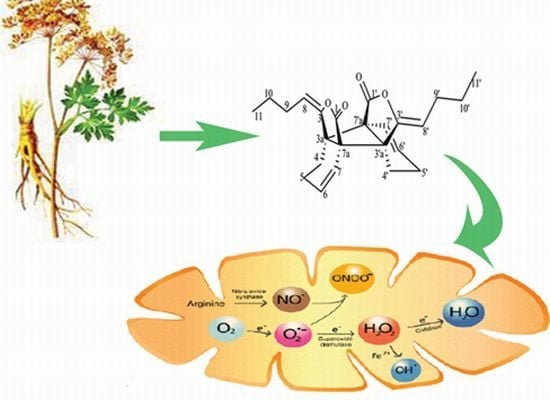
Antioxidant Properties of cis-Z,Z'-3a.7a',7a.3a'-Dihydroxy-ligustilide on Human Umbilical Vein Endothelial Cells in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Structure Elucidation

| No. | δC (DEPT) | δH J (Hz) | HMBC(H→C) * | NOESY | |
|---|---|---|---|---|---|
| 1 | 1' | 172.7 (s) | |||
| 3 | 3' | 148.9 (s) | |||
| 3a | 3'a | 51.7 (s) | |||
| 4 | 4' | 24.6 (t) | 1.92 (α-H, m); 1.85 (β-H, m) | C-3, 3a, 5, 6, 7, 7a | H-7, H-8 |
| 5 | 5' | 20.7 (t) | 2.13 (α-H, m); 1.92 (β-H, m) | C-6, 7, 7a | H-6, H-8 |
| 6 | 6' | 134.1 (d) | 6.19 (1H, ddd, 10.0, 6.5, 1.0) | C-1, 3a, 4, 5, 7 | H-5, H-7 |
| 7 | 7' | 120.9 (d) | 5.80 (1H, dd, 10.0, 1.0) | C-1, 5, 7a, | H-4', α, H-6 |
| 7a | 7'a | 50.4 (s) | |||
| 8 | 8' | 107.8 (d) | 4.85 (1H, t, 7.5) | C-3, 7, 7a, 10 | H-4, H-5, H-9 |
| 9 | 9' | 27.4 (t) | 2.20 (α-H, m); 2.13 (β-H, m) | C-3, 8 | H-8, H-10 |
| 10 | 10' | 22.5 (t) | 1.42 (2H, m) | C-8, 9, 11 | H-9, H-11 |
| 11 | 11' | 13.7 (q) | 0.91 (3H, t, 7.5) | C-9, 10 | H-10 |
2.1.2. Antioxidant Activity


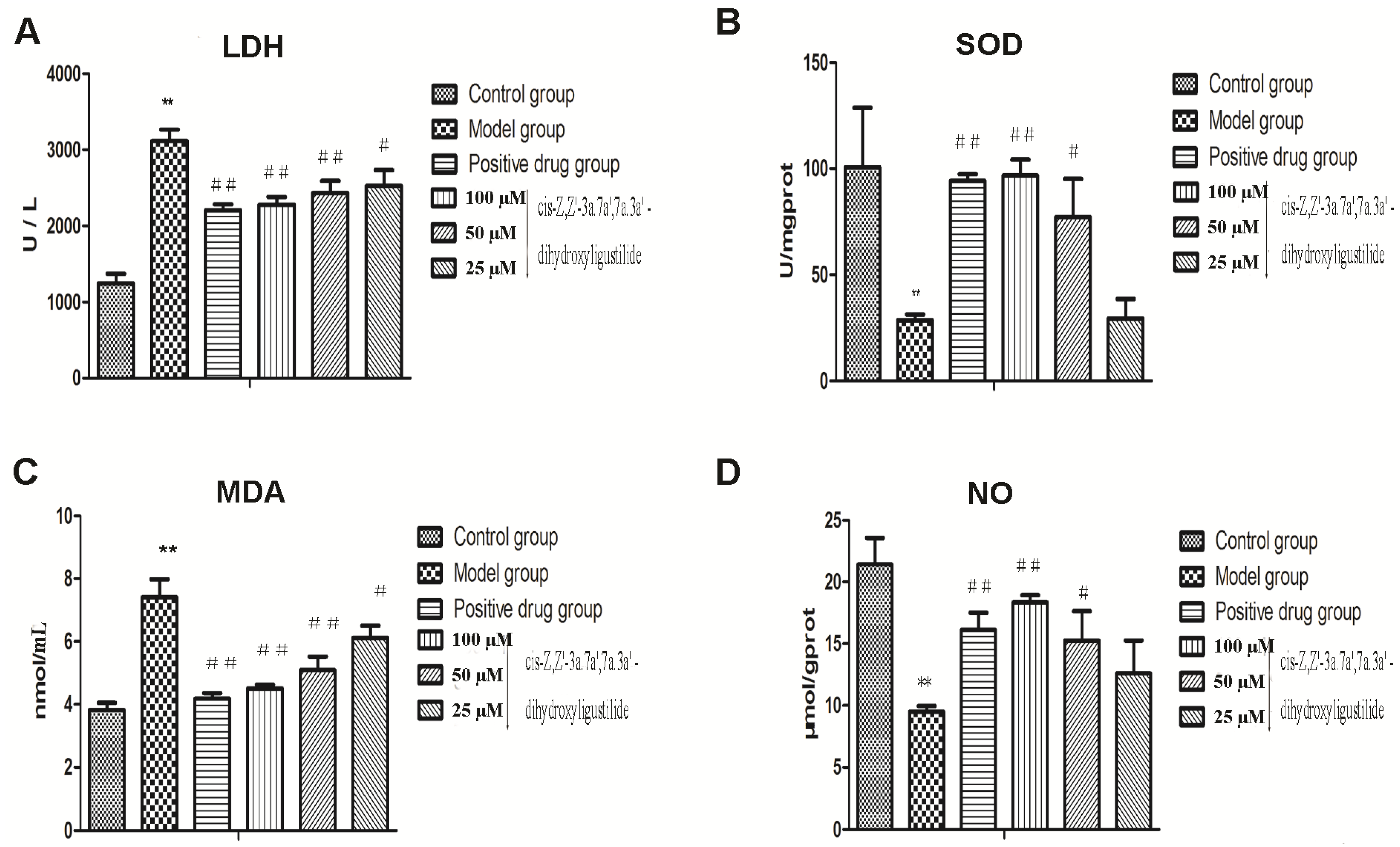
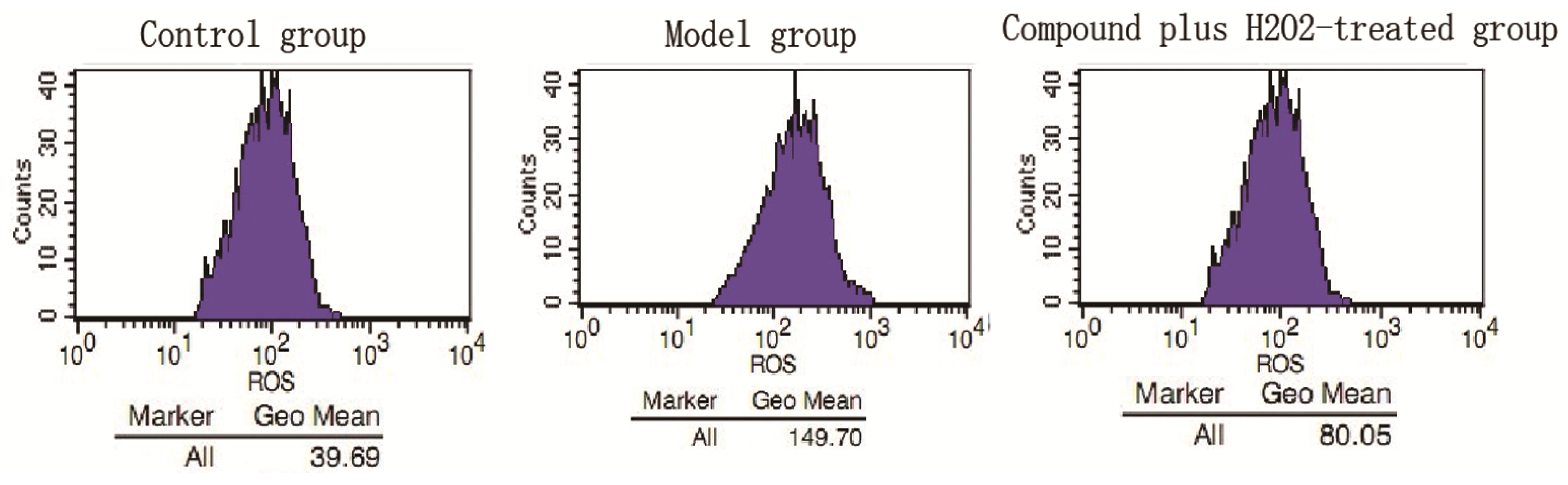

2.2. Discussion
| Number | Name | Monomer unit(s) | Linkage | References |
|---|---|---|---|---|
| 1 | Z,Z'-3.3'a,7.7'a-diligustilide | Z-ligustilide | 3a-7′a, 7a-3′a | [16] |
| 2 | Angelicide | Z-ligustilide | 6-8′, 7-3′ | [21] |
| 3 | Gelispirolide | Z-ligustilide and butylidenephthalide | 6-8′, 7-3′ | [22] |
| 4 | Riligustilide | Z-ligustilide | 6-8′, 7-3′ | [22] |
| Levistolide A | Z-ligustilide | 6-6′, 7-3′a | ||
| 5 | Levistolide B | Z-ligustilide and E-ligustilide | 6-6′, 7-3′a | [23] |
| 6 | Z-3′,8′,3′a,7′a-tetrahydro-6,3′,7,7′a-diligustilide-8′-one | Z-ligustilide | 6-3′, 7-7′a | [23] |
| 7 | Z,Z′-3.3′,8.8′-diligustilide | Z-ligustilide | 3-3′, 8-8′ | [22] |
| 8 | E,E′-3.3′,8.8′-diligustilide | E-ligustilide | 3-3′, 8-8′ | [24] |
| 9 | Senkyunolide O | Z-ligustilide and E-ligustilide | 6-6′, 7-3′a | [24] |
| 10 | Tokinolide B | Z-ligustilide | 3-3a′, 8-6′ | [24] |
| 11 | E-232 | Z-ligustilide and E-ligustilide | 3a-8′, 6-3′ | [25] |
| 12 | Sinaspirolide | Z-ligustilide and butylidenephthalide | 3-3′a, 8-7′a | [26] |
| 13 | Ansaspirolide | Z-ligustilide and butylidenephthalide | 3-3′a, 8-6′ | [27] |
| 14 | 3-3′ Z,-6.7′,7.6′-diligustilide | Z-ligustilide | 6.7′, 7.6′ | [28] |
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Antioxidant Activity in Vitro
3.4.1. Cell Cultures and MTT Assay
3.4.2. LDH Release Assay
3.4.3. SOD Activity Assay
3.4.4. Lipid Peroxidation Assay
3.4.5. NO Production Assay
3.4.6. ROS Production Assay
3.4.7. Flow Cytometric Analysis
3.5. Data Analysis
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds 1, 2 and 3 are available from the authors.
References and Notes
- The State Pharmacopoeia Commission of the People’s Republic of China, Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; Volume 1, p. 124.
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 10–15. [Google Scholar]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signal-ing. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, 1005–1028. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 9, 239–247. [Google Scholar]
- Deng, S.X. Phytochemical investigation of bioactive constituents from Angelica sinensis. Ph.D. Thesis, University of Illinois, Chicago, IL, USA, 2005. [Google Scholar]
- Zhang, S.; He, B.; Ge, J.B.; Li, H.B.; Luo, X.Y.; Zhang, H.; Li, Y.H.; Zhai, C.L.; Liu, P.G.; Liu, X.; et al. Extraction chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int. J. Biol. Macromol. 2010, 47, 546–550. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, T.; Hou, J.; Li, G.; Yu, S.; Xin, W. Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. Eur. J. Pharmacol. 2003, 467, 41–47. [Google Scholar] [CrossRef]
- Yu, Y.; Du, J.R.; Wang, C.Y.; Qian, Z.M. Protection against hydrogen peroxide-induced injury by Z-ligustilide in PC12 cells. Exp. Brain Res. 2008, 184, 307–312. [Google Scholar] [CrossRef]
- Beckman, E.J. Using CO2 to Produce Chemical products Sustainably. Environ. Sci. Technol. 2002, 36, 347–353. [Google Scholar] [CrossRef]
- Dhar-Mascareño, M.; Cárcamo, J.M.; Golde, D.W. Hypoxia-reoxygenation-induced mitochondrialdamage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic. Biol. Med. 2005, 38, 1311–1322. [Google Scholar] [CrossRef]
- Wassmann, S.; Wassmann, K.; Nickenig, G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 2004, 44, 381–386. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Coyle, C.H.; Martinez, L.J.; Coleman, M.C.; Spitz, D.R.; Weintraub, N.L.; Kader, K.N. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radic. Biol. Med. 2006, 40, 2206–2213. [Google Scholar] [CrossRef]
- Liu, H.T.; Li, W.M.; Xu, G.; Li, X.Y.; Bai, X.F.; Wei, P.; Chao, Y.; Yu, G.D. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol. Res. 2009, 59, 167–175. [Google Scholar] [CrossRef]
- Orrenius, S. Reactive oxygen species in mitochondria mediated-cell death. Drug Metab. Rev. 2007, 39, 443–455. [Google Scholar] [CrossRef]
- Yi, L.; Li, P.; Bi, Z.M. A new dimeric phthalide from Angelica sinensis. Chin. Chem. Lett. 2006, 17, 1579–1581. [Google Scholar]
- The crystallographic data have been deposited in the Cambridge Crystallographic Data Centre with Deposition No. CCDC-848087. Copies of data can be obtained on application to the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [Tel: +44(0)1223 762 911, or e-mail: [email protected]].
- Frederik, F.C.; Gijbels, M.J. cis- and trans-Neocnidilide; 1H- and 13C-NMR Data of Some Phthalides. Planta Med. 1987, 53, 77–80. [Google Scholar] [CrossRef]
- Lu, J.L.; Duan, J.A.; Tang, Y.P.; Yang, N.Y.; Zhang, L.B. Phthalide mono- and dimers from the radix of Angelica sinensis. Biochem. Syst. Ecol. 2009, 37, 405–411. [Google Scholar] [CrossRef]
- Zhang, L.; Du, J.R.; Wang, J.; Yu, D.K.; Chen, Y.S.; He, Y.; Wang, C.Y. Z-ligustilide extracted from Radix Angelica Sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi 2009, 129, 855–859. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H. Analysis of the chemical ingredients of Angelica sinensis—Analysis of nonvolatile constituents of roots. Gaodeng Xuexiao Huaxue Xuebao 1984, 5, 515–520. [Google Scholar]
- Deng, S.X.; Chen, S.N.; Lu, J.; Wang, Z.J.; Nikolic, D.; Van-Breemen, R.B.; Santarsiero, B.D.; Mesecar, A.; Fong, H.H.S.; Farnsworth, N.R.; et al. GABAergic phthalide dimers from Angelica sinensis (Oliv.)Diels. Phytochem. Anal. 2006, 17, 398–405. [Google Scholar] [CrossRef]
- Lin, L.Z.; He, X.G.; Lian, L.Z.; King, W.; Elliott, J. Liquid chromatographic-electrospray mass spectrometric study of the phthalides of Angelica sinensis and chemical changes of Z-ligustilide. J. Chromatogr. 1998, A810, 71–79. [Google Scholar]
- Su, D.M.; Yu, S.S.; Qin, H.L. New dimeric phthalide derivative from Angelica sinensis. Yao Xue Xue Bao 2005, 40, 141–144. [Google Scholar]
- Lu, X.H.; Zhang, J.J.; Zhang, X.X.; Liang, H.; Zhao, Y.Y. Study on biligustilides from Angelica sinensis. Zhongguo Zhong Yao Za Zhi 2008, 33, 2196–2201. [Google Scholar]
- Hon, P.M.; Lee, C.M.; Choang, T.F.; Chui, K.Y.; Wong, H.N.C. A ligustilide dimer from Angelica sinensis. Phytochemistry 1990, 29, 1189–1191. [Google Scholar] [CrossRef]
- Deng, S.; Chen, S.; Yao, P.; Bolton, J.; Nikolic, D.; Van-Breemen, R.; Fong, H.H.S.; Farnsworth, N.R.; Pauli, G.F. Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. J. Nat. Prod. 2006, 69, 536–541. [Google Scholar] [CrossRef]
- Li, X.N.; Chen, Y.Y.; Cheng, D.P.; Tong, S.Q.; Qu, H.B.; Yan, J.Z. Two phthalide dimers from the radix of Angelica sinensis. Nat. Prod. Res. 2011, 25, 1–5. [Google Scholar] [CrossRef]
- Craig, S.; Helfrich, L.A. Understanding Fish Nutrition, Feeds and Feeding; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2002; pp. 420–456. [Google Scholar]
- Cai, H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc. Res. 2005, 68, 26–36. [Google Scholar] [CrossRef]
- Mi, Y.L.; Zhang, C.Q. Protective effect of quercetin on aroclor 1254-induced oxidative damage in cultured chicken sermatogonial cells. Toxicol. Sci. 2005, 88, 545–550. [Google Scholar] [CrossRef]
- Rubbo, H.; Radi, R.; Trujillo, M.; Telleri, R.; Kalyanaraman, B.; Barnes, S.; Kirk, M.; Freeman, B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. J. Biol. Chem. 1994, 269, 26066–26075. [Google Scholar]
- Wu, Q.Z.; Huang, K.X. Protective effect of ebselen on cytotoxicity induced by cholestane-3β, 5α, 6β-triol in ECV-304 cells. Biochim. Biophys. Acta 2006, 1761, 350–359. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. NY Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Pantano, C.; Shrivastava, P.; McElhinney, B.; Janssen-Heininger, Y. Hydrogen peroxide signaling through tumor necrosis factor receptor 1 leads to selective activation of c-jun n-terminal kinase. J. Biol. Chem. 2003, 278, 44091–44096. [Google Scholar]
- Woo, S.H.; Park, I.C.; Park, M.-J.; An, S.; Lee, H.-C.; Jin, H.-O.; Park, S.-A.; Cho, H.; Lee, S.-J.; Gwak, H.-S.; et al. Arsenic tri-oxide sensitizes CD95/Fas-induced apoptosis through ros-mediated upregulation of CD95/Fas by NF-κB activation. Int. J. Cancer 2004, 112, 596–606. [Google Scholar] [CrossRef]
- Kamata, H.; Honda, S.I.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.; Wu, Y.; Liu, X.; Yan, C.; Liu, D.; Pan, Y.; Yang, G.; Yin, F.; Weng, Z.; Zhao, D.; et al. Antioxidant Properties of cis-Z,Z'-3a.7a',7a.3a'-Dihydroxy-ligustilide on Human Umbilical Vein Endothelial Cells in Vitro. Molecules 2013, 18, 520-534. https://doi.org/10.3390/molecules18010520
Li W, Wu Y, Liu X, Yan C, Liu D, Pan Y, Yang G, Yin F, Weng Z, Zhao D, et al. Antioxidant Properties of cis-Z,Z'-3a.7a',7a.3a'-Dihydroxy-ligustilide on Human Umbilical Vein Endothelial Cells in Vitro. Molecules. 2013; 18(1):520-534. https://doi.org/10.3390/molecules18010520
Chicago/Turabian StyleLi, Weidong, Yu Wu, Xuedong Liu, Cuiping Yan, Dan Liu, Yang Pan, Guangming Yang, Fangzhou Yin, Zebin Weng, Ding Zhao, and et al. 2013. "Antioxidant Properties of cis-Z,Z'-3a.7a',7a.3a'-Dihydroxy-ligustilide on Human Umbilical Vein Endothelial Cells in Vitro" Molecules 18, no. 1: 520-534. https://doi.org/10.3390/molecules18010520