 24.24 |
| b3 | 1.22 | 0.0848 | −0.0744 | −3.90 | −7.32 |
| b12 | - | - | - | - | −0.00 |
| b22 | - | 0.0243 | - | - | −0.66 |
| b32 | 0.05 | - | 0.0107 | - | 0.06 |
| b12 | - | 0.0022 | - | - | −0.05 |
| b13 | −0.02 | −0.0005 | −0.0063 | - | 0.01 |
| b23 | −0.39 | - | - | 0.50 | −0.15 |
| R2 | 0.750 | 0.824 | 0.925 | 0.780 | 0.979 |
| p-value | 0.027 * | 0.011 * | 0.027 * | 0.038 * | 0.004 * |
| Lack of fit (p-value) | 0.188 | 0.499 | 0.236 | 0.353 | 0.508 |
| Variables | Main effects | Quadratic effects | Interaction effects | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x11 | x22 | x33 | x1x2 | x1x3 | x2x3 | ||
| Volume Weighted mean (μm) | p-value | 0.020 | 0.024 | 0.557 * | - | - | 0.021 | - | 0.042 | 0.014 |
| F-ratio | 7.919 | 7.328 | 0.372 | - | - | 7.795 | - | 5.636 | 9.272 | |
| Span | p-value | 0.853 * | 0.003 | 0.013 | - | 0.034 | - | 0.023 | 0.046 | - |
| F-ratio | 0.036 | 15.674 | 9.400 | - | 6.215 | - | 7.513 | 5.373 | - | |
| Solubility (80 °C, %) | p-value | 0.011 | - | 0.859 * | - | - | 0.042 | - | 0.010 | - |
| F-ratio | 9.200 | - | 0.033 | - | - | 5.303 | - | 9.600 | - | |
| WHC | p-value | - | 0.014 | 0.011 | - | - | - | - | - | 0.007 |
| F-ratio | - | 8.433 | 9.223 | - | - | - | - | - | 10.857 | |
| OHC | p-value | 0.243 * | 0.002 | 0.002 | 0.005 | 0.002 | 0.005 | 0.017 | 0.011 | 0.034 |
| F-ratio | 1.753 | 38.267 | 35.557 | 22.591 | 31.629 | 22.307 | 12.215 | 15.288 | 8.346 | |

2.2. Span or Particle Size Distribution

2.3. Solubility
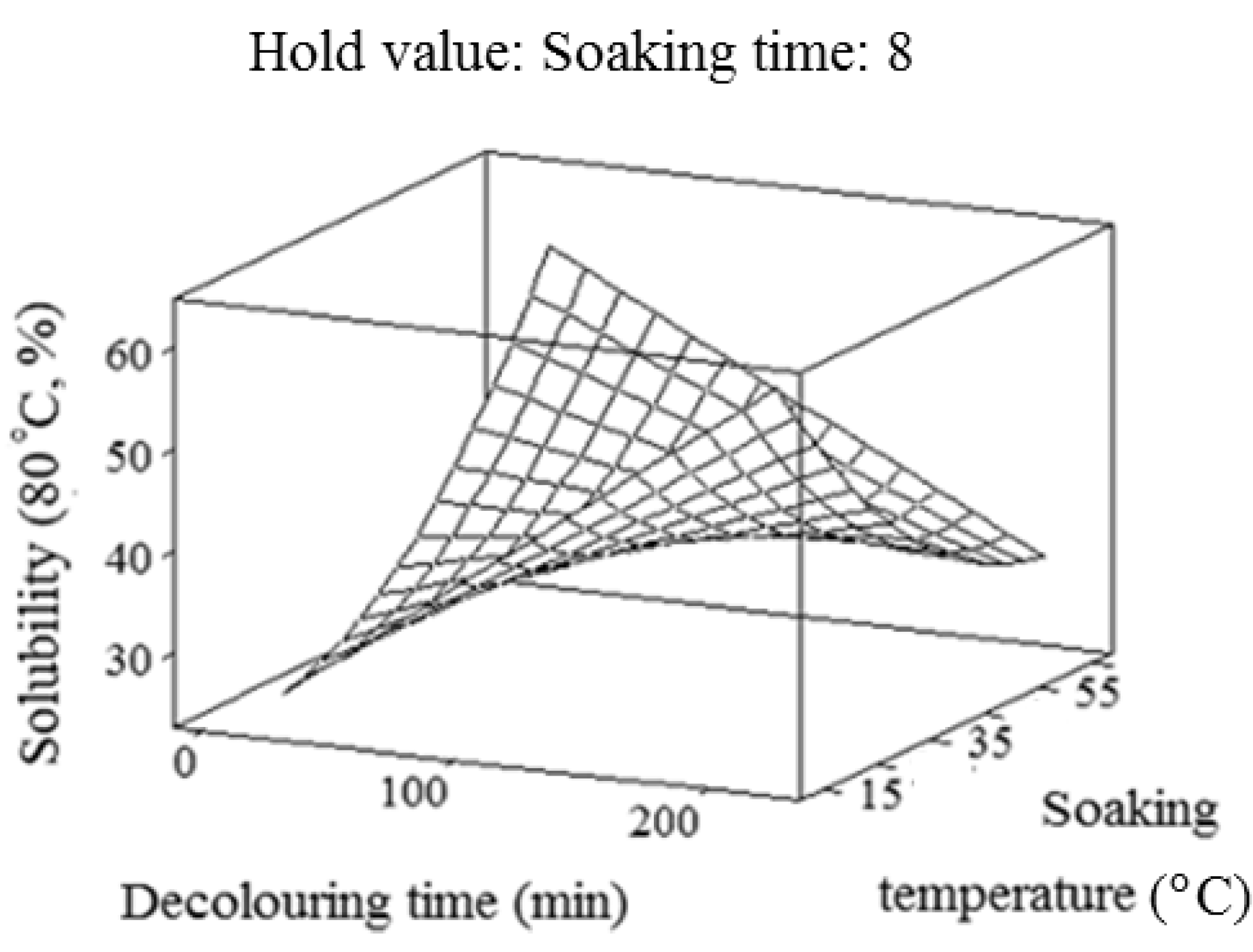
2.4. Water-Holding Capacity (WHC)
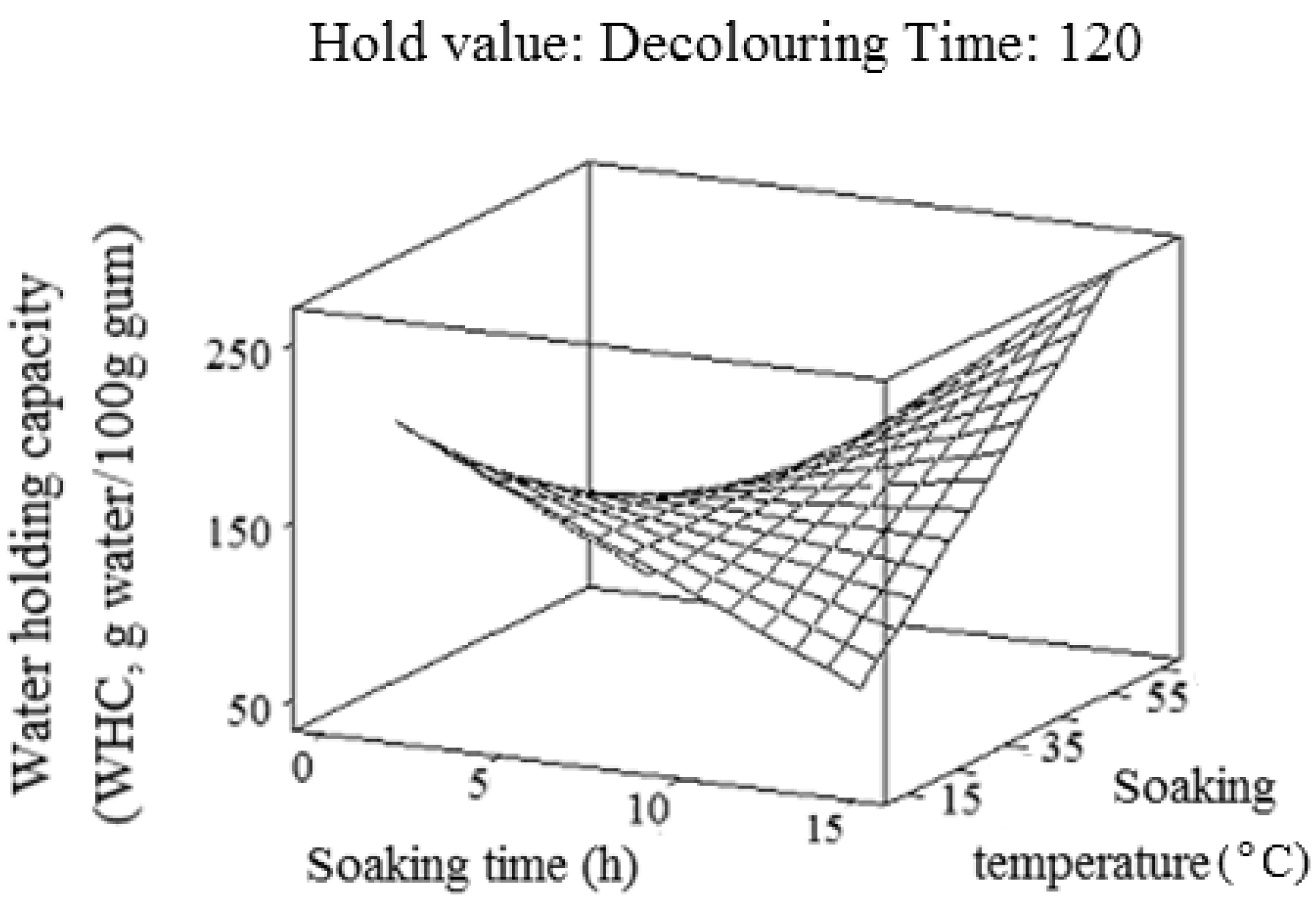
2.5. Oil-Holding Capacity (OHC)
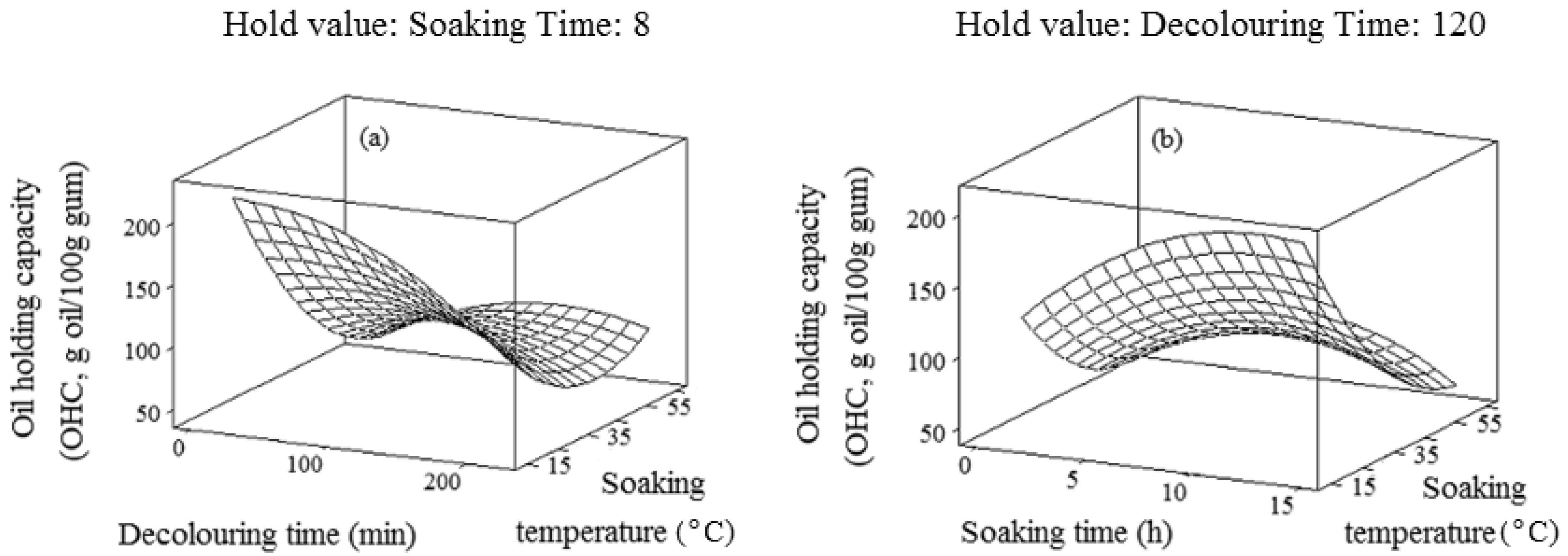
3. Experimental
3.1. Chemicals and Standards
3.2. Chemical Extraction of Crude Durian Seed Gum

3.3. Analytical Tests
3.3.1. Volume-Weighted Means (D [4,3])
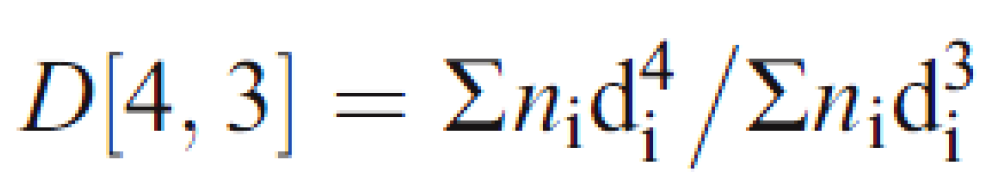
3.3.2. Span

3.3.3. Solubility
3.3.4. Water- and Oil-Holding Capacity

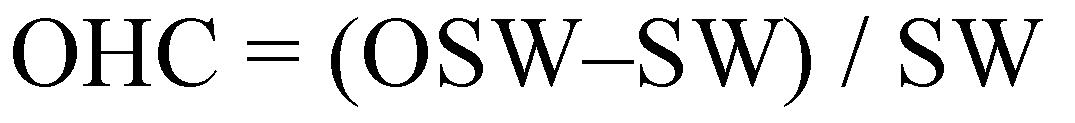
3.4. Experimental Design and Data Analysis
| Runs | Blocks | Decolouring time (x1, min) | Soaking time (x2, h) | Soaking temperature (x3, °C) |
|---|---|---|---|---|
| 1 * | 1 | 120.0 | 8.0 | 40.0 |
| 2 | 1 | 60.0 | 4.0 | 25.0 |
| 3 | 1 | 180.0 | 12.0 | 25.0 |
| 4 | 1 | 60.0 | 12.0 | 55.0 |
| 5 | 1 | 180.0 | 4.0 | 55.0 |
| 6 * | 1 | 120.0 | 8.0 | 40.0 |
| 7 | 2 | 180.0 | 12.0 | 55.0 |
| 8 | 2 | 60.0 | 4.0 | 55.0 |
| 9 * | 2 | 120.0 | 8.0 | 40.0 |
| 10 | 2 | 60.0 | 12.0 | 25.0 |
| 11 | 2 | 180.0 | 4.0 | 25.0 |
| 12 * | 2 | 120.0 | 8.0 | 40.0 |
| 13 | 3 | 120.0 | 14.5 | 40.0 |
| 14 | 3 | 218.0 | 8.0 | 40.0 |
| 15 * | 3 | 120.0 | 8.0 | 40.0 |
| 16 | 3 | 120.0 | 8.0 | 64.5 |
| 17 | 3 | 120.0 | 8.0 | 15.5 |
| 18 * | 3 | 120.0 | 8.0 | 40.0 |
| 19 | 3 | 22.0 | 8.0 | 40.0 |
| 20 | 3 | 120.0 | 1.5 | 40.0 |
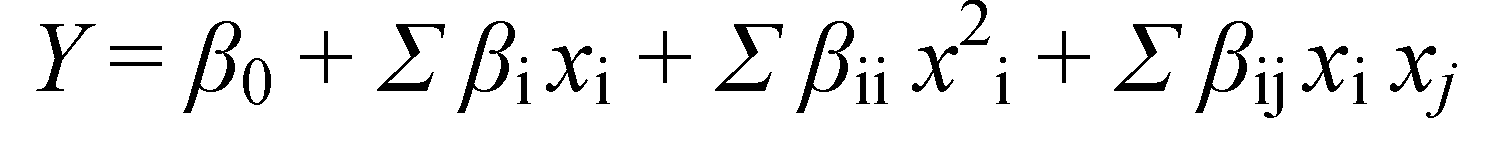
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds (i.e., Durian seed gum) are available from the authors.
References and Notes
- Ibañez, M.C.; Ferrero, C. Extraction and characterization of the hydrocolloid from Prosopis flexuosa DC seeds. Food Res. Int. 2003, 36, 455–460. [Google Scholar] [CrossRef]
- Rana, V.; Rai, P.; Tiwary, A.K.; Singh, R.S.; Kennedy, J.F.; Knill, C.J. Modified gums: Approaches and applications in drug delivery. Carbohydr. Polym. 2011, 83, 1031–1047. [Google Scholar] [CrossRef]
- Jun, T.Y.; Arumugam, S.D.; Abdul Latip, N.H.; Abdullah, A.M.; Abdul Latif, P. Effect of activation temperature and heating duration on physical characteristics of activated carbon prepared from agriculture waste. Environ. Asia 2010, 3, 143–148. [Google Scholar]
- Glicksman, M. A Series of Monographs: Gum Technology in the Food Industry; Academic Press, Inc.: London, UK, 1969. [Google Scholar]
- Vardhanabhuti, B.; Ikeda, S. Isolation and characterization of hydrocolloids from monoi (Cissampelos pareira) leaves. Food Hydrocol. 2006, 20, 885–891. [Google Scholar] [CrossRef]
- Amin, A.M.; Ahmad, A.S.; Yin, Y.Y.; Yahya, N.; Ibrahim, N. Extraction, purification and characterization of durian (Durio zibethinus) seed gum. Food Hydrocol. 2007, 21, 273–279. [Google Scholar] [CrossRef]
- Somboonpanyakul, P.; Wang, Q.; Cui, W.; Barbut, S.; Jantawat, P. Malva nut gum. (Part I): Extraction and physicochemical characterization. Carbohydr. Polym. 2006, 64, 247–253. [Google Scholar] [CrossRef]
- Mohammadzadeh Milani, J.; Emam-Djomeh, Z.; Rezaee, K.; Safari, M.; Ganbarzadeh, B.; Gunasekaran, S. Extraction and physicochemical properties of Barijeh (Ferula galbaniflua) gum. Int. J. Agric. Biol. 2007, 9, 80–83. [Google Scholar]
- Ho, C.H.L.; Cacacea, J.E.; Mazza, G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water. LWT Food Sci. Technol. 2007, 40, 1637–1647. [Google Scholar] [CrossRef]
- Kawamura, Y. Food Safety and Quality:Carob Bean Gum Chemical and Technical Assessment(CTA) for the Joint FAO/WHO Expert Committee on Food Additives (JECFA). In Food and Agriculture Organization of the United State; 2008. [Google Scholar]
- Chaires-Martínez, L.; Salazar-Montoya, J.A.; Ramos-Ramírez, E.G. Physicochemical and functional characterization of the galactomannan obtained from mesquite seeds (Prosopis pallida). Eur. Food Res. Technol. 2008, 227, 1669–1676. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Mortazavi, S.A.; Matia-Merino, L.; Hosseini-Parvar, S.H.; Motamedzadegan, A.; Khanipour, E. Optimisation study of gum extraction from Basil seeds (Ocimum basilicum L.). Int. J. Food Sci. Technol. 2009, 44, 1755–1762. [Google Scholar] [CrossRef]
- Galla, N.R.; Dubasi, G.R. Chemical and functional characterization of gum karaya (Sterculia urens L.) seed meal. Food Hydrocol. 2010, 24, 479–485. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Aghlara, A.; Hamid, N.S.A.; Yusof, S.; Boo, H.C. Influence of pectin and CMC on physical stability, turbidity loss rate, cloudiness and flavor release of orange beverage emulsion during storage. Carbohydr. Polym. 2008, 73, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Characterization of the influence of main emulsion components on cloudiness, size index, conductivity and emulsion stability of orange beverage emulsion using response surface methodology. Food Hydrocol. 2009, 23, 271–280. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Naghshineh, M. Influence of pectin and CMC content on physicochemical properties of orange beverage emulsion. J. Food Agric. Environ. 2010, 8, 134–139. [Google Scholar]
- Mirhosseini, H.; Tan, C.P. Effect of various hydrocolloids on physicochemical characteristics of orange beverage emulsion. J. Food Agric. Environ. 2010, 8, 308–313. [Google Scholar]
- Mirhosseini, H.; Tan, C.P.; Taherian, A.R. Effect of glycerol and vegetable oil on physicochemical properties of Arabic gum-based beverage emulsion. Eur. Food Res. Technol. 2008, 228, 19–28. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Characterization of the main emulsion components on cloudiness, size index, conductivity and emulsion stability of orange beverage emulsion using response surface methodology. Food Hydrocol. 2009, 23, 271–280. [Google Scholar] [CrossRef]
- Tabatabaee Amid, B.; Mirhosseini, H. Optimization of aqueous extraction of gum from Durian (Durio zibethinus) seed: A potential, low cost source of hydrocolloid. Food Chem. 2012, 132, 1258–1268. [Google Scholar] [CrossRef]
- Chew, N.Y.K.; Chan, H.K. Effect of powder polydispersity on Aerosol Generation. J. Pharm. Sci. 2002, 5, 162–168. [Google Scholar]
- Malvern Instruments Operators Guide, Man. 0247 Issue 2.0; Malvern Instruments Ltd.: Worcestershire, UK, 1999.
- Sarkar, A.; Rano, R.; Mishra, K.K.; Sinha, I.N. Particle size distribution profile of some Indian fly ash: A comparative study to assess their possible uses. Fuel Proc. Technol. 2005, 86, 1221–1238. [Google Scholar] [CrossRef]
- Ghorbani Gorji, E.; Mohammadifar, M.A.; Ezzatpanah, H. Influence of gum tragacanth, Astragalus gossypinus, addition on stability of non-fat Doogh, an Iranian fermented milk drink. Int. J. Dairy Technol. 2011, 64, 262–368. [Google Scholar] [CrossRef]
- Laaman, T.R. Hydrocolloids: Fifteen Practical Tips. In Hydrocolloids in Food Processing; Blackwell Publishing Ltd. and Institute of Food Technologists, Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- Maier, H.; Anderson, M.; Karl, C.; Magnuson, K.; Whistler, R.L. Guar, Locust Bean, Tara and Fenugreek Gums. In Industrial Gums, Polysaccharides and their Derivates; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: San Diego, CA, USA, 1933; pp. 205–215. [Google Scholar]
- Koocheki, A.; Taherian, A.R.; Bostan, A. Studies on the steady shear flow behaviour and functional properties of Lepidium perfoliatum seed gum. Food Res. Int. 2011. [Google Scholar] [CrossRef]
- Ophardt, C.E. Temperature and Pressure Effects on Solubility. Virtual Chembook. Available online: http://www.elmhurst.edu/~chm/vchembook/174temppres.html (accessed on 5 March 2010).
- Knudsen, K.E.B. Dietary Fibre in Nutrition and Health of Piglets. Available online: http://www.pig333.com/nutrition/dietary-fibre-in-nutrition-and-health-of-piglets_1256/ (accessed on 27 May 2009).
- Singh, U. Functional properties of grain legume flours. J. Food Sci. Technol. 2001, 38, 191–199. [Google Scholar]
- Simas-Tosin, F.F.; Barraza, R.R.; Petkowicz, C.L.O.; Silveira, J.L.M.; Sassaki, G.L.; Santos, E.M.R.; Gorin, P.A.J.; Iacomini, M. Rheological and structural characteristics of peach tree gum exudates. Food Hydrocol. 2010, 24, 486–493. [Google Scholar] [CrossRef]
- Torio, M.A.O.; Saez, J.; Merc, F.E. Physicochemical characterization of galactomannan from sugar palm (Arenga saccharifera Labill.) endosperm at different stages of nut maturit. Philippine J. Sci. 2006, 135, 19–30. [Google Scholar]
- Grigelmo-Miguel, N.; Martin-Belloso, O. Characterization of dietary fibre from orange juice extraction. Food Res. Int. 1999, 31, 355–361. [Google Scholar] [CrossRef]
- Shad, M.A.; Nawaz, H.; Hussain, M.; Yousuf, B. Proximate composition and functional properties of rhizomes of lotus (Nelumbo nucifera) from Punjab, Pakistan. Pakistan J. Bot. 2011, 43, 895–904. [Google Scholar]
- Kinsella, J.E. Relationships between Structural and Functional Properties of Food Proteins. In Food Protein; Fox, P.F., Condon, J.J., Eds.; Applied Science Publishers: London, UK, 1982; pp. 51–103. [Google Scholar]
- Janaki, B.; Sashidhar, R.B. Physicochemical analysis of gum Kondagogu (Cochlospermum gossypium): A potential food additive. Food Chem. 1998, 61, 231–236. [Google Scholar] [CrossRef]
- Hermansson, A.M.; Harbitz, O.; Langton, M. Formation of two types of bovine myosin gels. J. Sci. Food Agric. 1986, 37, 69–83. [Google Scholar] [CrossRef]
- Chang, Y.H.; Cui, S.W.; Roberts, K.T.; Ng, P.K.W.; Wang, Q. Evaluation of extrusion-modified fenugreek gum. Food Hydrocol. 2011, 25, 1296–1301. [Google Scholar] [CrossRef]
- Thanatcha, R.; Pranee, A. Extraction and characterization of mucilage in Ziziphus mauritiana Lam. Int. Food Res. J. 2011, 18, 201–212. [Google Scholar]
- Asha, D.; Shastri, P.N. Changes in structure, fat binding and water absorption of starch during roasting of wheat and legume flour. J. Food Sci. Technol. 2004, 41, 681–683. [Google Scholar]
- Thongsombat, W.; Sirichote, A.; Chanthachum, S. The production of guava juice fortified with dietary fiber. Songklanakarin J. Sci. Technol. 2007, 29, 187–196. [Google Scholar]
- Figuerola, F.; Hurtado, M.L; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Bravo, L.; Saura-Calixto, F. High dietary fibre powders from orange and lime peels, associated polyphenols and antioxidant capacity. Food Res. Int. 1996, 29, 757–762. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; McKenna, B. The application of peleg’s equation to model water absorption during the soaking of red kidney beans (Phaseolus vulgaris L.). J. Food Eng. 1997, 32, 391–401. [Google Scholar] [CrossRef]
- Bressani, R.; Benavides, V.; Acevedo, E.; Oritiz, M.A. Changes in selected nutrient content and in protein quality of common and quality protein maize during torilla preparation. Cereal Chem. 1990, 6, 515–518. [Google Scholar]
- Kinsella, J.E. Functional properties of proteins in foods: A survey. Crit. Rev. Food Sci. Nut. 1976, 4, 219–280. [Google Scholar]
- Lazos, E.S. Certain functional properties of defatted pumpkin seed flour. Plant Food Hum. Nut. 1992, 42, 257–273. [Google Scholar] [CrossRef]
- Singh, V.; Singh, S.K.; Maurya, S. Microwave induced poly (acrylic acid) modification of Cassia javanica seed gum for efficient Hg (II) removal from solution. Chem. Eng. J. 2010, 160, 129–137. [Google Scholar] [CrossRef]
- Kováčová, R.; Synytsya, A.; Štětina, J. Characterisation of whey proteins-pectin interaction in relation to emulsifying properties of whey proteins. Czech J. Food Sci. 2009, 27, S4–S8. [Google Scholar]
- León-Martínez, F.M.; Rodríguez-Ramírez, J.; Medina-Torres, L.L.; Méndez Lagunas, L.L.; Bernad-Bernad, M.J. Effects of drying conditions on the rheological properties of reconstituted mucilage solutions (Opuntia ficus-indica). Carbohydr. Polym. 2011, 84, 439–445. [Google Scholar] [CrossRef]
- Dakia, P.A.; Blecker, C.; Roberta, C.; Watheleta, B.; Paquota, M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocol. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Maldonado, F.; Ribotta, P.D.; Pérez, G.T.; León, A.E. Chemical composition and functional properties of Gleditsia triacanthos gum. Food Hydrocol. 2009, 23, 306–313. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed; Wiley: New York, NY, USA, 2001; pp. 455–492. [Google Scholar]
- Mirhosseini, H.; Tan, C.P.; Taherian, A.R.; Boo, H.C. Modeling the physicochemical properties of orange beverage emulsion as function of main emulsion components using response surface methodology. Carbohydr. Polym. 2009, 75, 512–520. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P. Discrimination of orange beverage emulsions with different formulations using multivariate analysis. J. Sci. Food Agric. 2010, 90, 1308–1316. [Google Scholar] [CrossRef]
- Joglekar, A.M.; May, A.T. Product excellence through design of experiments. Cereal Food. World 1987, 32, 857–868. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mirhosseini, H.; Amid, B.T. Influence of Chemical Extraction Conditions on the Physicochemical and Functional Properties of Polysaccharide Gum from Durian (Durio zibethinus) Seed. Molecules 2012, 17, 6465-6480. https://doi.org/10.3390/molecules17066465
Mirhosseini H, Amid BT. Influence of Chemical Extraction Conditions on the Physicochemical and Functional Properties of Polysaccharide Gum from Durian (Durio zibethinus) Seed. Molecules. 2012; 17(6):6465-6480. https://doi.org/10.3390/molecules17066465
Chicago/Turabian StyleMirhosseini, Hamed, and Bahareh Tabatabaee Amid. 2012. "Influence of Chemical Extraction Conditions on the Physicochemical and Functional Properties of Polysaccharide Gum from Durian (Durio zibethinus) Seed" Molecules 17, no. 6: 6465-6480. https://doi.org/10.3390/molecules17066465




