Repeated Oral Administration of Oleanolic Acid Produces Cholestatic Liver Injury in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dose-Response of Liver Injury Produced by OA
2.1.1. Animal Body Weight and Liver Weight
2.1.2. Serum Biochemistry
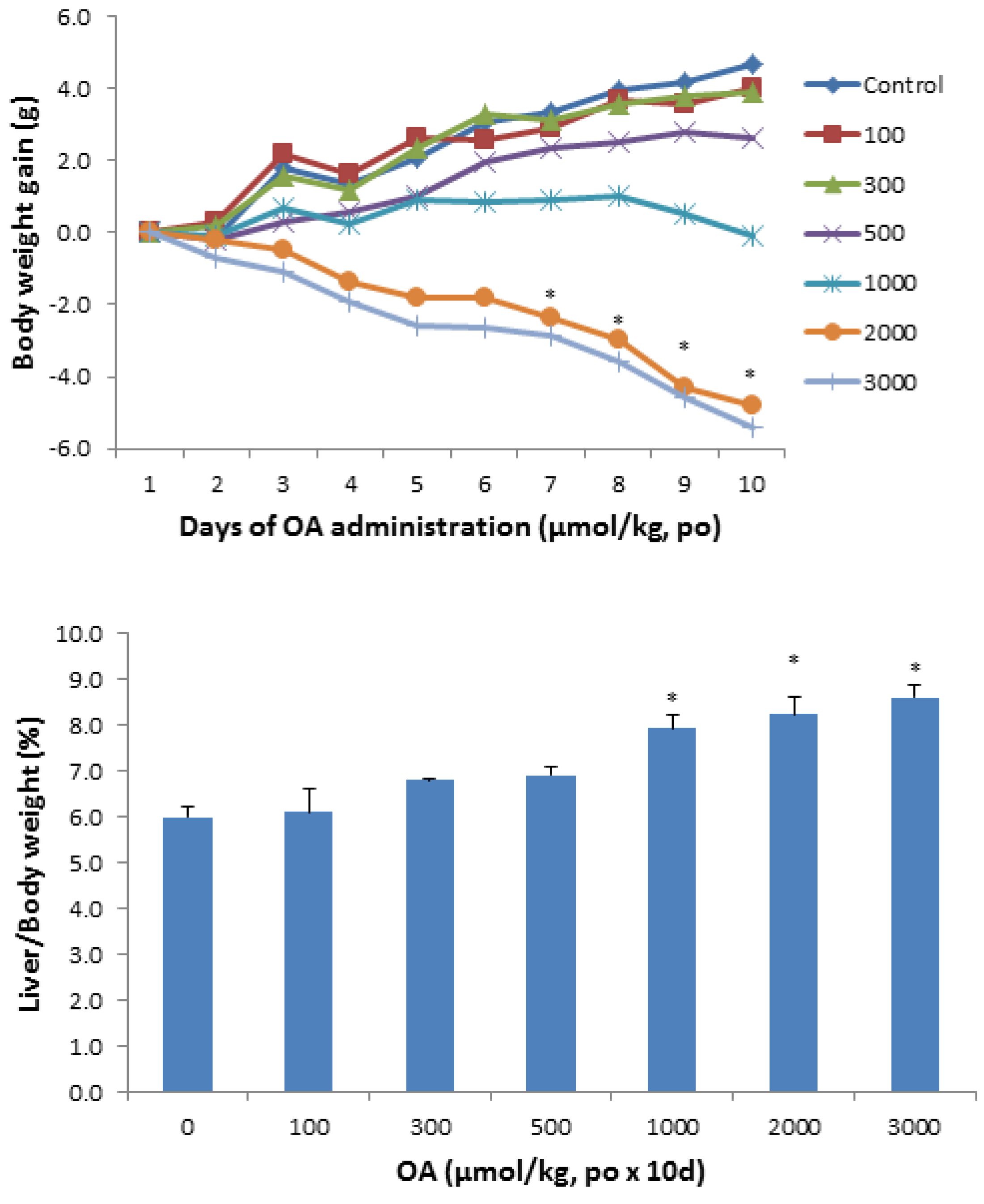

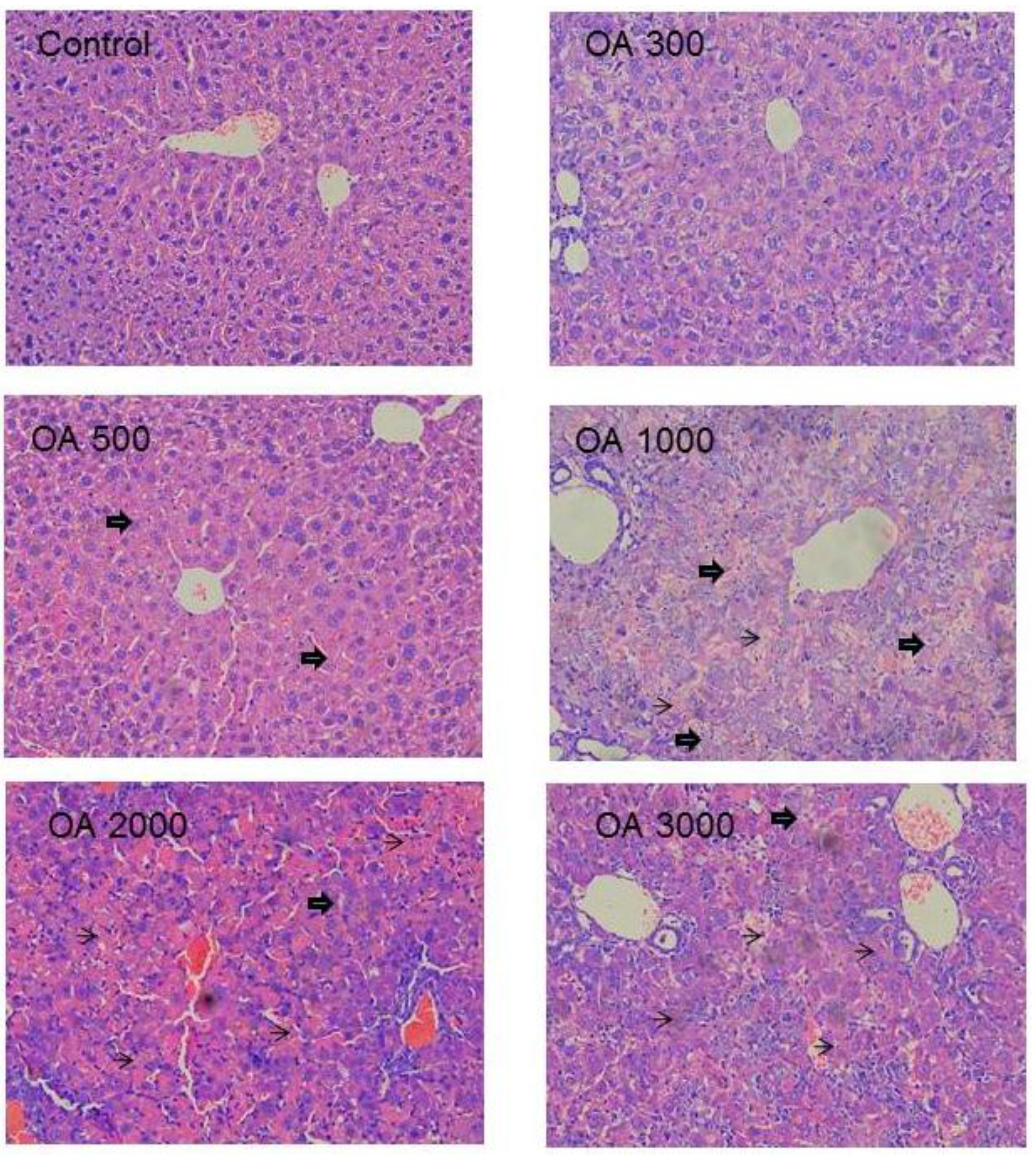
2.1.3. Histopathology
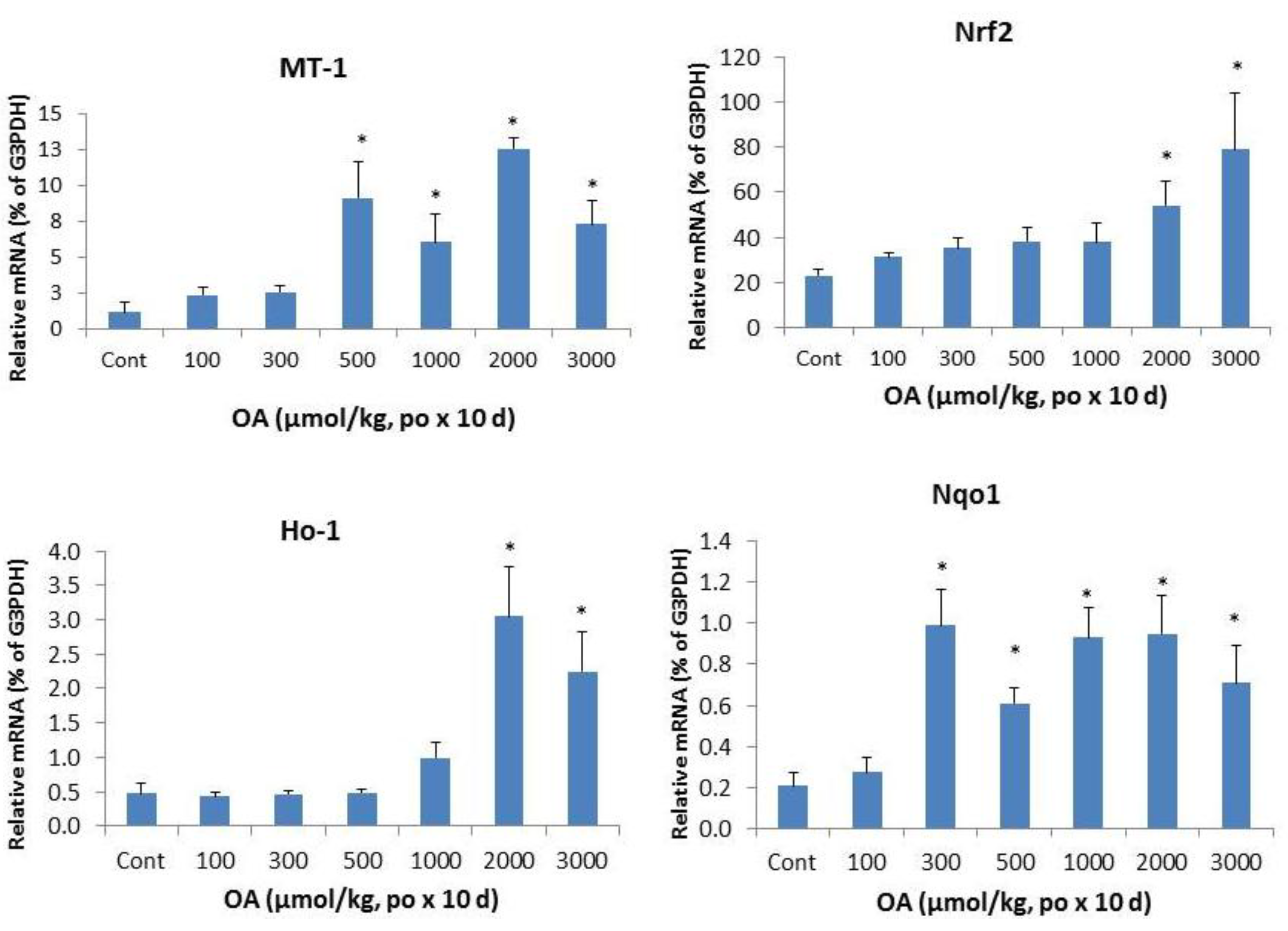
2.2. Effects of OA on Acute-Phase Protein Genes and Inflammatory Markers
2.3. Effects of OA on Bile Acid Metabolism Genes
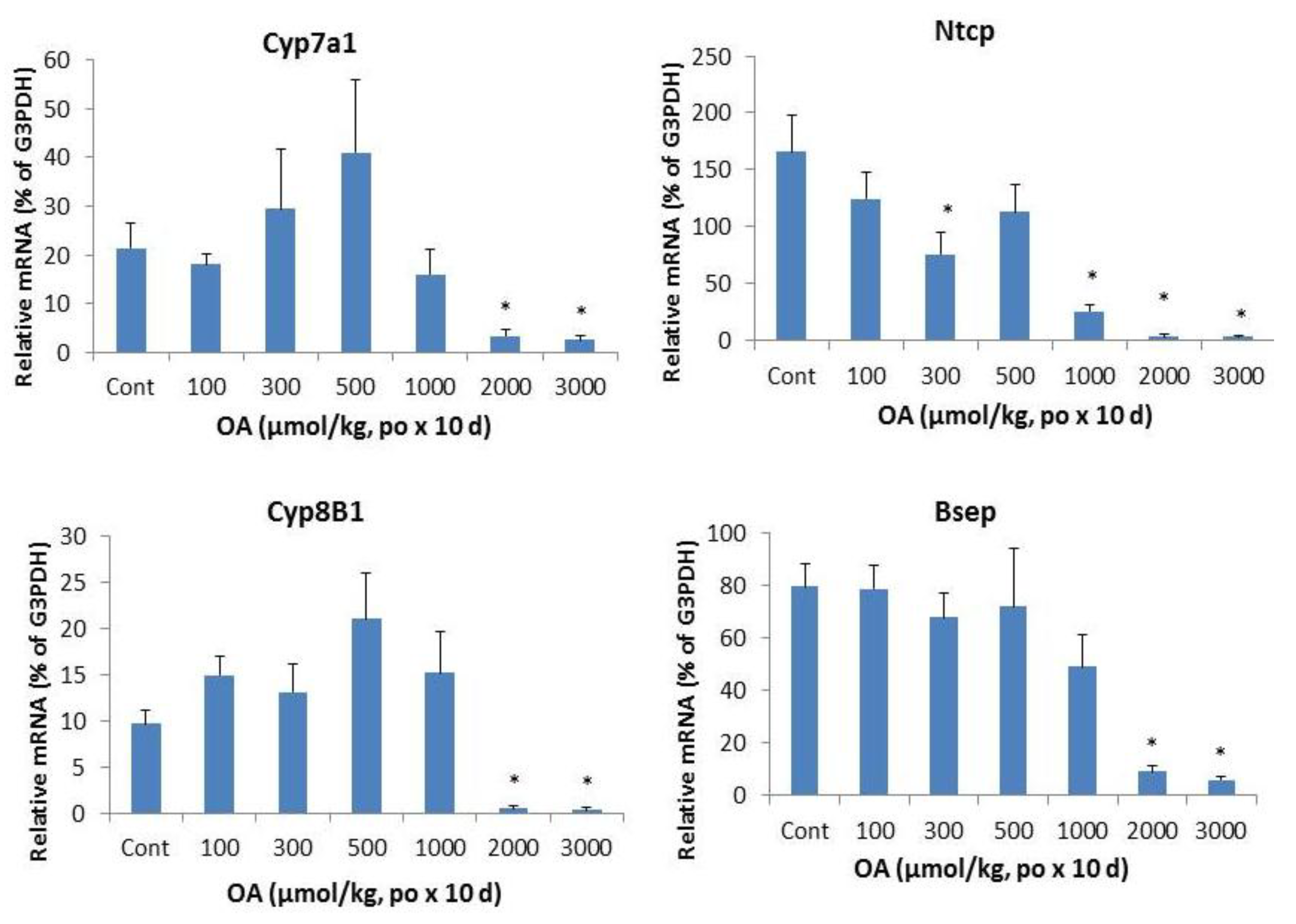
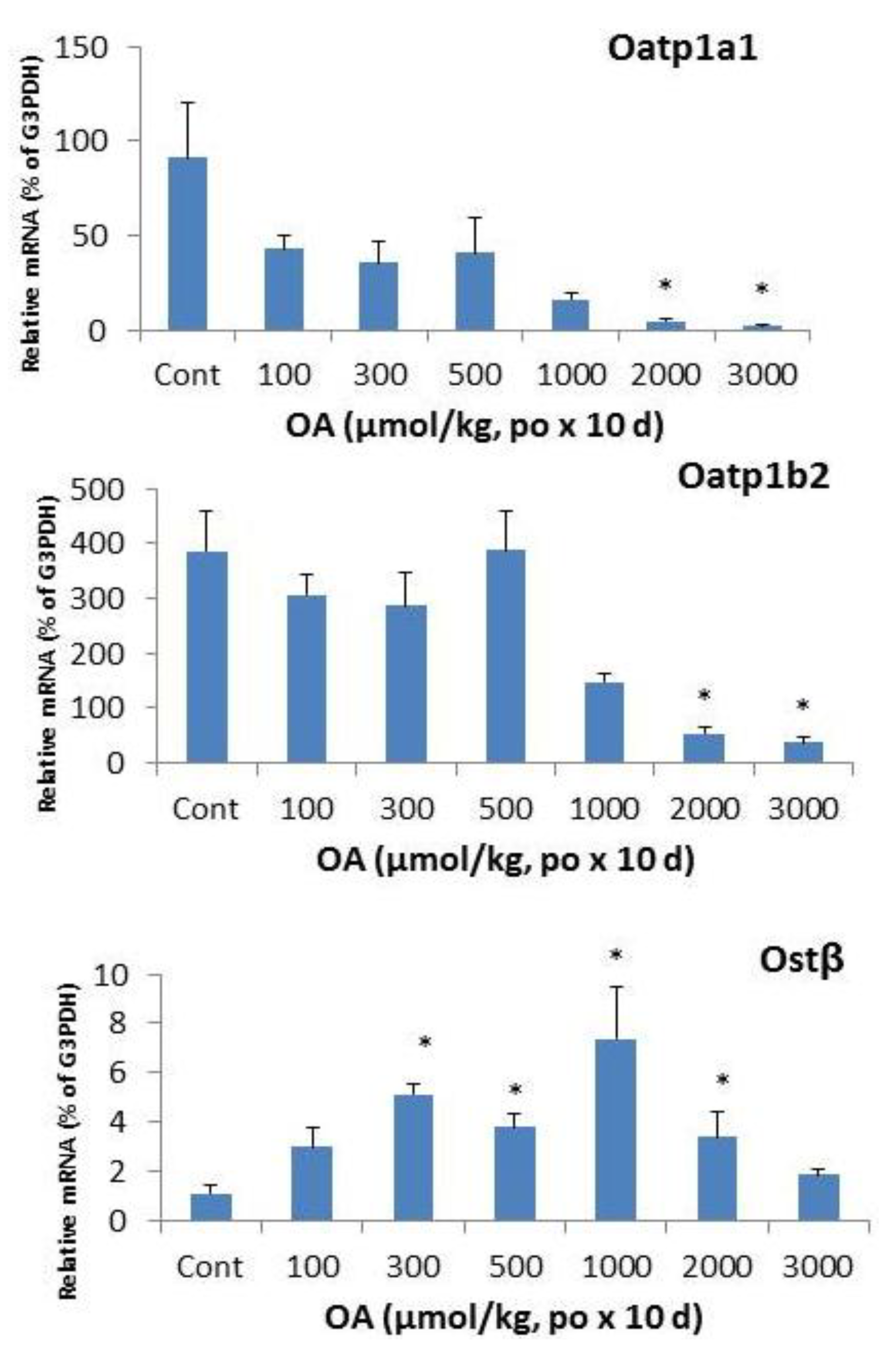
2.4. Effects of OA on Hepatic Transporters
2.5. Discussion
3. Experimental
3.1. Chemicals
3.2. Animals and Treatments
3.3. Serum Biochemistry
3.4. Histopathology
3.5. RNA Isolation
3.6. Real-Time RT-PCR Analysis
3.7. Statistical Analysis
| Gene | GenBank# | Forward | Reverse |
|---|---|---|---|
| Besp | NM_021022 | GGACAATGATGTGCTTGTGG | CACACAAAGCCCCTACCAGT |
| Cyp7a1 | NM_007824 | ATCCTGGCAAACAGAAATCG | GGCCAAGTCTGGTTTCTCTG |
| Cyp8b1 | NM_010012 | AGTTGCAGCGTCTCTTCCAT | CCTTGCTCCCTCAGAAACTG |
| G3PDH | M32599 | AACTTTGGCATTGTGGAAGG | GGATGCAGGGATGATGTTCT |
| HO-1 | M33203 | CCTCACTGGCAGGAAATCATC | CCTCGTGGAGACGCTTTACATA |
| MT-1 | NM_013602 | CTCCGTAGCTCCAGCTTCAC | AGGAGCAGCAGCTCTTCTTG |
| Nqo1 | BC004579 | TATCCTTCCGAGTCATCTCTAGCA | TCTGCAGCTTCCAGCTTCTTG |
| Nrf2 | BC026943 | CGAGATATACGCAGGAGAGGTAAGA | GCTCGACAATGTTCTCCAGCTT |
| Ntcp | NM_011387 | GGTGCCCTACAAAGGCATTA | ACAGCCACAGAGAGGGAGAA |
| Oatp1a1 | NM_013797 | ATCCAGTGTGTGGGGACAAT | GCAGCTGCAATTTTGAAACA |
| Oatp1b2 | NM_020495 | CAAACTCAGCATCCAAGCAA | GGCTGCCAAAAATATCCTGA |
| Ostâ | NM_178933 | ATCCTGGCAAACAGAAATCG | GGCCAAGTCTGGTTTCTCTG |
4. Conclusions
Acknowledgments
References
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Sultana, N.; Ata, A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzyme Inhib. Med. Chem. 2008, 23, 739–756. [Google Scholar] [CrossRef]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.L.; Wu, H.; Liu, J.Z.; Hu, J.X.; Liao, N.; Peng, J.; Cao, P.P.; Liang, X.; Hai, C.X. Antidiabetic effect of oleanolic acid: A promising use of a traditional pharmacological agent. Phytother Res. 2011, 25, 1031–1040. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Kinjo, J.; Okawa, M.; Udayama, M.; Sohno, Y.; Hirakawa, T.; Shii, Y.; Nohara, T. Hepatoprotective and hepatotoxic actions of oleanolic acid-type triterpenoidal glucuronides on rat primary hepatocyte cultures. Chem. Pharm. Bull. (Tokyo) 1999, 47, 290–292. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, O.P.; Dawra, R.K.; Bhat, T.K. Disposition of lantadene A, the pentacyclic triterpenoid hepatotoxin, orally administered to guinea pigs. Toxicol. Lett. 1999, 105, 59–66. [Google Scholar] [CrossRef]
- Milkiewicz, P.; Heathcote, J. Cholestasis induced by Chinese herbal remedy Xia-Ku-Hua-Tan-Pian. Liver Int. 2011, 31, 746–747. [Google Scholar] [CrossRef]
- Zoja, C.; Corna, D.; Nava, V.; Locatelli, M.; Abbate, M.; Gaspari, F.; Carrara, F.; Sangalli, F.; Remuzzi, G.; Benigni, A. Analogues of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am. J. Physiol. Renal Physiol. 2012. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Mao, Q.; Klaassen, C.D. The effects of 10 triterpenoid compounds on experimental liver injury in mice. Fundam. Appl. Toxicol. 1994, 22, 34–40. [Google Scholar] [CrossRef]
- Copple, B.L.; Jaeschke, H.; Klaassen, C.D. Oxidative stress and the pathogenesis of cholestasis. Semin. Liver Dis. 2010, 30, 195–204. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Novel insight into mechanisms of cholestatic liver injury. World J. Gastroenterol. 2012, 18, 4985–4993. [Google Scholar] [CrossRef]
- Brambila, E.; Munoz-Sanchez, J.L.; Waalkes, M.P.; Albores, A. Effect of surgically induced cholestasis on the levels of hepatic zinc and metallothionein in rat liver. Biol. Trace Elem. Res. 2000, 78, 255–264. [Google Scholar] [CrossRef]
- Froh, M.; Conzelmann, L.; Walbrun, P.; Netter, S.; Wiest, R.; Wheeler, M.D.; Lehnert, M.; Uesugi, T.; Scholmerich, J.; Thurman, R.G. Heme oxygenase-1 overexpression increases liver injury after bile duct ligation in rats. World J. Gastroenterol. 2007, 13, 3478–3486. [Google Scholar]
- Aleksunes, L.M.; Slitt, A.L.; Maher, J.M.; Dieter, M.Z.; Knight, T.R.; Goedken, M.; Cherrington, N.J.; Chan, J.Y.; Klaassen, C.D.; Manautou, J.E. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones 2006, 11, 356–363. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Csanaky, I.L.; Lu, H.; Zhang, Y.; Ogura, K.; Choudhuri, S.; Klaassen, C.D. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology 2011, 53, 272–281. [Google Scholar] [CrossRef]
- Lam, P.; Soroka, C.J.; Boyer, J.L. The bile salt export pump: Clinical and experimental aspects of genetic and acquired cholestatic liver disease. Semin. Liver Dis. 2010, 30, 125–133. [Google Scholar] [CrossRef]
- Lu, H.; Choudhuri, S.; Ogura, K.; Csanaky, I.L.; Lei, X.; Cheng, X.; Song, P.Z.; Klaassen, C.D. Characterization of organic anion transporting polypeptide 1b2-null mice: Essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol. Sci. 2008, 103, 35–45. [Google Scholar]
- Liu, J.; Lu, Y.F.; Zhang, Y.C.; Wu, C.; Klaassen, C.D. Oleanolic acid produces cholestatic liver injury in C57 mice. Toxicologist 2013, 132, A1087. [Google Scholar]
- Xi, J.; Tang, H.; Zheng, Y. Oral dosage forms of oleanolic acid and their pharmacokinetics. Chin. J. New Drugs 2009, 18, 507–515. [Google Scholar]
- Wang, J.B.; Zhao, H.P.; Zhao, Y.L.; Jin, C.; Liu, D.J.; Kong, W.J.; Fang, F.; Zhang, L.; Wang, H.J.; Xiao, X.H. Hepatotoxicity or hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. in treating rat liver injury. PLoS One 2011, 6, e24498. [Google Scholar] [CrossRef]
- Chitturi, S.; Farrell, G.C. Herbal hepatotoxicity: An expanding but poorly defined problem. J. Gastroen. Hepatol. 2000, 15, 1093–1099. [Google Scholar] [CrossRef]
- Teschke, R.; Frenzel, C.; Schulze, J.; Eickhoff, A. Spontaneous reports of primarily suspected herbal hepatotoxicity by Pelargonium sidoides: Was causality adequately ascertained? Regul. Toxicol. Pharmacol. 2012, 63, 1–9. [Google Scholar] [CrossRef]
- Li, M.K.; Crawford, J.M. The pathology of cholestasis. Semin. Liver Dis. 2004, 24, 21–42. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Gui, C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 2008, 38, 778–801. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef]
- Boyer, J.L.; Trauner, M.; Mennone, A.; Soroka, C.J.; Cai, S.Y.; Moustafa, T.; Zollner, G.; Lee, J.Y.; Ballatori, N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1124–G1130. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the oleanolic acid are available from the authors as well as from comercial resources (Sigma or Chinese Pharmaceutical companies).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, Y.-F.; Wan, X.-L.; Xu, Y.; Liu, J. Repeated Oral Administration of Oleanolic Acid Produces Cholestatic Liver Injury in Mice. Molecules 2013, 18, 3060-3071. https://doi.org/10.3390/molecules18033060
Lu Y-F, Wan X-L, Xu Y, Liu J. Repeated Oral Administration of Oleanolic Acid Produces Cholestatic Liver Injury in Mice. Molecules. 2013; 18(3):3060-3071. https://doi.org/10.3390/molecules18033060
Chicago/Turabian StyleLu, Yuan-Fu, Xiao-Li Wan, Yasha Xu, and Jie Liu. 2013. "Repeated Oral Administration of Oleanolic Acid Produces Cholestatic Liver Injury in Mice" Molecules 18, no. 3: 3060-3071. https://doi.org/10.3390/molecules18033060




