Synthesis and Characterization of Oil-Chitosan Composite Spheres
Abstract
:1. Introduction

2. Results and Discussion
2.1. Morphology


2.2. Characterization
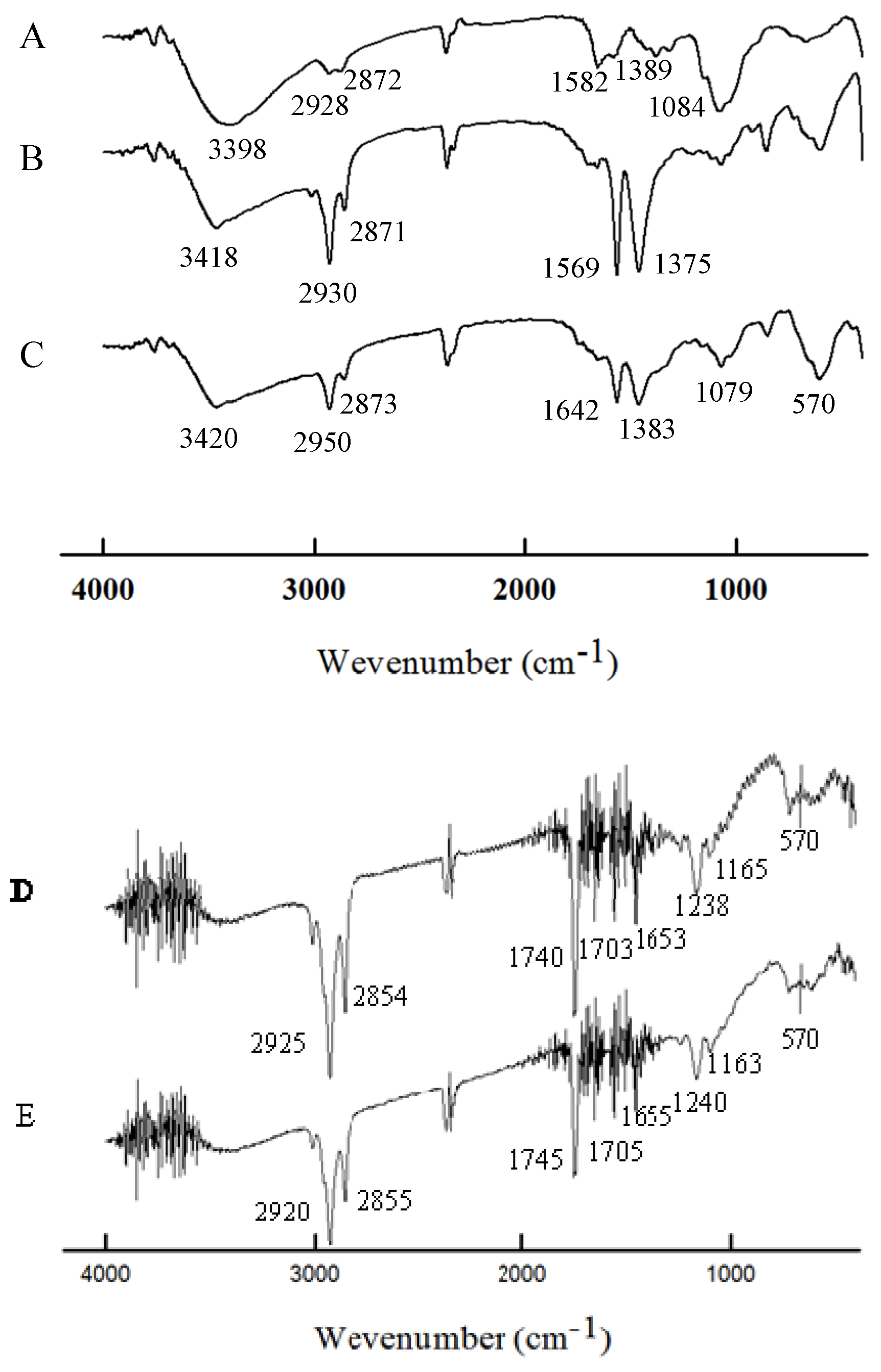
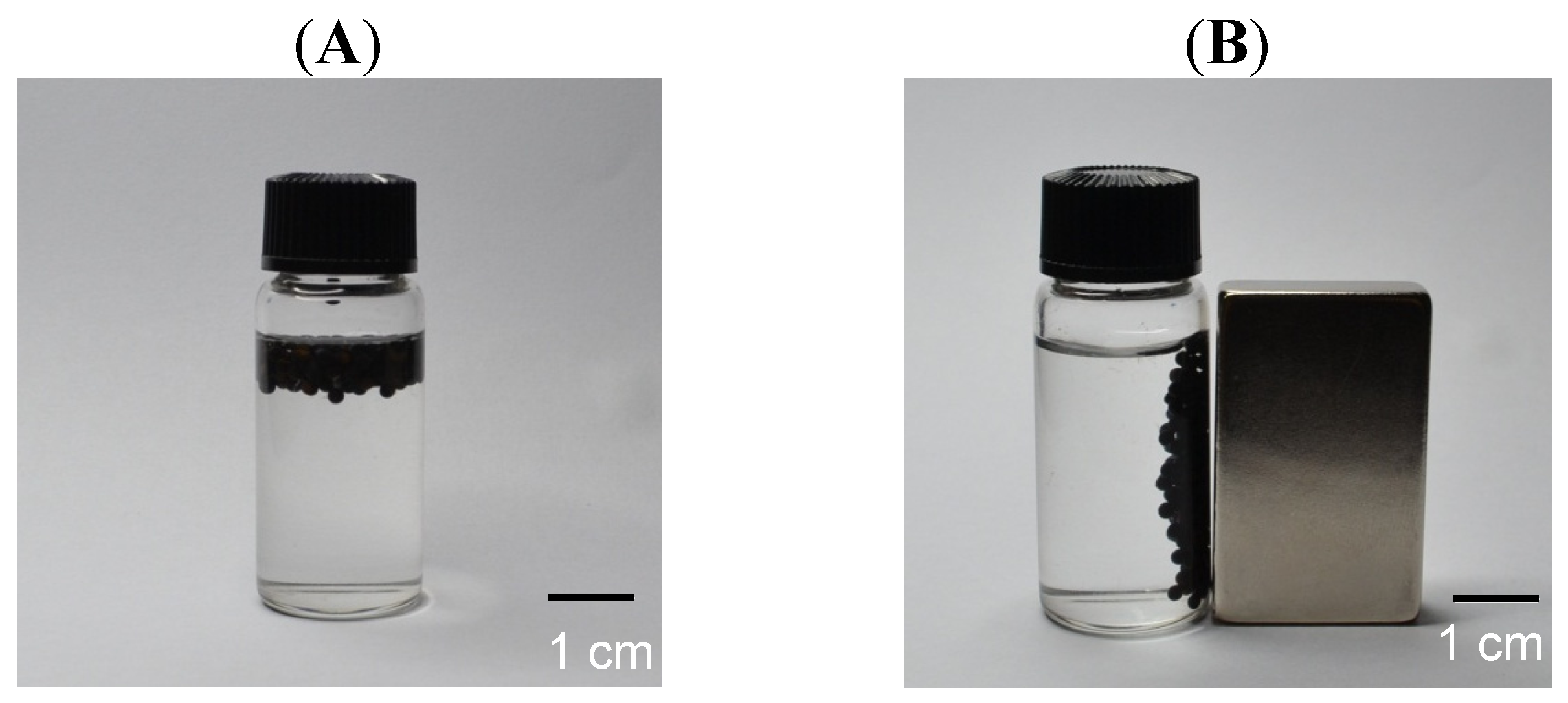
2.3. Magnetic Response and Dual Encapsulation Properties
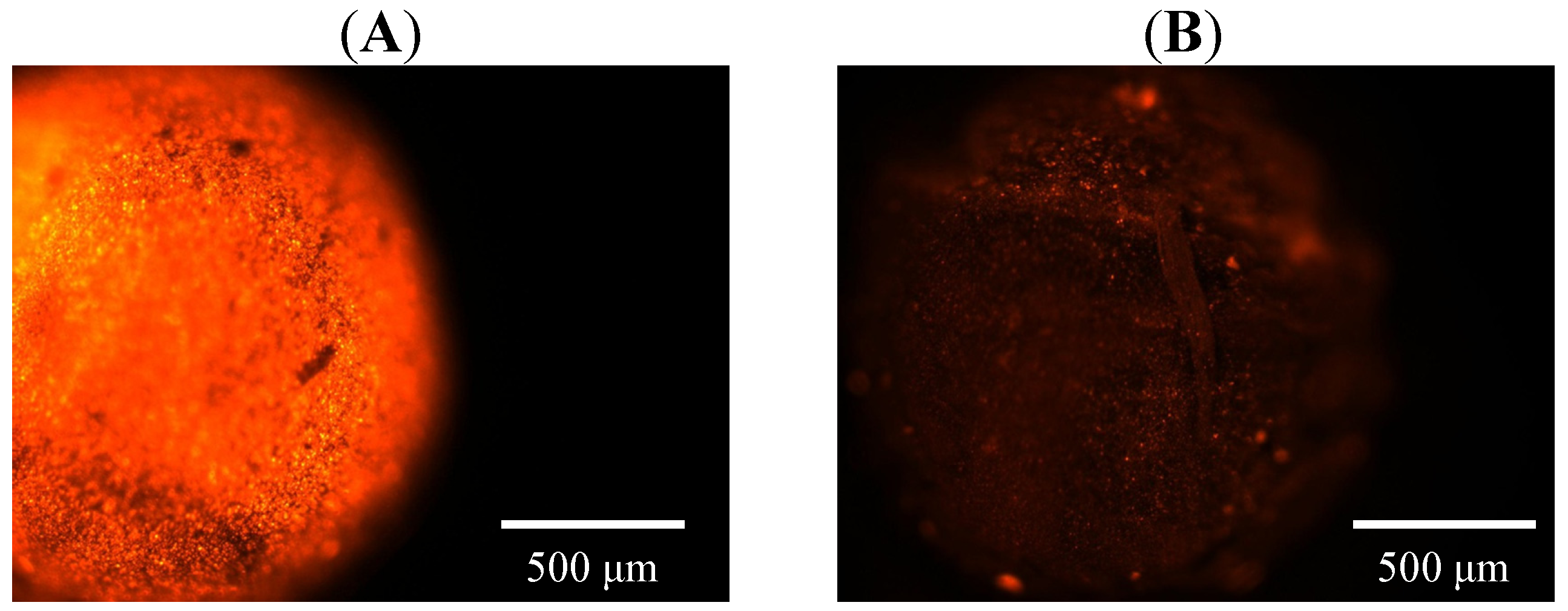
3. Experimental
3.1. Materials
3.2. Synthesis of Chitosan Spheres
3.3. Synthesis of Oil-Chitosan Composite Spheres
3.4. Synthesis of Superparamagnetic Oil-Chitosan Composite Spheres
3.5. Preparation of Epirubicin and Encapsulated Iron Oxide Oil-Chitosan Composites
3.6. Instruments
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Yang, C.H.; Huang, K.S.; Wang, C.Y.; Hsu, Y.Y.; Chang, F.R.; Lin, Y.S. Microfluidic-assisted synthesis of hemispherical and discoidal chitosan microparticles at an oil/water interface. Electrophoresis 2012, 33, 3173–3180. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Guzman-Morales, J.; Tran-Khanh, N.; Lavallee, G.; Jolicoeur, M.; Lavertu, M. Chitosan rate of uptake in HEK293 cells is influenced by soluble versus microparticle state and enhanced by serum-induced cell metabolism and lactate-based media acidification. Molecules 2013, 18, 1015–1035. [Google Scholar] [CrossRef]
- Kim, G.O.; Kim, N.; Kim D.Y.; Kwon, J.S.; Min, B.H. An electrostatically crosslinked chitosan hydrogel as a drug carrier. Molecules (Basel, Switzerland) 2012, 17, 13704–13711. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, C.Y.; Huang, K.S.; Yeh, C.S.; Wang, A.H.J.; Wang, W.T.; Lin, M.Y. Facile synthesis of radial-like macroporous superparamagnetic chitosan spheres with in-situ co-precipitation and gelation of ferro-gels. PLoS One 2012, e49329. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zhang, H.F.; Shi, Y.P. Magnetic retrieval of chitosan: extraction of bioactive constituents from green tea beverage samples. The Analyst 2012, 137, 910–916. [Google Scholar] [CrossRef]
- Rozenberg, B.A.; Tenne, R. Polymer-assisted fabrication of nanoparticles and nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Huang, H.Y.; Shieh, Y.T.; Shih, C.M.; Twu, Y.K. Magnetic chitosan/iron (II, III) oxide nanoparticles prepared by spray-drying. Carbohydr. Polym. 2010, 81, 906–910. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Das, M.; Mishra, D.; Banerjee, I.; Sahu, S.K.; Maiti, T.K.; Pramanik, P. Folate receptor targeted, carboxymethyl chitosan functionalized iron oxide nanoparticles: a novel ultradispersed nanoconjugates for bimodal imaging. Nanoscale 2011, 3, 1653–1662. [Google Scholar]
- Wang, C.Y.; Yang, C.H.; Huang, K.S.; Yeh, C.S.; Wang, A.H.J.; Chen, C.H. Electrostatic droplets assisted in situ synthesis of superparamagnetic chitosan microparticles for magnetic-responsive controlled drug release and copper ion removal. J. Mater. Chem. B 2013, 1, 2205–2212. [Google Scholar] [CrossRef]
- Arami, H.; Stephen, Z.; Veiseh, O.; Zhang, M. Chitosan-Coated Iron Oxide Nanoparticles for Molecular Imaging and Drug Delivery. In Chitosan for Biomaterials I, Jayakumar, R.; Prabaharan, M., MuzzarelliR, A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 243, pp. 163–184. [Google Scholar]
- Kievit, F.M.; Zhang, M. Surface Engineering of Iron Oxide Nanoparticles for Targeted Cancer Therapy. Acc. Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Fang, Z.; Zhang, X.; Zhang, B. Magnetic Chitosan Nanocomposites: A Useful Recyclable Tool for Heavy Metal Ion Removal. Langmuir 2008, 25, 3–8. [Google Scholar]
- Zhou, H.; Yu, W.; Guo, X.; Liu, X.; Li, N.; Zhang, Y.; Ma, X. Synthesis and Characterization of Amphiphilic Glycidol−Chitosan−Deoxycholic Acid Nanoparticles as a Drug Carrier for Doxorubicin. Biomacromolecules 2010, 11, 3480–3486. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; Park, R.W.; Kim, I.S.; Kim, K.; Kwon, I.C. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Controlled Release 2008, 127, 41–49. [Google Scholar]
- Hsiao, M.H.; Larsson, M.; Larsson, A.; Evenbratt, H.; Chen, Y.Y.; Chen, Y.Y.; Liu, D.M. Design and characterization of a novel amphiphilic chitosan nanocapsule-based thermo-gelling biogel with sustained in vivo release of the hydrophilic anti-epilepsy drug ethosuximide. J. Controlled Release 2012, 161, 942–948. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, S.Y.; Lin, Y.L.; Liu, D.M. Synthesis and Characterization of Amphiphatic Carboxymethyl-hexanoyl Chitosan Hydrogel: Water-Retention Ability and Drug Encapsulation. Langmuir 2006, 22, 9740–9745. [Google Scholar] [CrossRef]
- Asthana, S.; Jaiswal, A.K.; Gupta, P.K.; Pawar, V.K.; Dube, A.; Chourasia, M.K. Immunoadjuvant Chemotherapy of Visceral Leishmaniasis in Hamsters Using Amphotericin B-Encapsulated Nanoemulsion Template-Based Chitosan Nanocapsules. Antimicrob. Agents Chemother. 2013, 57, 1714–1722. [Google Scholar] [CrossRef]
- Abdelghany, S.M.; Schmid, D.; Deacon, J.; Jaworski, J.; Fay, F.; McLaughlin, K.M.; Gormley, J. A.; Burrows, J.F.; Longley, D.B.; Donnelly, R.F.; Scott, C.J. Enhanced Antitumor Activity of the Photosensitizer meso-Tetra(N-methyl-4-pyridyl) Porphine Tetra Tosylate through Encapsulation in Antibody-Targeted Chitosan/Alginate Nanoparticles. Biomacromolecules 2013, 14, 302–310. [Google Scholar] [CrossRef]
- Bulmer, C.; Margaritis, A.; Xenocostas, A. Encapsulation and Controlled Release of Recombinant Human Erythropoietin from Chitosan-Carrageenan Nanoparticles. Curr. Drug Deliv. 2012, 9, 527–537. [Google Scholar] [CrossRef]
- Domaratzki, R.E.; Ghanem, A. Encapsulation and release of cladribine from chitosan nanoparticles. J. Appl. Polym. Sci. 2012, 128, 2173–2179. [Google Scholar]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Argüelles-Monal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Koppolu, B.; Zaharoff, D.A. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials 2013, 34, 2359–2369. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; D'Ath, L.; Lawrence, A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Neufeld, R.J.; Arnaud, P.; Chaumeil, J.C. Microencapsulation of lipophilic drugs in chitosan-coated alginate microspheres. Int. J. Pharm. 1999, 187, 115–123. [Google Scholar] [CrossRef]
- Sanyakamdhorn, S.; Agudelo, D.; Tajmir-Riahi, H.A. Encapsulation of antitumor drug doxorubicin and its analogue by chitosan nanoparticles. Biomacromolecules 2013, 14, 557–563. [Google Scholar] [CrossRef]
- Shi, X.Y.; Tan, T.W. Preparation of chitosan/ethylcellulose complex microcapsule and its application in controlled release of Vitamin D2. Biomaterials 2002, 23, 4469–4473. [Google Scholar] [CrossRef]
- Klaypradit, W.; Huang, Y.W. Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT Food Sci. Technol. 2008, 41, 1133–1139. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; McClements, D.J.; Decker, E.A. Stability of Spray-Dried Tuna Oil Emulsions Encapsulated with Two-Layered Interfacial Membranes. J. Agric. Food. Chem. 2005, 53, 8365–8371. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Chang, C.P.; Gao, Y.L. Controlled release properties of Chitosan encapsulated volatile Citronella Oil microcapsules by thermal treatments. Colloids Surf., B 2006, 53, 209–214. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, D.H. Recovery of gold(III) ions by a chitosancoated magnetic nano-adsorbent. Gold Bull. 2006, 39, 98–102. [Google Scholar] [CrossRef]
- Huang, K.S.; Lin, Y.S.; Yang, C.H.; Tsai, C.W.; Hsu, M.-Y. In situ synthesis of twin monodispersed alginate microparticles. Soft Matter 2011, 7, 6713–6718. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Lin, Y.S.; Wang, C.Y.; Lu, K.; Chang, Y.F.; Wang, Y.L. Electrostatic droplets assisted synthesis of alginate microcapsules. Drug. Deliv. Transl. Res. 2011, 1, 289–298. [Google Scholar] [CrossRef]
- Lin, Y.S.; Huang, K.S.; Yang, C.H.; Wang, C.Y.; Yang, Y.S.; Hsu, H.C.; Liao, Y.J.; Tsai, C.W. Microfluidic Synthesis of Microfibers for Magnetic-Responsive Controlled Drug Release and Cell Culture. PloS one 2012, 7, e33184. [Google Scholar]
- Wang, C.Y.; Yang, C.H.; Lin, Y.S.; Chen, C.H.; Huang, K.S. Anti-inflammatory effect with high intensity focused ultrasound-mediated pulsatile delivery of diclofenac. Biomaterials 2012, 33, 1547–1553. [Google Scholar]
- Yang, C.H.; Lin, Y.S.; Huang, K.S.; Huang, Y.C.; Wang, E.C.; Jhong, J.Y.; Kuo, C.Y. Microfluidic emulsification and sorting assisted preparation of monodisperse chitosan microparticles. Lab on a Chip 2009, 9, 145–150. [Google Scholar] [CrossRef]
- Pantazaki, A.A.; Dimopoulou, M.I.; Simou, O.M.; Pritsa, A.A. Sunflower seed oil and oleic acid utilization for the production of rhamnolipids by Thermus thermophilus HB8. Appl. Microbiol. Biotechnol. 2010, 88, 939–951. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573-574, 459–465. [Google Scholar] [CrossRef]
- Sigma-Aldrich Chemicals. Rhodamine B base data sheet. Available online: Available online: http://www.sigmaaldrich.com/catalog/product/aldrich/234141?lang=en®ion=TW (accessed on 29 March 2013).
- Zhang, G.Q.; Yang, M.M.; Xi, X.L.; Yang, P. Preliminary Research on the interaction between epirubicin-Mg2+ system and DNA. Chin. J. Inorg. Chem. 2011, 27, 79–86. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, K.-S.; Wang, C.-Y.; Yang, C.-H.; Grumezescu, A.M.; Lin, Y.-S.; Kung, C.-P.; Lin, I.-Y.; Chang, Y.-C.; Weng, W.-J.; Wang, W.-T. Synthesis and Characterization of Oil-Chitosan Composite Spheres. Molecules 2013, 18, 5749-5760. https://doi.org/10.3390/molecules18055749
Huang K-S, Wang C-Y, Yang C-H, Grumezescu AM, Lin Y-S, Kung C-P, Lin I-Y, Chang Y-C, Weng W-J, Wang W-T. Synthesis and Characterization of Oil-Chitosan Composite Spheres. Molecules. 2013; 18(5):5749-5760. https://doi.org/10.3390/molecules18055749
Chicago/Turabian StyleHuang, Keng-Shiang, Chih-Yu Wang, Chih-Hui Yang, Alexandru Mihai Grumezescu, Yung-Sheng Lin, Chao-Pin Kung, I-Yin Lin, Yi-Ching Chang, Wei-Jie Weng, and Wei-Ting Wang. 2013. "Synthesis and Characterization of Oil-Chitosan Composite Spheres" Molecules 18, no. 5: 5749-5760. https://doi.org/10.3390/molecules18055749





