Metabolites from Alternaria Fungi and Their Bioactivities
Abstract
:1. Introduction
2. Classification and Occurrence
| Metabolite class | Metabolite name | Alternaria species | Reference |
|---|---|---|---|
| Nitrogen-containing Metabolites | AAL-toxin TA1 (1) | A. alternata f.sp. lycopersici | [16,17] |
| AAL-toxin TA2 (2) | A. alternata f.sp. lycopersici | [16,17] | |
| AAL-toxin TB1 (3) | A. alternata f.sp. lycopersici | [16,17] | |
| AAL-toxin TB2 (4) | A. alternata f.sp. lycopersici | [16,17] | |
| AAL-toxin TC1 (5) | A. alternata f.sp. lycopersici | [18] | |
| AAL-toxin TC2 (6) | A. alternata f.sp. lycopersici | [18] | |
| AAL-toxin TD1 (7) | A. alternata f.sp. lycopersici | [18] | |
| AAL-toxin TD2 (8) | A. alternata f.sp. lycopersici | [18] | |
| AAL-toxin TE1 (9) | A. alternata f.sp. lycopersici | [18] | |
| AAL-toxin TE2 (10) | A. alternata f.sp. lycopersici | [18] | |
| Fumonisin B1 (11) | A. alternata | [19] | |
| A. alternata f.sp. lycopersici | [20] | ||
| Altersetin (12) | Alternaria sp. | [21] | |
| N-Acetyltyramine (13) | A. tenuissima | [22] | |
| Pyrophen (14) | A. alternata | [23] | |
| Tenuazonic acid = TeA = TA = AAC-toxin (15) | A. alternata | [24,25,26,27,28] | |
| A. citri | [29] | ||
| A, crassa | [30] | ||
| A. linicola | [31] | ||
| A. tenuissima | [24] | ||
| Deprenylzinnimide (16) | A. porri | [32] | |
| Zinnimide (17) | A. porri | [32] | |
| Cichorine (18) | A. cichorii | [33] | |
| Zinnimidine (19) | A. cichorii | [33] | |
| A. porri | [32,34,35] | ||
| Z-Hydroxyzinnimidine (20) | A. cichorii | [33] | |
| Porritoxin (21) | A. porri | [7,36] | |
| Porritoxin sulfonic acid (22) | A. porri | [35] | |
| ACT-toxin I (23) | A. alternata | [37,38] | |
| ACT-toxin II (24) | A. alternata | [37,38] | |
| AK-toxin I (25) | A. kikuchiana (A. alternata) | [39] | |
| AK-toxin II (26) | A. kikuchiana (A. alternata) | [39] | |
| AS-I toxin (27) | A. alternata | [40] | |
| (2S,3S,4R,2'R)-2-(2'-hydroxytetracosanoylamino) Octadecane-1,3,4-triol (28) | Alternaria sp. | [41] | |
| Cerebroside B (29) | Alternaria sp. | [41] | |
| Cerebroside C (30) | Alternaria sp. | [41] | |
| AI-77-B (31) | A. tenuis | [42] | |
| AI-77-F (32) | A. tenuis | [42] | |
| Sg17-1-4 (33) | A. tenuis | [42] | |
| Cyclo-(Pro-Ala-) (34) | A. alternata | [10] | |
| A. tenuissima | [22] | ||
| Nitrogen-containing Metabolites | Cyclo-(Pro-Pro-) (35) | A. tenuissima | [22] |
| Cyclo-(Phe-Ser-) (36) | Alternaria sp.FL25 | [43] | |
| Cyclo-(l-Leu-trans-4-hydroxy-l-Pro-) (37) | A. alternata | [44] | |
| A. tenuissima | [22] | ||
| Cyclo-(S-Pro-R-Val-) (38) | A. alternata | [10] | |
| A. tenuissima | [22] | ||
| Cyclo-(Pro-Leu-) (39) | A. tenuissima | [22] | |
| Cyclo-(Pro-Homoleucine-) (40) | A. alternata | [10] | |
| Cyclo-(S-Pro-R-Ile-) (41) | A. tenuissima | [22] | |
| Cyclo-(Pro-Phe-) (42) | A. alternata | [10] | |
| A. tenuissima | [22] | ||
| Maculosin = Cyclo-(l-Pro-l-Tyr-) (43) | A. alternata | [10] | |
| Cyclo-(l-Phe-trans-4-hydroxy-l-Pro-) (44) | A. alternata | [44] | |
| Cyclo-(l-Ala-trans-4-hydroxy-L-Pro-) (45) | A. alternata | [44] | |
| AM-toxin I (46) | A. mali (A. alternata) | [39] | |
| AM-toxin II (47) | A. mali (A. alternata) | [39] | |
| AM-toxin III (48) | A. mali (A. alternata) | [39] | |
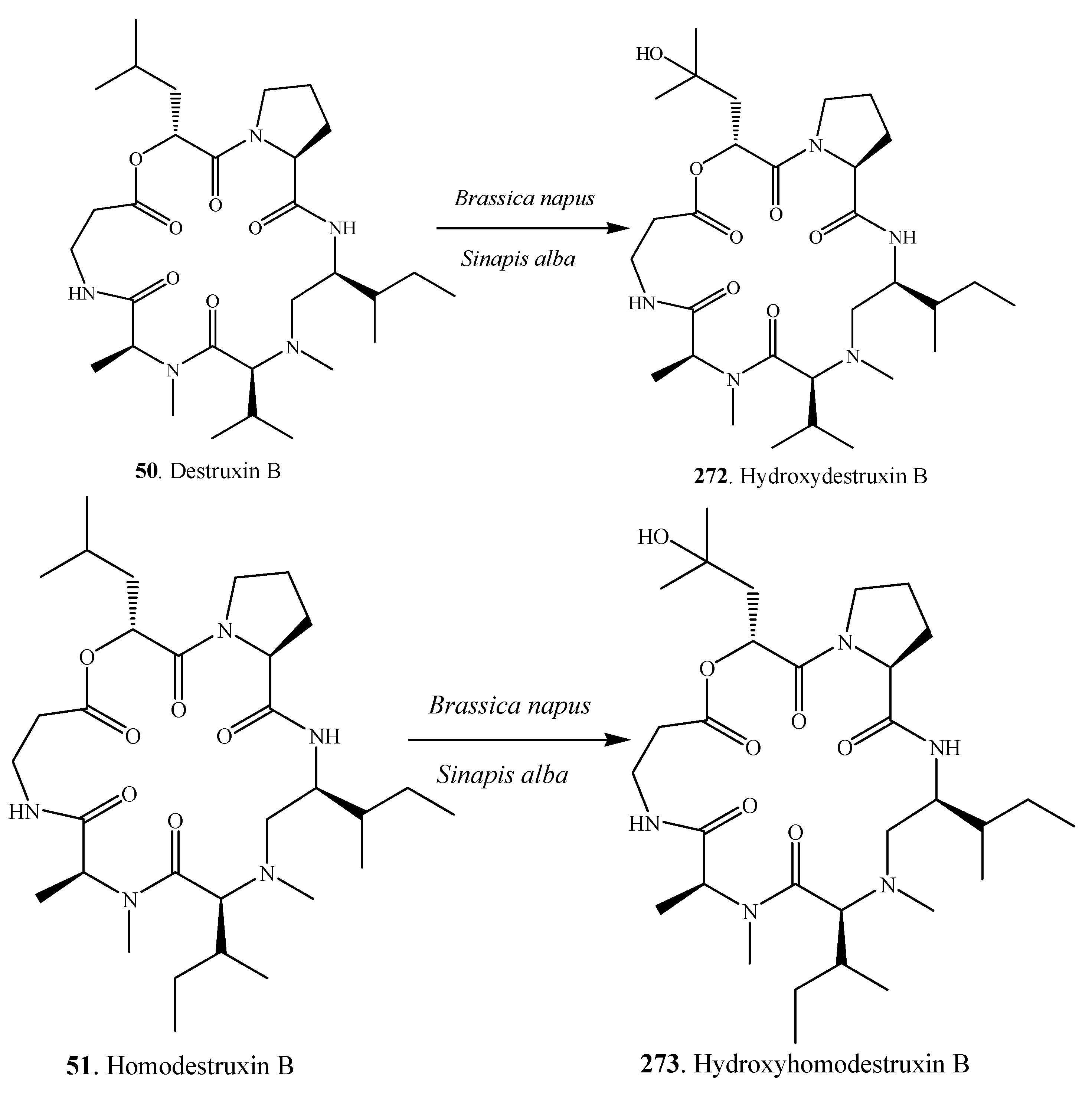
| Destruxin A (49) | A. linicola | [31] | |
| Destruxin B (50) | A. brassicae | [45] | |
| A. linicola | [31] | ||
| Homodestruxin B (51) | A. brassicae | [46] | |
| Desmethyldestruxin B (52) | A. brassicae | [46] | |
| Tentoxin (53) | A. alternata | [47] | |
| A. citri | [29] | ||
| A. linicola | [31] | ||
| A. porri | [48] | ||
| Isotentoxin (54) | A. porri | [48] | |
| Dihydrotentoxin (55) | A. citri | [29] | |
| A. porri | [47,48] | ||
| Uridine (56) | A. alternata | [49] | |
| Adenosine (57) | A. alternata | [49] | |
| Brassicicolin A (58) | A. brassicicola | [50,51] | |
| Fumitremorgin B (59) | Alternaria sp. FL25 | [52] | |
| Fumitremorgin C (60) | Alternaria sp. FL25 | [52] | |
| Paclitaxel = Taxol (61) | A. alternata var. monosporus | [53] | |
| Steroids | Ergosterol (62) | A. alternata | [27,54] |
| Ergosta-4,6,8(14),22-tetraen-3-one (63) | A. alternata | [27,54] | |
| Ergosta-4,6,8(9),22-tetraen-3-one (64) | A.alternata | [49] | |
| Ergosta-7,24(28)-dien-3-ol ( 65 ) | A. alternata | [49] | |
| 3β-Hydroxy-ergosta-5,8(9),22-trien-7-one (66) | A. brassicicola ML-P08 | [55] | |
| 3β,5α-Dihydroxy-ergosta-7,22-dien-6-one (67) | A. brassicicola ML-P08 | [55] | |
| Cerevisterol (68) | A. brassicicola ML-P08 | [55] | |
| Terpenoids | Bicycloalternarene 1 (69) | A. alternata | [56] |
| Bicycloalternarene 11 (70) | A. alternata | [56] | |
| Bicycloalternarene 2 (71) | A. alternata | [56] | |
| Bicycloalternarene 3 = ACTG toxin A (72) | A. alternata | [56] | |
| Bicycloalternarene 4 (73) | A. alternata | [56] | |
| Bicycloalternarene 10 (74) | A. alternata | [56] | |
| Bicycloalternarene 5 (75) | A. alternata | [56] | |
| Bicycloalternarene 8 (76) | A. alternata | [56] | |
| Bicycloalternarene 9 = ACTG toxin B (77) | A. alternata | [56] | |
| Bicycloalternarene 6 (78) | A. alternata | [56] | |
| Bicycloalternarene 7 (79) | A. alternata | [56] | |
| Tricycloalternarene 1a (80) | A. alternata | [57] | |
| Tricycloalternarene 1b (81) | A. alternata | [57,58] | |
| Tricycloalternarene 11a (82) | A. alternata | [59] | |
| Tricycloalternarene 11b (83) | A. alternata | [59] | |
| Tricycloalternarene 2a (84) | A. alternata | [57] | |
| Tricycloalternarene 2b (85) | A. alternata | [57,58] | |
| Tricycloalternarene 3a (86) | A. alternata | [57] | |
| Tricycloalternarene 3b = ACTG toxin G (87) | A. alternata | [57,60] | |
| A. citri | [61] | ||
| ACTG toxin H (88) | A. citri | [61] | |
| Tricycloalternarenal (89) | A. alternata | [60] | |
| Tricycloalternarene 4a (90) | A. alternata | [57] | |
| Tricycloalternarene 4b (91) | A. alternate | [57] | |
| Tricycloalternarene 10b (92) | A. alternate | [59] | |
| Tricycloalternarene 5a (93) | A. alternate | [57] | |
| Tricycloalternarene 5b (94) | A. alternate | [57] | |
| Tricycloalternarene 8a (95) | A. alternate | [59] | |
| Tricycloalternarene 9b (96) | A. alternate | [59] | |
| Tricycloalternarene 6a (97) | A. alternate | [59] | |
| Tricycloalternarene 6b (98) | A. alternate | [59] | |
| Tricycloalternarene 7a (99) | A. alternate | [59] | |
| Tricycloalternarene 7b (100) | A. alternate | [59] | |
| Tricycloalternarene A (101) | A. alternata Ly83 | [58] | |
| Tricycloalternarene B (102) | A. alternata Ly83 | [58] | |
| Tricycloalternarene C (103) | A. alternata Ly83 | [58] | |
| Tricycloalternarene D (104) | A. alternata Ly83 | [58] | |
| Tricycloalternarene E (105) | A. alternata Ly83 | [58] | |
| Brassicicene A (106) | A. brassicicola | [62] | |
| Brassicicene B (107) | A. brassicicola | [62] | |
| Brassicicene C (108) | A. brassicicola | [62] | |
| Brassicicene D (109) | A. brassicicola | [62] | |
| Brassicicene E (110) | A. brassicicola | [62] | |
| Brassicicene F (111) | A. brassicicola | [62] | |
| Brassicicene G (112) | A. brassicicola | [51] | |
| Brassicicene H (113) | A. brassicicola | [51] | |
| Brassicicene I (114) | A. brassicicola | [51] | |
| Abscisic acid = ABA (115) | A. brassicae | [63] | |
| (1aS,2S,6R,7R,7aR,7bR)-1a,2,4,5,6,7,7a,7b-Octahydro-7,7a-dimethyl-1a-(1-methylethenyl)-naphth[1,2-b]oxirene-2,6-diol (116) | A. citri | [61] | |
| Helvolic acid (117) | Alternaria sp. FL25 | [43] | |
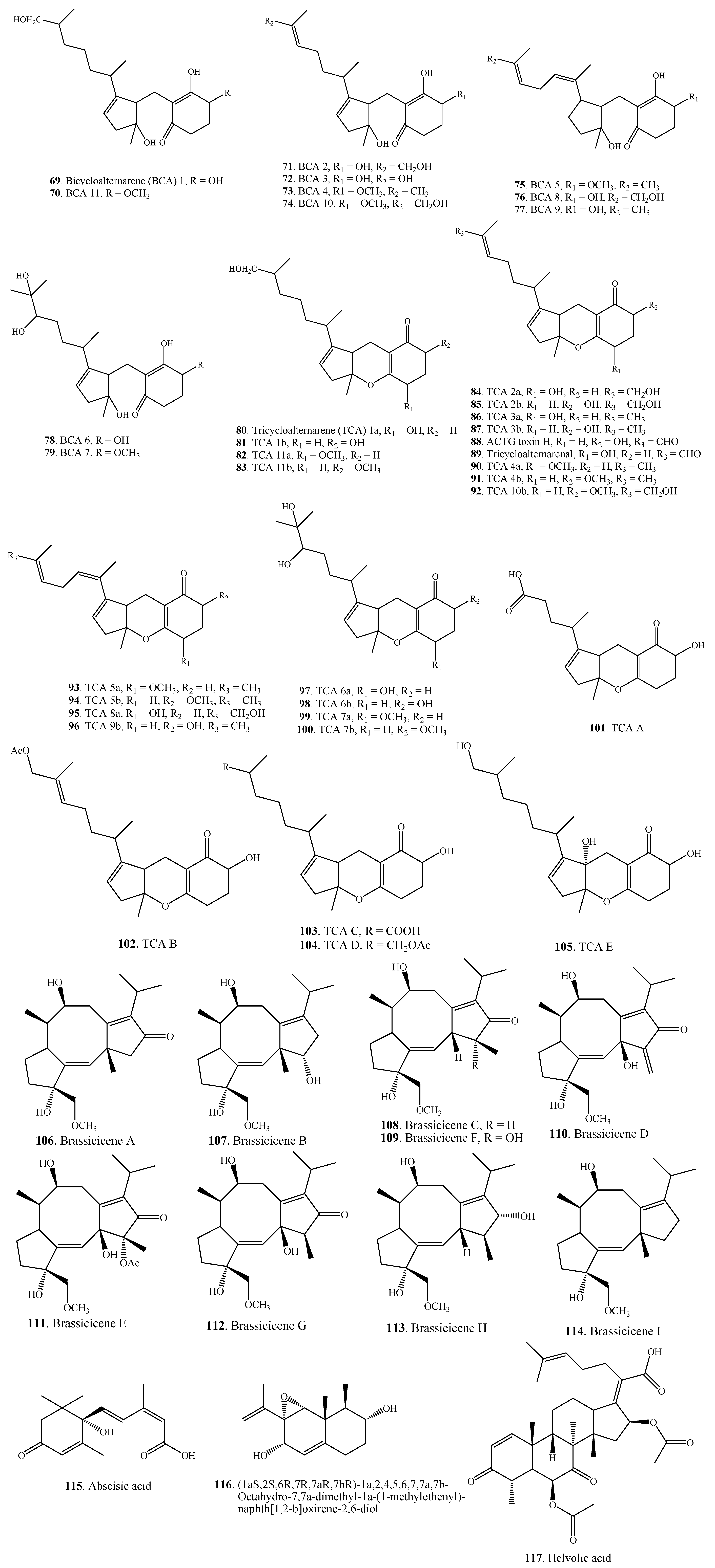
| Pyranones | Radicinin (118) | A. chrysanthemi | [64,65] |
| A. helianthi | [66] | ||
| A. radicina | [67] | ||
| Deoxyradicinin (119) | Alternaria sp. CIB 108 | [68] | |
| A. helianthi | [66,69] | ||
| Radicinol (120) | A. chrysanthemi | [64,65] | |
| A. radicina | [67] | ||
| Deoxyradicinol (121) | A. helianthi | [66] | |
| 3-Epiradicinol (122) | Alternaria sp. CIB 108 | [68] | |
| A. chrysanthemi | [65] | ||
| A. radicina | [67] | ||
| 3-Epideoxyradicinol (123) | Alternaria sp. CIB 108 | [68] | |
| A. helianthi | [70] | ||
| 3-Methoxy-3-epiradicinol (124) | A. chrysanthemi | [65] | |
| 9,10-Epoxy-3-methoxy-3-epiradicinol (125) | A. chrysanthemi | [65] | |
| Radianthin (126) | A. helianthi | [66] | |
| 3-Butyryl-6-[rel-(1S,2S)-1,2-dihydroxypropyl]-4-hydroxy-2H-pyran-2-one (127) | Alternaria sp. CIB 108 | [68] | |
| Phomapyrone A = Phomenenin A (128) | A. brassicicola | [51] | |
| A. infectoria | [71] | ||
| Phomenenin B (129) | A. infectoria | [71] | |
| Phomapyrone G (130) | A. brassicicola | [51] | |
| Infectopyrone (131) | A.arbusti | [72] | |
| A. conjuncta | [72] | ||
| A. infectoria | [72,73] | ||
| A. intercepta | [72] | ||
| A. metachromatica | [72] | ||
| A. novae-zelandiae | [72] | ||
| A. oregonensis | [72] | ||
| A. triticimaculans | [72] | ||
| A. viburni | [72] | ||
| Herbarin A (132) | A. brassicicola ML-P08 | [55] | |
| Alternaric acid (133) | A. solani | [74] | |
| Novae-zelandin A (134) | A. cetera | [72] | |
| A. infectoria | [72] | ||
| A. intercepta | [72] | ||
| A. novae-zelandiae | [72] | ||
| A. triticimaculans | [72] | ||
| A. viburni | [72] | ||
| Novae-zelandin B (135) | A. cetera | [72] | |
| A. infectoria | [72] | ||
| A. intercepta | [72] | ||
| A. novae-zelandiae | [72] | ||
| A. triticimaculans | [72] | ||
| A. viburni | [72] | ||
| 4 Z-Infectopyrone (136) | A. arbusti | [72] | |
| A. conjuncta | [72] | ||
| A. infectoria | [72] | ||
| A. intercepta | [72] | ||
| A. metachromatica | [72] | ||
| A. novae-zelandiae | [72] | ||
| A. oregonensis | [72] | ||
| A. triticimaculans | [72] | ||
| A. viburni | [72] | ||
| Pyrenocine A (137) | A. infectoria | [72] | |
| Pyrenocine B (138) | A. infectoria | [72] | |
| Pyrenocine C (139) | A. infectoria | [72] | |
| ACRL toxin I (140) | A. citri | [75] | |
| ACRL toxin II (141) | A. citri | [76] | |
| ACRL toxin III (142) | A. citri | [76] | |
| ACRL toxin IV (143) | A. citri | [76] | |
| ACRL toxin IV’ (144) | A. citri | [76] | |
| Solanapyrone A (145) | A. solani | [77] | |
| Solanapyrone B (146) | A. solani | [77] | |
| Solanapyrone C (147) | A. solani | [77] | |
| Solanapyrone D (148) | A. solani | [78] | |
| Solanapyrone E (149) | A. solani | [78] | |
| Tenuissimasatin (150) | A. tenuissima | [22] | |
| Altechromone A (151) | A. brassicicola ML-P08 | [55] | |
| 2,5-Dimethyl-7-hydroxychromone (152) | Alternaria sp. | [79] | |
| Phomapyrone F (153) | A. brassicicola | [51] | |
| Altenuisol (154) | Alternaria sp. | [80] | |
| A. tenuis | [81] | ||
| Altertenuol (155) | A. tenuis | [82] | |
| Dehydroaltenusin (156) | A. tenuis | [83] | |
| Alternariol =AOH (157) Alternariol 5-O-sulfate (158) | Alternaria sp. | [41,84] | |
| A. alternata | [25,27,85] | ||
| Alternaria sp. | [84] | ||
| Alternariol 9-methyl ether = AME = Djalonensone (159) | Alternaria sp. | [41,84,86] | |
| A. alternata | [25,27,85] | ||
| A. linicola | [31] | ||
| A. tenuis | [87] | ||
| A. tenuissima | [86] | ||
| Alternariol 5-O-methyl ether-4'-O-sulfate (160) | Alternaria sp. | [84] | |
| 3'-Hydroxyalternariol (161) | Alternaria sp. | [84] | |
| Altenuene = ATL (162) | Alternaria sp. | [84] | |
| A. alternata | [85] | ||
| Isoaltenuene (163) | A. alternata | [88] | |
| 4'-Epialtenuene (164) | Alternaria sp. | [84] | |
| 5'-Epialtenuene (165) | A. alternata | [89] | |
| Neoaltenuene (166) | A. alternata | [89] | |
| Rubrofusarin B (167) | A. alternata | [23] | |
| Fonsecin (168) | A. alternata | [23] | |
| Fonsecin B (169) | A. alternata | [23] | |
| Aurasperone A (170) | A. alternata | [23] | |
| Aurasperone B (171) | A. alternata | [23] | |
| Aurasperone C (172) | A. alternata | [23] | |
| Aurasperone F (173) | A. alternata | [23] | |
| Quinones | Macrosporin (174) | Alternaria sp. ZJ-2008003 | [90] |
| A. porri | [32] | ||
| A. solani | [91] | ||
| Demethylmacrosporin (175) | A. porri | [32] | |
| Dihydroaltersolanol A (176) | Alternaria sp. ZJ-2008003 | [90] | |
| Tetrahydroaltersolanol B (177) | Alternaria sp. ZJ-2008003 | [90] | |
| A. solani | [92] | ||
| Tetrahydroaltersolanol C (178) | Alternaria sp. ZJ-2008003 | [90] | |
| Tetrahydroaltersolanol D (179) | Alternaria sp. ZJ-2008003 | [90] | |
| Tetrahydroaltersolanol E (180) | Alternaria sp. ZJ-2008003 | [90] | |
| Tetrahydroaltersolanol F (181) | Alternaria sp. ZJ-2008003 | [90] | |
| Bostrycin (182) | A. eichhorniae | [93] | |
| 4-Deoxybostrycin (183) | A. eichhorniae | [93] | |
| Hydroxybostrycin (184) | A. solani | [94] | |
| Altersolanol A = Stemphylin (185) | A. porri | [95] | |
| A. solani | [94,96,97] | ||
| Altersolanol B = Dactylarin (186) | Alternaria sp. ZJ-2008003 | [90] | |
| A. porri | [95] | ||
| A. solani | [94,96,97] | ||
| Altersolanol C = Dactylariol (187) | Alternaria sp. ZJ-2008003 | [90] | |
| A. porri | [95,98] | ||
| A. solani | [94,96,97] | ||
| Altersolanol D (188) | A. solani | [94,96,97] | |
| Altersolanol E (189) | A. solani | [94,96,97] | |
| Altersolanol F (190) | A. solani | [94,96,97] | |
| Altersolanol G (191) | A. solani | [94] | |
| Altersolanol H (192) | A. solani | [94] | |
| Altersolanol L (193) | Alternaria sp. ZJ-2008003 | [90] | |
| Ampelanol (194) | Alternaria sp. ZJ-2008003 | [90] | |
| Alterporriol A/B (195) | A. porri | [32] | |
| A. solani | [94,99] | ||
| Alterporriol C (196) | Alternaria sp. ZJ-2008003 | [90] | |
| A. porri | [32] | ||
| A. solani | [99] | ||
| Alterporriol D/E (197) | A. porri | [32] | |
| Alterporriol F (198) | A. porri | [32] | |
| Alterporriol K (199) | Alternaria sp. ZJ9-6B | [100] | |
| Alterporriol L (200) | Alternaria sp. ZJ9-6B | [100] | |
| Alterporriol M (201) | Alternaria sp. ZJ9-6B | [100] | |
| Alterporriol N (202) | Alternaria sp. ZJ-2008003 | [90] | |
| Alterporriol O (203) | Alternaria sp. ZJ-2008003 | [90] | |
| Alterporriol P (204) | Alternaria sp. ZJ-2008003 | [90] | |
| Alterporriol Q (205) | Alternaria sp. ZJ-2008003 | [90] | |
| Alterporriol R (206) | Alternaria sp. ZJ-2008003 | [90] | |
| Alterperylenol (207) | Alternarial sp. | [79,101] | |
| Alternaria sp. M6 | [102] | ||
| A. alternata | [27] | ||
| A. cassiae | [103] | ||
| A. tenuissima | [22] | ||
| 8β-Chloro-3,6aα,7β,9β,10-pentahydroxy-9,8,7,6a-tetrahydroperylen-4(6aH)-one (208) | Alternaria sp. M6 | [102] | |
| Dihydroalterperylenol (209) | Alternarial sp. | [101] | |
| Alternaria sp. M6 | [102] | ||
| A. alternate | [104] | ||
| Stemphyperylenol (210) | Alternaria sp. | [79] | |
| A. alternata | [105] | ||
| A. cassiae | [103] | ||
| 6-Epi-stemphytriol (211) | A. alternata | [105] | |
| Altertoxin I = ATX-I (212) | Alternaria sp. | [79,80,106] | |
| A. alternata | [26,27,104,105,107] | ||
| A. cassiae | [103] | ||
| A. tenuissima | [22] | ||
| Alteichin (213) | A. alternata | [26,107] | |
| A. eichorniae | [108] | ||
| Alterlosin I (214) | A. alternata | [26] | |
| Alterlosin II (215) | A. alternata | [26] | |
| Phenolics | p-Hydroxybenzoic acid (219) | A. tagetica | [109] |
| Tyrosol (220) | A. tagetica | [109] | |
| α-Acetylorcinol (221) | A. tenuissima | [22] | |
| 2-Carboxy-3-(2-hydroxypropanyl) phenol (222) | Alternaria sp. HS-3 | [110] | |
| Methyl eugenol (223) | Alternaria sp. | [111] | |
| Tagetolone (224) | A. tagetica | [109] | |
| Tagetenolone (225) | A. tagetica | [109] | |
| Zinniol (226) | A. carthami | [112,113] | |
| A. cichorii | [33] | ||
| A. cirsinoxia | [114] | ||
| A. dauci | [115] | ||
| A. macrospora | [113] | ||
| A. porri | [113,116] | ||
| A. solani | [113,117,118] | ||
| A. tagetica | [113,116,119] | ||
| A. zinniae | [120] | ||
| 8-Zinniol 2-(phenyl)-ethyl ether (227) | A. solani | [118] | |
| A. tagetica | [116] | ||
| 8-Zinniol methyl ether (228) | A. solani | [118] | |
| A. tagetica | [116] | ||
| 8-Zinniol acetate (229) | A. tagetica | [116] | |
| 7-Zinniol acetate (230) | A. tagetica | [116] | |
| Homozinniol (231) | A. solani | [117] | |
| Zinnol (232) | A. cichorii | [33] | |
| 8-Zinnol methyl ether (233) | A. solani | [118] | |
| A. tagetica | [116] | ||
| Zinnidiol (234) | A. cichorii | [33] | |
| 2-(2'',3''-dimethyl-but-1-enyl)-Zinniol (235) | A. solani | [118] | |
| Bis-7-O-8''.8-O-7''-zinniol (236) | A. tagetica | [121] | |
| Bis-7-O-7''.8-O-8''-zinniol (237) | A. tagetica | [121] | |
| 4-Acetyl-5-hydroxy-3,6,7-trimethylbenzofuran-2(3 H)-one (238) | Alternaria sp. HS-3 | [110] | |
| 5-Methyl-6-hydroxy-8-methoxy-3-methylisochroman (239) | Alternaria sp. HS-3 | [110] | |
| Alternarian acid (240) | Alternaria sp. | [79] | |
| Altenusin (241) | Alternaria sp. | [79,84,122,123] | |
| A. mali | [124] | ||
| A. tenuis | [82] | ||
| Desmethylaltenusin (242) | Alternaria sp. | [84] | |
| Porric acid D (243) | Alternaria sp. | [123] | |
| Alterlactone (244) | Alternaria sp. | [84] | |
| Alternethanoxin A (245) | A. sonchi | [125] | |
| Alternethanoxin B (246) | A. sonchi | [125] | |
| Alternarienonic acid (247) | Alternaria sp. | [79,84] | |
| Talaroflavone (248) | Alternaria sp. | [84] | |
| Curvularin (249) | A. cinerariae | [126] | |
| A. tomato | [127] | ||
| (4S)-α,β-Dehydrocurvularin (250) | Alternaria sp. | [86] | |
| A. cinerariae | [126,128] | ||
| A. tenuissima | [86] | ||
| A. tomato | [127] | ||
| A. zinniae | [129] | ||
| β-Hydroxycurvularin (251) | A. tomato | [127] | |
| Resveratrol (252) | Alternaria sp. MG1 | [130] | |
| 6-(3',3'-dimethylallyloxy)-4-Methoxy-5-methylphthalide (253) | A. porri | [7] | |
| A. solani | [117] | ||
| A. tagetica | [116] | ||
| Porritoxinol (254) | A. porri | [131] | |
| 5-(3',3'-dimethylallyloxy)-7-Methoxy-6-methylphthalide (255) | A. porri | [7,32,34] | |
| A. solani | [118] | ||
| A. tagetica | [116] | ||
| Porriolide (256) | A. porri | [7,32] | |
| Miscellaneous Metabolites | Depudecin (257) | A. brassicicola | [132] |
| Altenin (258) | A. kikuchiana | [133] | |
| Brefeldin A (259) | A. carthami | [112] | |
| A. zinniae | [129] | ||
| 7-Dehydrobrefeldin A (260) | A. carthami | [112] | |
| α-Linoleic acid (261) | A. infectoria | [71] | |
| α-Linolenic acid (262) | A. infectoria | [71] | |
| AF-toxin I (263) | A. alternata | [134,135] | |
| AF-toxin II (264) | A. alternata | [134,135] | |
| AF-toxin III (265) | A. alternata | [134] | |
| Xanalteric acid I (266) | Alternaria sp. | [79] | |
| Xanalteric acid II (267) | Alternaria sp. | [79] | |
| Cladosporol (268) | A. alternate var. monosporus | [53] |
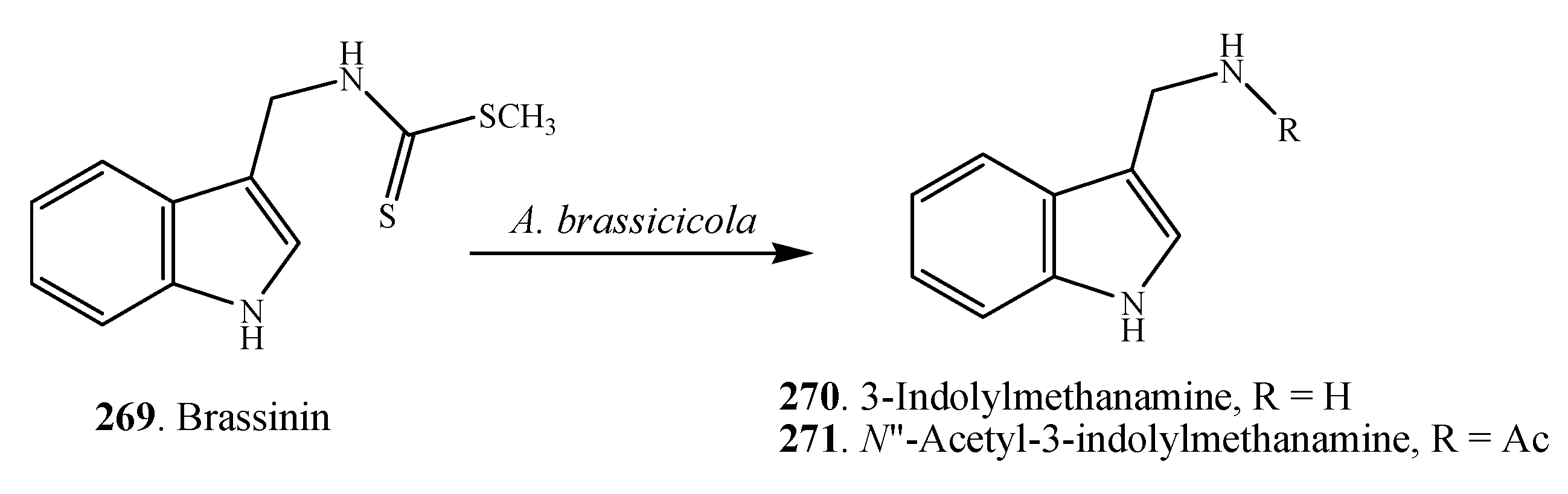
2.1. Nitrogen-Containing Metabolites
2.1.1. Amines and Amides
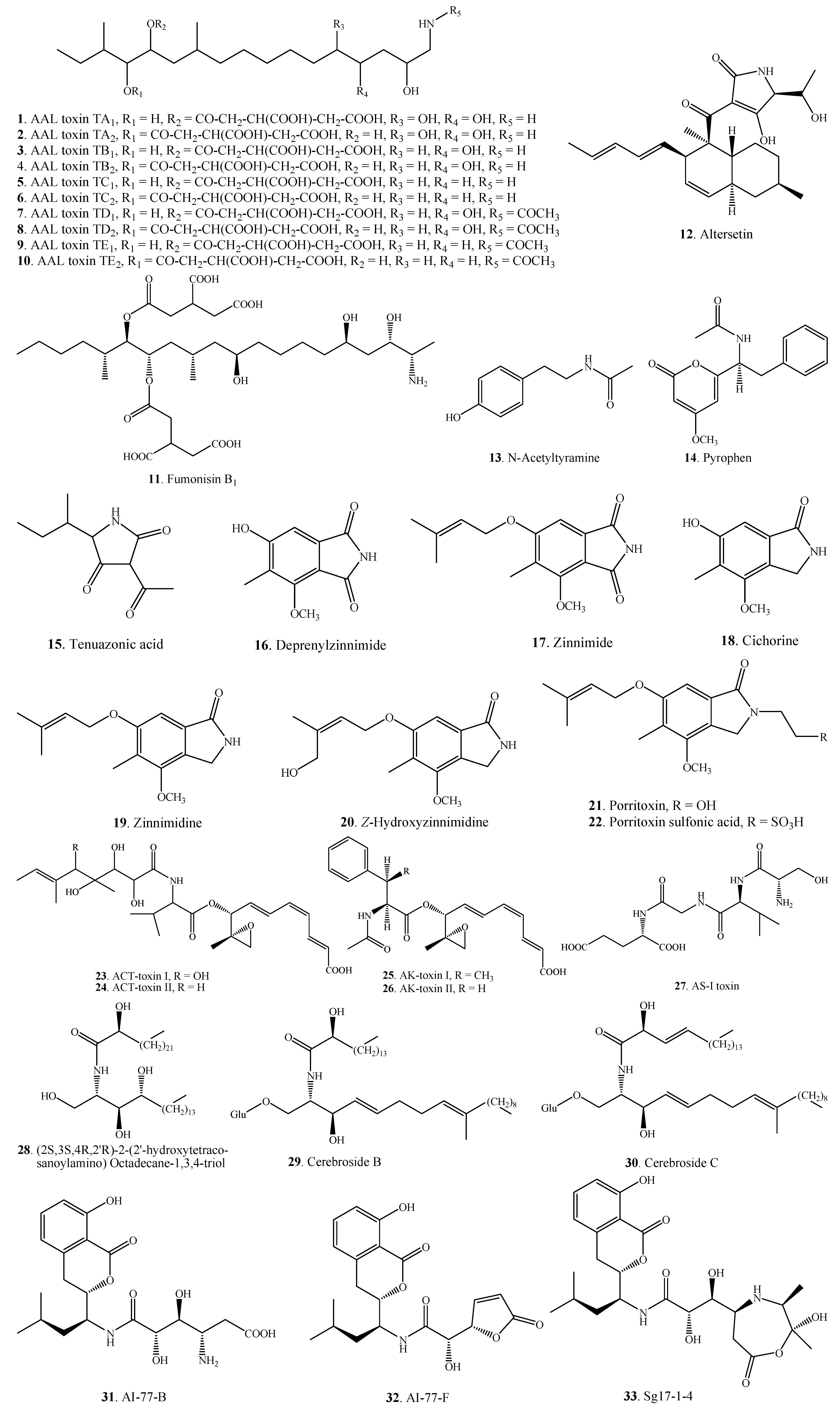
2.1.2. Cyclopeptides
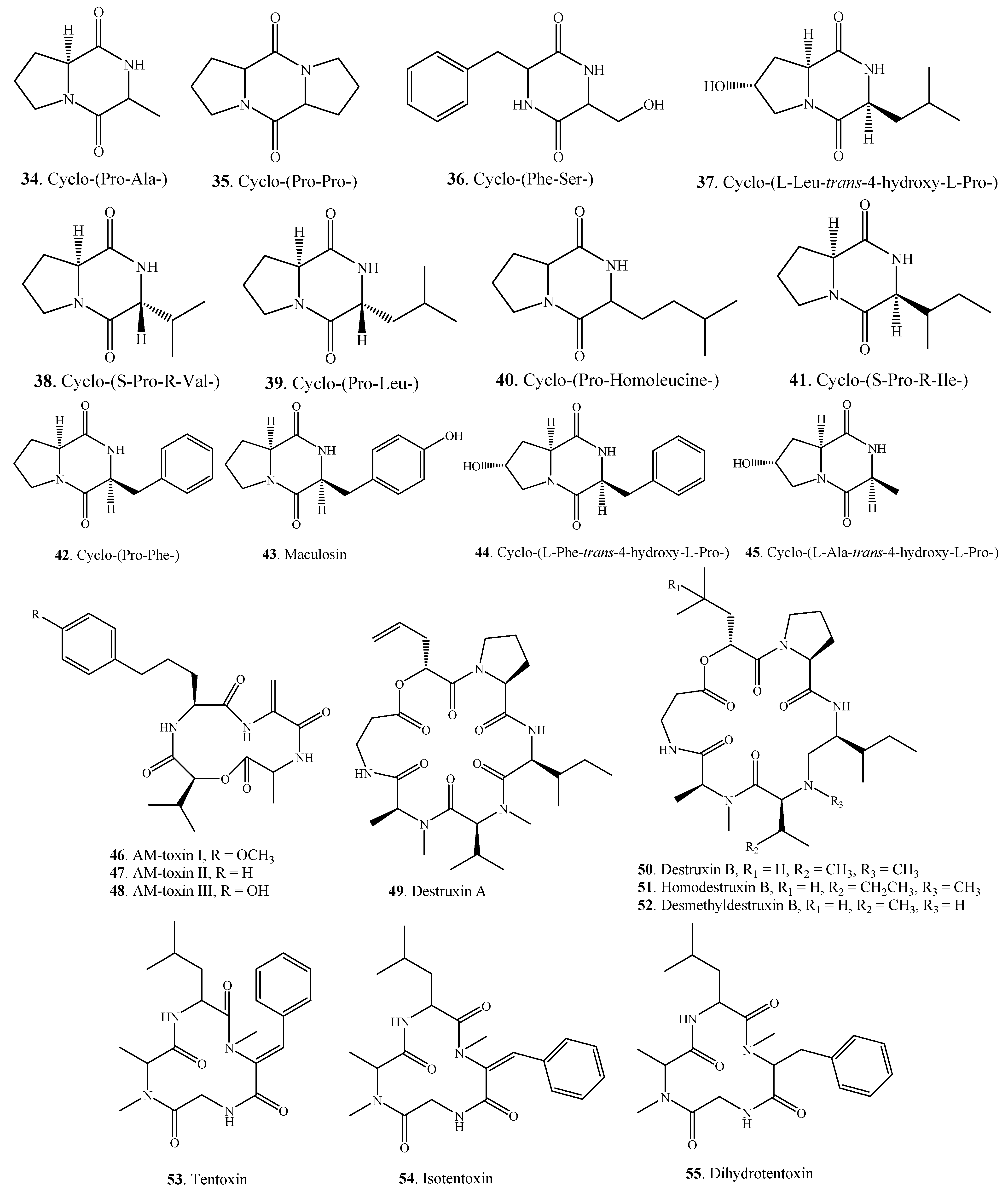
2.1.3. Other Nitrogen-Containing Metabolites
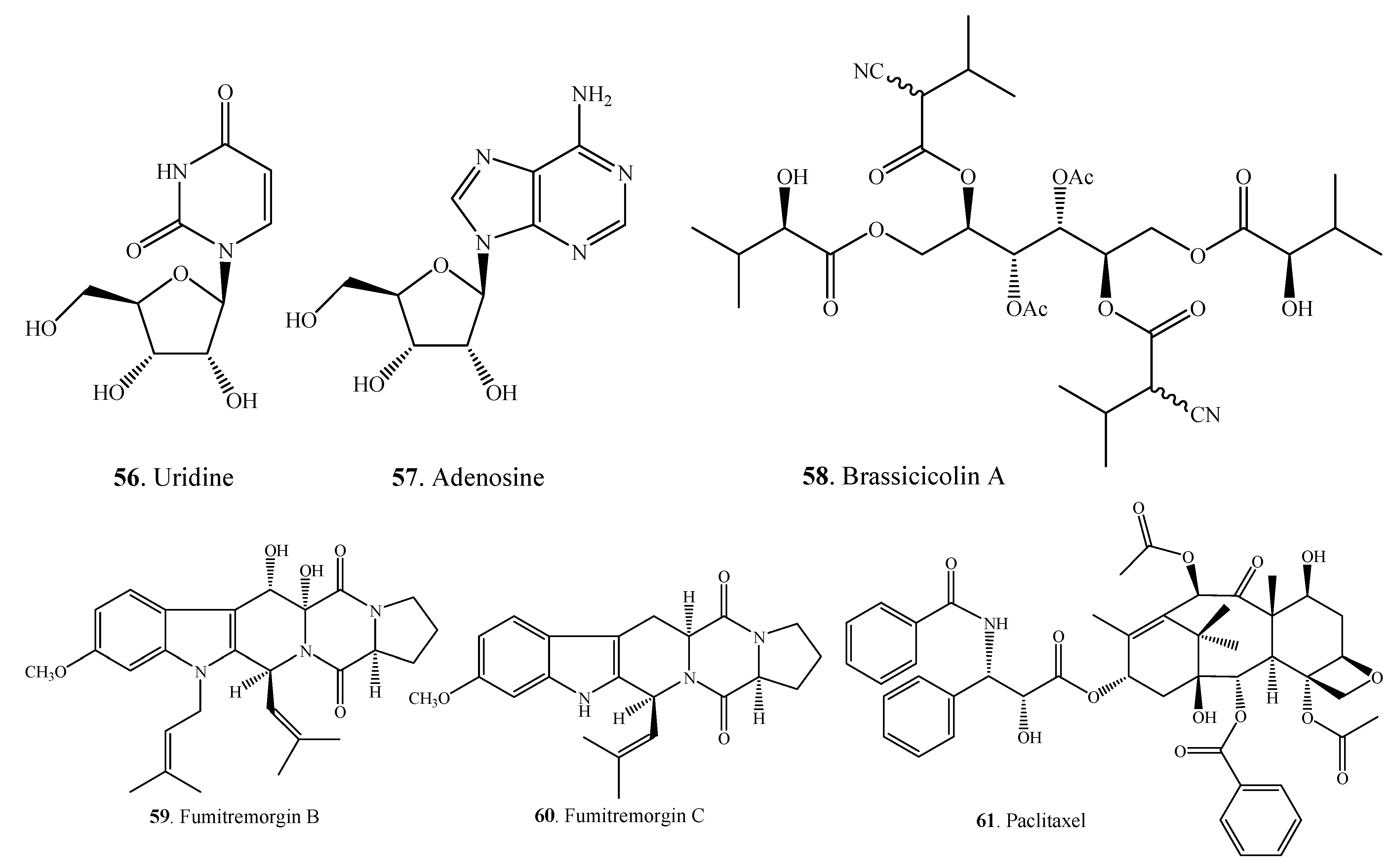
2.2. Steroids
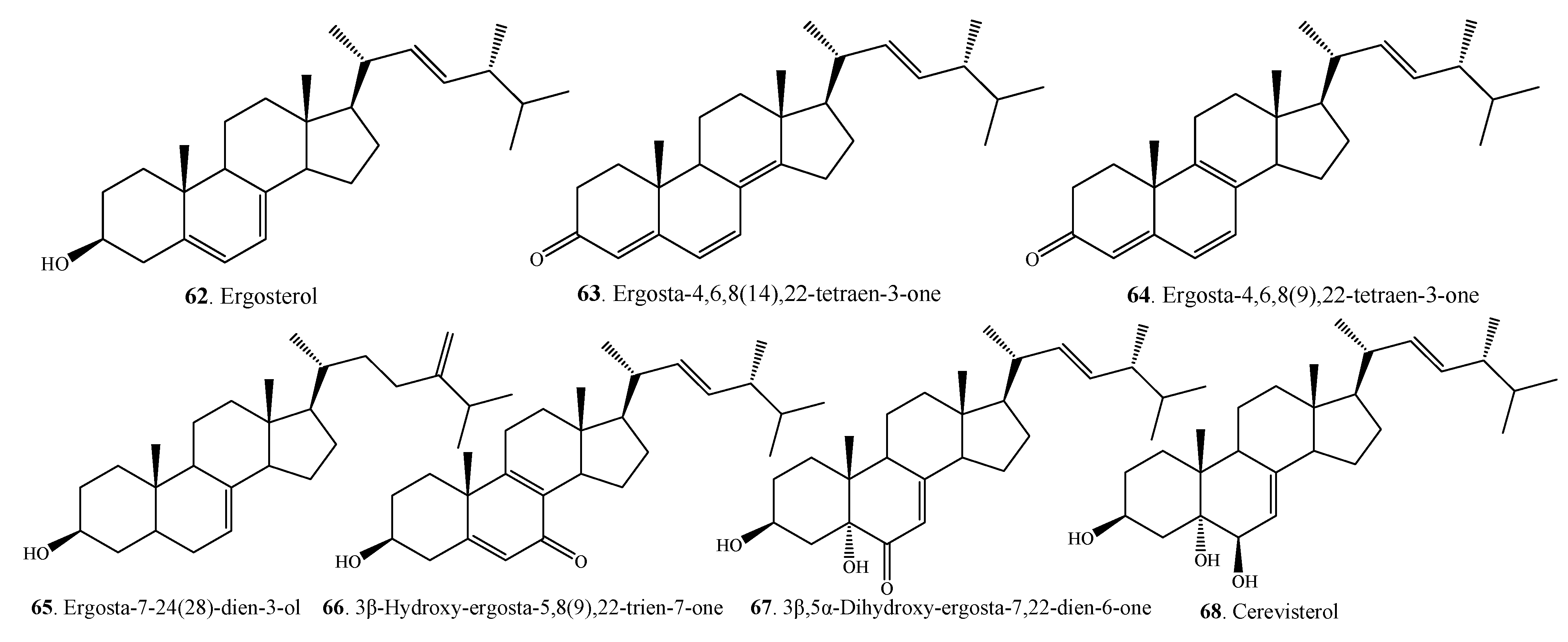
2.3. Terpenoids
2.4. Pyranones
2.4.1. Simple Pyranones
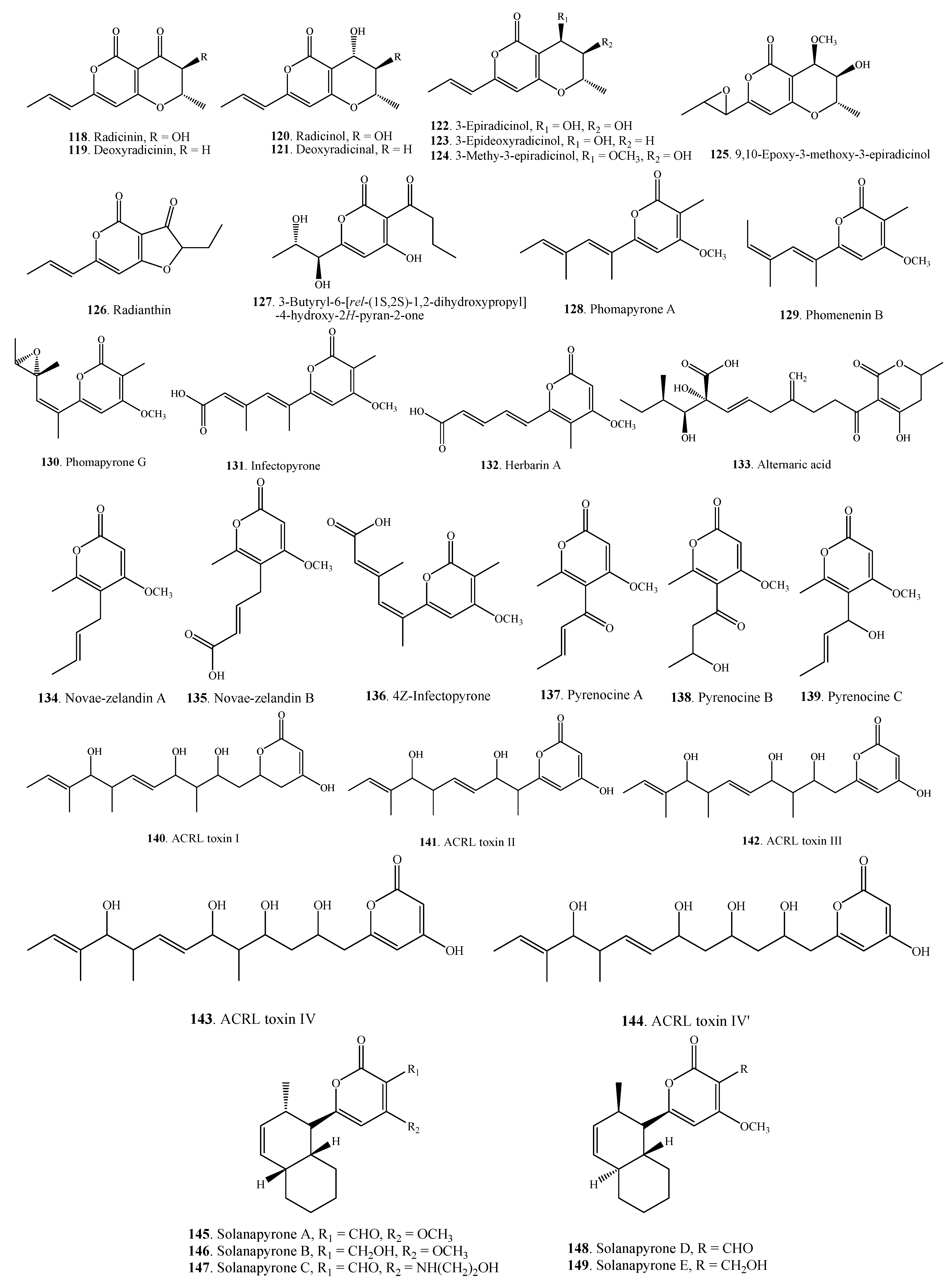
2.4.2. Monobenzopyranones
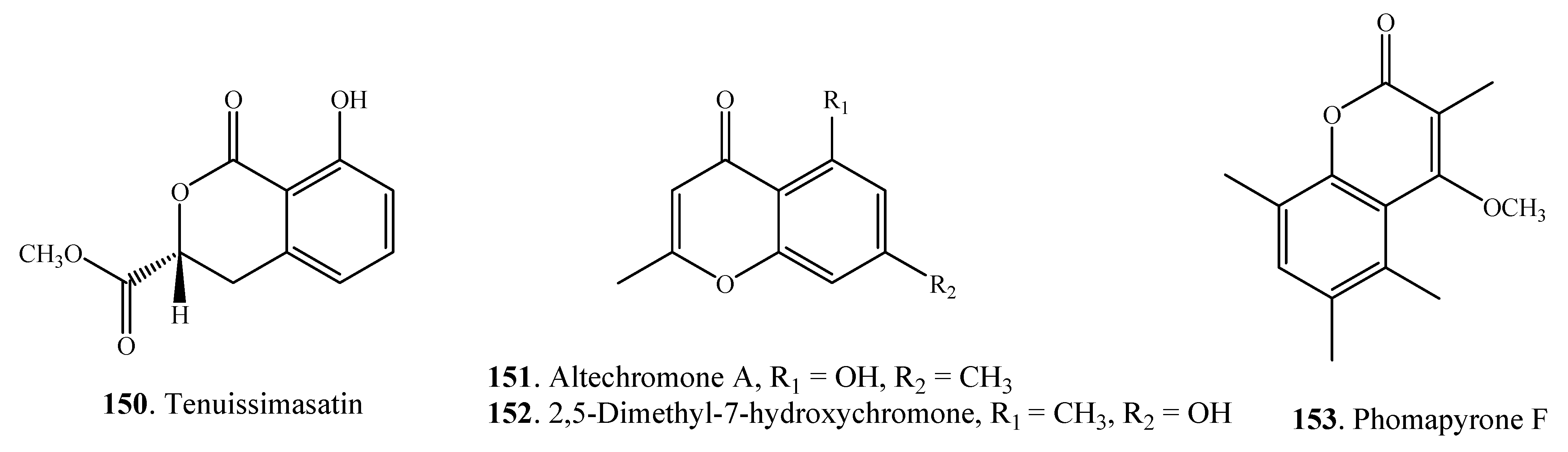
2.4.3. Dibenzopyranones
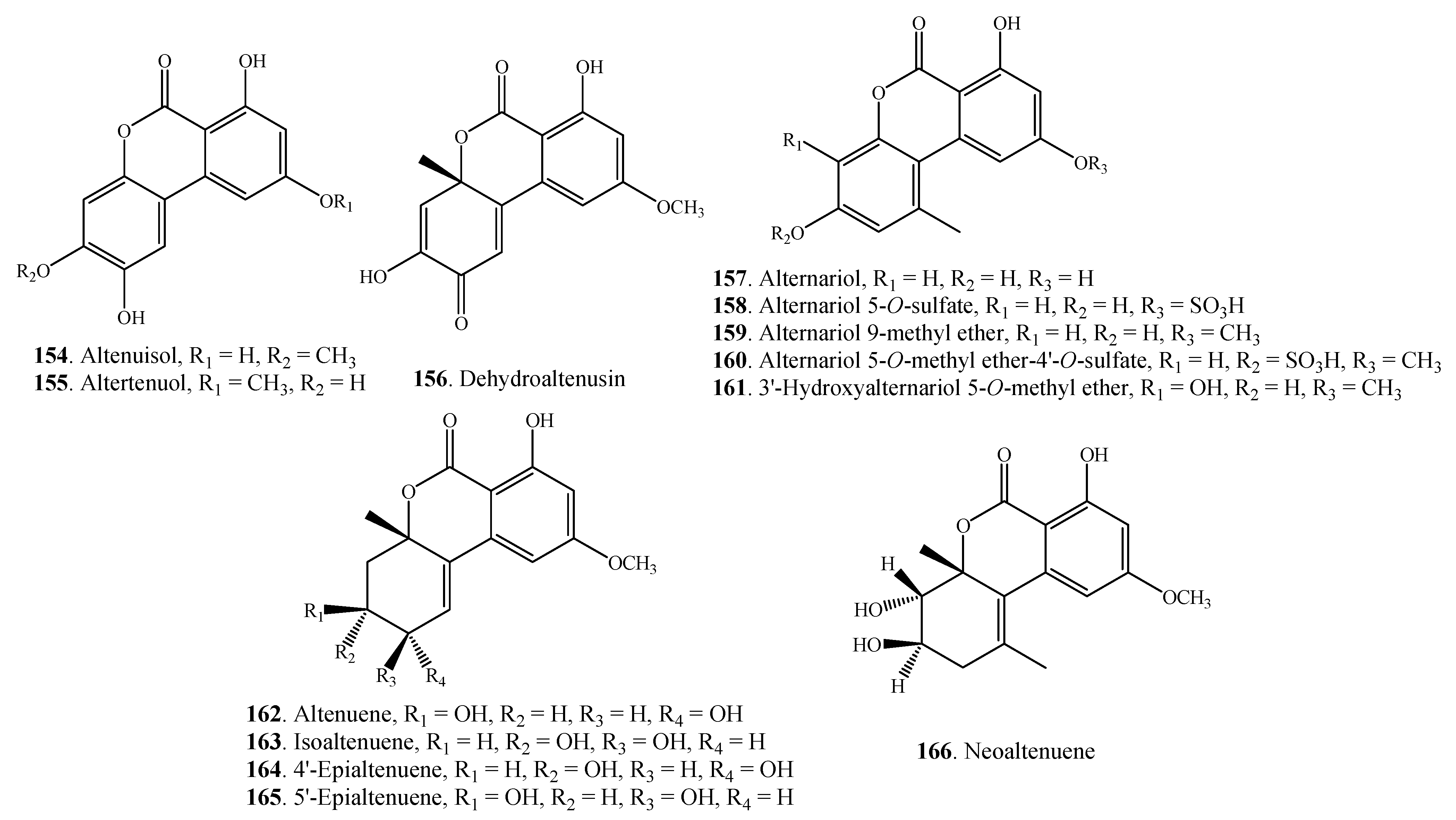
2.4.4. Naphthopyranones

2.5. Quinones
2.5.1. Anthraquinones
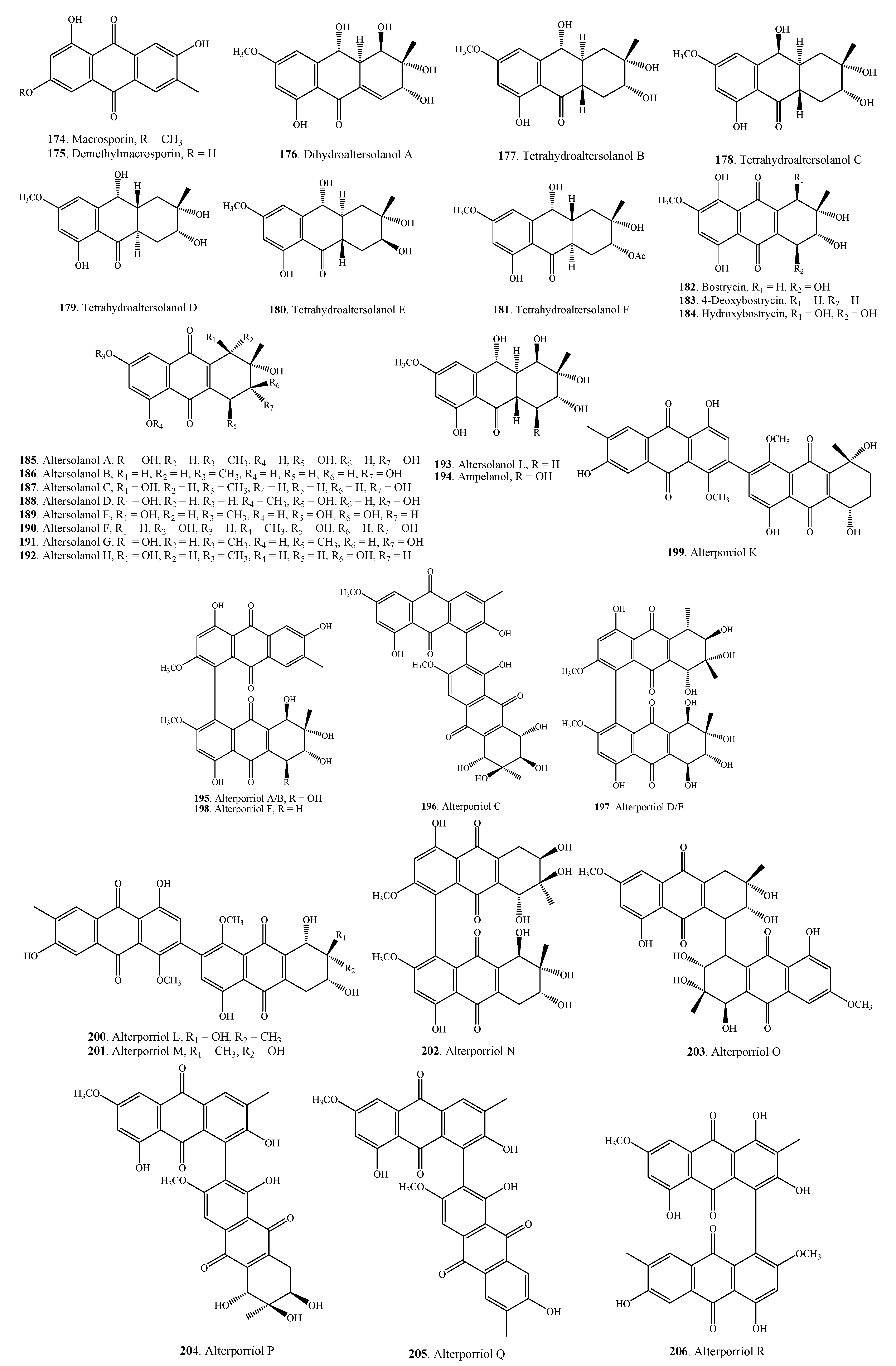
2.5.2. Perylenequinones
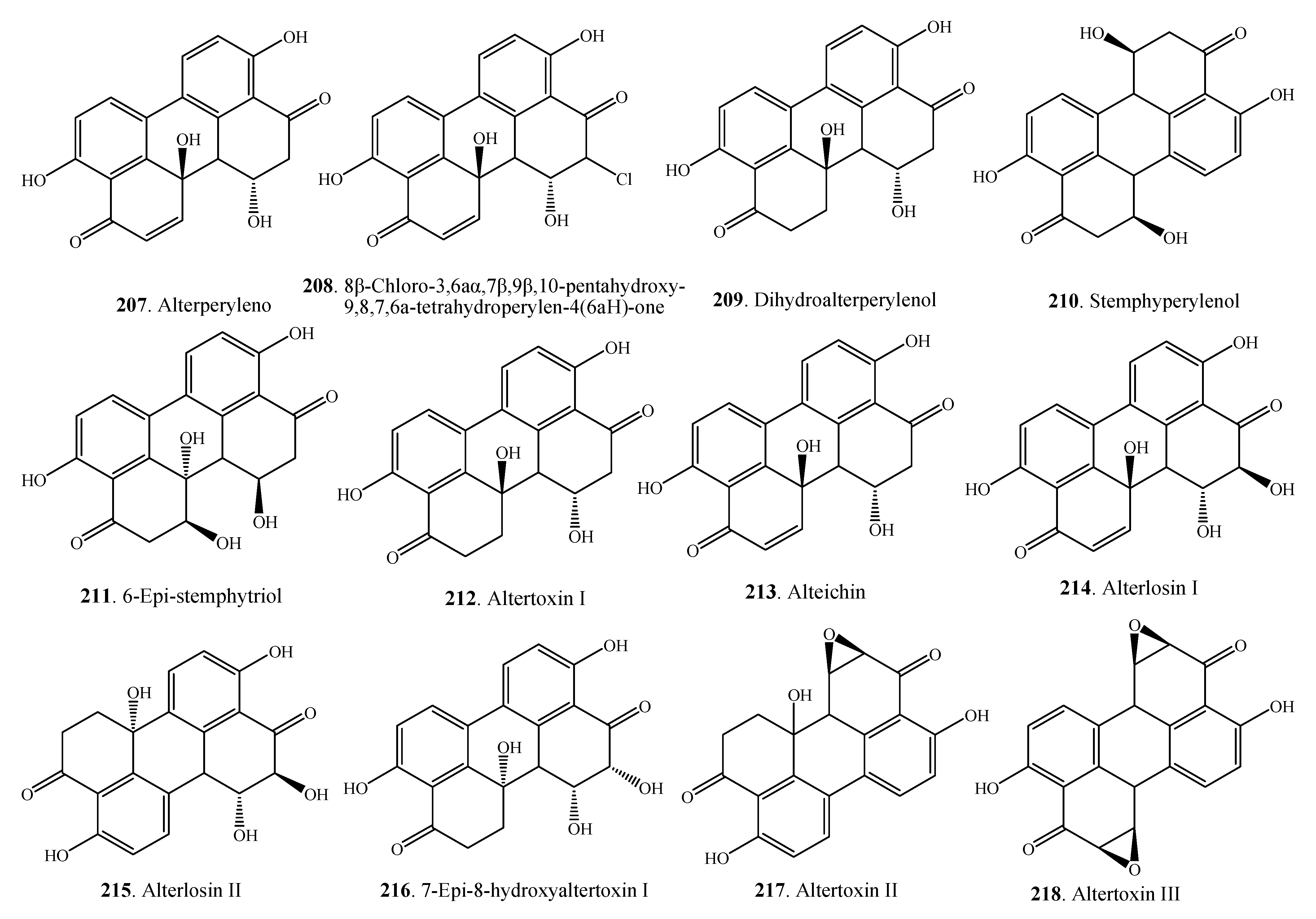
2.6. Phenolics

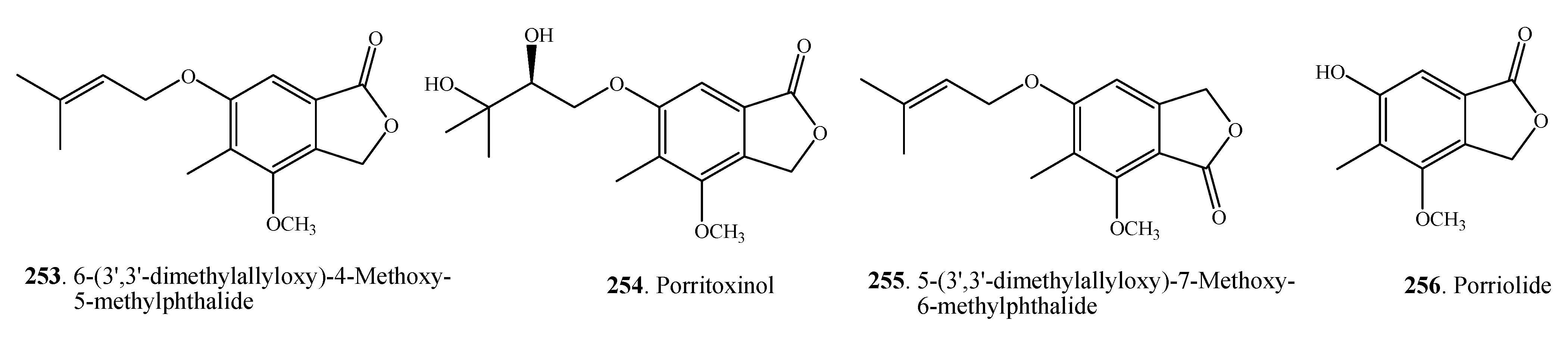
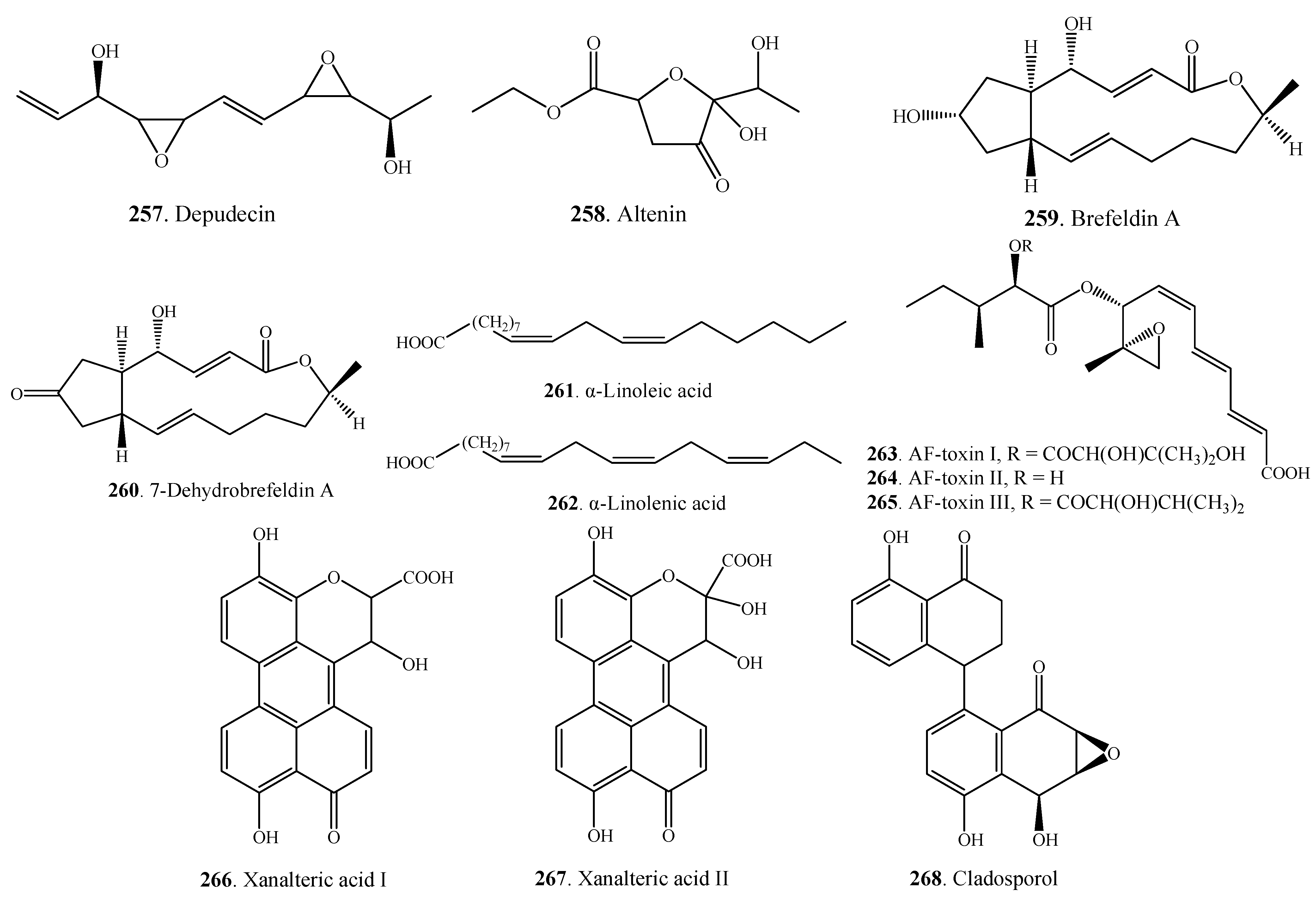
2.7. Miscellaneous Metabolites
3. Biological Activities and Functions
3.1. Effects on Plants
| Phytotoxin name | Alternaria species | Host plant | Plant disease | Reference |
|---|---|---|---|---|
| AAL-toxins TA1 (1), TA2 (2), TB1 (3), TB2 (4), TC1 (5), TC2 (6), TD1 (7), TD2 (8), TE1 (9), TE2 (10) | A. alternata f.sp. lycopersici | Tomato (Solanum lycopersicum) | Stem canker disease of tomato | [16,17,18] |
| ACT-toxins I (23) and II (24) | A. citri (A. alternata) | Mandarins and tangerine (Citrus spp.) | Brown spot of tangerine | [37,38] |
| AK-toxins I (25) and II (26) | A. kikuchiana (A. alternata) | Japanese pear (Pyrus serotina) | Black spot disease | [39,135] |
| AS-I toxin (27) | A. alternata | Sunflower (Helianthus annuus) | Necrotic spots on sunflower leaves | [40] |
| Maculosin (43) | A. alternata | Spotted knapweed (Centaurea maculosa) | Black leaf blight | [10,26] |
| AM-toxins I (46), II (47) and III (48) | A.mali (A. alternata) | Apple (Malus pumila) | Alternaria blotch of apple | [39] |
| Destruxin A (49), Destruxin B (50), Homodestruxin B (51), Desmethyldestruxin B (52) | A. brassicae | Brassica juncea; Brassica napus; Brassica rapa | Alternaria blackspot disease of Brassica | [46,148] |
| ACRL toxins I (140), II (141), III (142), IV (143), IV’(144) | A. citri | Rough lemon (Citrus limon) | Brown spot disease of Citrus | [75,76] |
| AF-toxins I (263), II (264) and III (265) | A. alternata | Strawberry (Fragaria spp.) | Alternaria balck spot of strawberry | [134,135] |
| Phytotoxin name | Alternaria species | Target weed species | Reference |
|---|---|---|---|
| AAL-toxins ( 1–10) | A. alternata | Jimson weed ( Datura stramonium) | [166] |
| Tenuazonic acid ( 15) | A. alternata | Lantana camara | [12] |
| Maculosin ( 43) | A. alternata | Spotted knapweed ( Centaurea maculosa) | [10] |
| Tentoxin ( 53) | A. alternata | Galium aparine | [11] |
| Isotentoxin ( 54) | A. alternata | Galium aparine | [11] |
| Alteichin ( 213) | A. eichorniae | Water hyacinth ( Eichhornia crassipes) | [108] |
| Alternethanoxin A ( 245) | A. sonchi | Sonchus arvensis | [125] |
| Alternethanoxin B ( 246) | A.sonchi | Sonchus arvensis | [125] |
| Brefeldin A ( 259) | A. zinniae | Xanthium occidentale | [129] |
3.2. Cytotoxic Activity
3.3. Antimicrobial Activity
3.4. Other Bioactivities
4. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Strange, R.N. Phytotoxins produced by plant pathogens. Nat. Prod. Rep. 2007, 24, 127–144. [Google Scholar] [CrossRef]
- Mobius, N.; Hertweck, C. Fungal phytotoxins as mediators of virulence. Curr. Opin. Plant Biol. 2009, 12, 390–398. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. Modes of action of microbially-produced phytotoxins. Toxins 2011, 3, 1038–1064. [Google Scholar] [CrossRef]
- Brase, S.; Encinas, A.; Keck, J.; Nising, C.F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Ohtani, K.; Kodama, M.; Akagi, Y.; Egusa, M.; Yamamoto, M.; Otani, H. Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef]
- Horiuchi, M.; Tokuda, H.; Ohnishi, K.; Yamashita, M.; Nishino, H.; Maoka, T. Porritoxins, metabolites of Alternaria porri, as anti-tumor-promoting active compounds. Nat. Prod. Res. 2006, 20, 161–166. [Google Scholar]
- Monneret, C. Histone deacetylase inhibitors. Eur. J. Med. Chem. 2005, 40, 1–13. [Google Scholar] [CrossRef]
- Lakshmi, A.I.; Madhusudhan, T.; Kumar, P.D.; Padmavathy, J.; saravanan, D.; Kumar, P.C. Histone deacetylase inhibitors in cancer therapy: an update. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 38–44. [Google Scholar]
- Stierle, A.C.; Cardellina II, J.H.; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef]
- Liebermann, B.; Ellinger, R.; Pinet, E. Isotentoxin, a conversion product of the phytotoxin tentoxin. Phytochemistry 1996, 42, 1537–1540. [Google Scholar]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Pandey, A.K.; Bhogendra, G.; Prasad, K.S.; Bisen, P.S. Isolation and characterization of tenuazonic acid produced by Alternaria alternata, a potential bioherbicidal agent for control Lantana camara. J. Plant Prot. Res. 2010, 50, 133–139. [Google Scholar]
- Montemurro, N.; Visconti, A. Alternaria metabolites—Chemical and biological data. In Alternaria: Biology, Plant Disease and Metabolites; Chelkowski, J., Visconti, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 449–557. [Google Scholar]
- Nishimura, S.; Kohmoto, K. Host-specific toxins and chemical structures from Alternaria species. Ann. Rev. Phytopathol. 1983, 21, 87–116. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: and overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Bottini, A.T.; Gilchrist, D.G. Phytotoxins. I. A 1-aminodimethylheptadecapentol from Alternaria alternata f.sp. lycopersici. Tetrahedron Lett. 1981, 22, 2719–2722. [Google Scholar] [CrossRef]
- Bottini, A.T.; Bowen, J.R.; Gilchrist, D.G. Phytotoxins. II. Characterization of a phytotoxic fraction from Alternaria alternata f.sp. lycopersici. Tetrahedron Lett. 1981, 22, 2723–2726. [Google Scholar] [CrossRef]
- Caldas, E.D.; Jones, A.D.; Ward, B.; Winter, C.K.; Gilchrist, D.G. Structural characterization of three new AAL toxins produced by Alternaria alternata f.sp. lycopersici. J. Agric. Food Chem. 1994, 42, 327–333. [Google Scholar]
- Abbas, H.K.; Riley, R.T. The presence and phytotoxicity of fumonisins and AAL-toxin in Alternaria alternata. Toxicon 1996, 34, 133–136. [Google Scholar] [CrossRef]
- Chen, J.; Mirocha, C.J.; Xie, W.; Hogge, L.; Olson, D. Production of the mycotoxin fumonisin B1 by Alternaria alternata f.sp. lycopersici. Appl. Environ. Microbiol. 1992, 58, 3928–3931. [Google Scholar]
- Hellwig, V.; Grothe, T.; Mayer-Bartschmid, A.; Endermann, R.; Geschke, F.-U.; Henkel, T.; Stadler, M. Altersetin, a new antibiotic from cultures of endophytic Alternaria spp. taxonomy, Fermentation, Isolation, Structure elucidation and biological activities. J. Antibiot. 2002, 55, 881–892. [Google Scholar]
- Fang, Z.F.; Yu, S.S.; Zhou, W.Q.; Chen, X.G.; Ma, S.G.; Li, Y.; Qu, J. A new isocoumarin from metabolites of the endophytic fungus Alternaria tenuissima (Nee & T. Nee: Fr) Wiltshire. Chin. Chem. Lett. 2012, 23, 317–320. [Google Scholar]
- Shaaban, M.; Shaaban, K.A.; Abdel-Aziz, M.S. Seven naphtho-γ-pyrones from the marine-derived fungus Alternaria alternata: Structure elucidation and biological properties. Org. Med. Chem. Lett. 2012, 2, 6. [Google Scholar]
- Davis, N.D.; Diener, U.L.; Morgan-Jones, G. Tenuazonic acid production by Alternaria alternata and Alternaria tenuissima isolated from cotton. Appl. Environ. Microbiol. 1977, 34, 155–157. [Google Scholar]
- Griffin, G.F.; Chu, F.S. Toxicity of the Alternaria metabolites alternariol, Alternariol methyl ether, Altenuene, and tenuazonic acid in the chicken embryo assay. Appl. Environ. Microbiol. 1983, 46, 1420–1422. [Google Scholar]
- Stierle, A.C.; Cardellina II, J.H.; Strobel, G.A. Phytotoxins from Alternaria alternata, a pathogen of spotted knapweed. J. Nat. Prod. 1989, 52, 42–47. [Google Scholar]
- Qin, J.-C.; Zhang, Y.-M.; Hu, L.; Ma, Y.-T.; Gao, J.-M. Cytotoxic metabolites produced by Alternaria no.28, an endophytic fungus isolated from Ginkgo biloba. Nat. Prod. Commun. 2009, 4, 1473–1476. [Google Scholar]
- Sonaimuthu, V.; Parihar, S.; Thakur, J.P.; Luqman, S.; Saikia, D.; Chanotiya, C.S.; Jhonpaul, M.; Negi, A.S. Tenuazonic acid: A promising antitubercular principle from Alternaria alternata. Microbiol. Res. 2010, 2, 63–65. [Google Scholar]
- Kono, Y.; Gardner, J.M.; Takeuchi, S. Nonselective phytotoxins simultaneously produced with host-selective ACTG-toxins by a pathotype of Alternaria citri causing brown spot disease of mandarins. Agric. Biol. Chem. 1986, 50, 2401–2403. [Google Scholar]
- Sattar, A.; Alam, M.; Janardhanan, K.K.; Husain, A. Isolation of tenuazonic acid, a phytotoxin from Alternaria crassa (Sacc.) rands causing leaf blight and fruit rot of Datura stramonium Mill. Curr. Sci. 1986, 55, 195–196. [Google Scholar]
- Evans, N.; Mcroberts, N.; Hill, R.A.; Marshall, G. Phytotoxin production by Alternaria linicola and phytoalexin production by the linseed host. Ann. Appl. Biol. 1996, 129, 415–431. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Rangsan, J.; Siripong, P.; Tip-pyang, S. New antitumour fungal metabolites from Alternaria porri. Nat. Prod. Res. 2009, 23, 1063–1071. [Google Scholar] [CrossRef]
- Stierle, A.; Hershenhorn, J.; Strobel, G. Zinniol-related phytotoxins from Alternaria cichorii. Phytochemistry 1993, 32, 1145–1149. [Google Scholar]
- Suemitsu, R.; Ohnishi, K.; Morikawa, Y.; Nagatomo, S. Zinnimidine and 5-(3’,3’-dimethylallyloxy)-7-methoxy-6-methylphthalide from Alternaria porri. Phytochemistry 1995, 38, 495–497. [Google Scholar]
- Horiuchi, M.; Ohnishi, K.; Iwase, N.; Nakajima, Y.; Tounai, K.; Yamashita, M.; Yamada, Y. A novel isoindoline, porritoxin sulfonic acid, from Alternaria porri and the structure-phytotoxicity correlation of its related compounds. Biosci. Biotechnol. Biochem. 2003, 67, 1580–1583. [Google Scholar]
- Horiuchi, M.; Maoka, T.; Iwase, N.; Ohnishi, K. Reinvestigation of structure of porritoxin, a phytotoxin of Alternaria porri. J. Nat. Prod. 2002, 65, 1204–1205. [Google Scholar] [CrossRef]
- Kohmoto, K.; Itoh, Y.; Shimomura, N.; Kondoh, Y.; Otani, H.; Kodama, M.; Nishimura, S.; Nakatsuka, S. Isolation and biological activities of two host-specific toxins from the tangerine pathotype of Alternaria alternata. Phytopathology 1993, 83, 495–502. [Google Scholar] [CrossRef]
- Masunaka, A.; Ohtani, K.; Peever, T.L.; Timmer, L.W.; Tsuge, T.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. An isolate of Alternaria alternata that is pathogenic to both tangerines and rough lemon and produces two host-selective toxins, ACT- and ACR-toxins. Phytopathology 2005, 95, 241–247. [Google Scholar] [CrossRef]
- Ueno, T. Secondary metabolites related to host selection by plant pathogenic fungi. Pure Appl. Chem. 1990, 62, 1347–1352. [Google Scholar] [CrossRef]
- Liakopoulou-Kyriakides, M.; Lagopodi, A.L.; Thanassoulopoulos, C.C.; Stavropoulos, G.S.; Magafa, V. Isolation and synthesis of a host-selective toxin produced by Alternaria alternata. Phytochemistry 1997, 45, 37–40. [Google Scholar]
- Wu, S.; Chen, Y.; Li, Z.; Yang, L.; Li, S. Metabolites of the endophytic fungus Alternaria sp. PR-14 of Paeonia delavayi. Nat. Prod. Res. Dev. 2011, 23, 850–852. [Google Scholar]
- Huang, Y.-F.; Li, L.-H.; Tian, L.; Qiao, L.; Hua, H.-M.; Pei, Y.-H. Sg17–1-4, a novel isocoumarin from a marine fungus Alternaria tenuis Sg17–1. J. Antibiot. 2006, 59, 355–357. [Google Scholar] [CrossRef]
- Feng, C.; Ma, Y. Isolation and anti-phytopathogenic activity of secondary metabolites from Alternaria sp. FL25, an endophytic fungus in Ficus carica. Chin. J. Appl. Environ. Biol. 2010, 16, 76–78. [Google Scholar] [CrossRef]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; Di Toppi, L.S.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: an ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar]
- Buchwaldt, L.; Green, H. Phytotoxicity of destruxin B and its possible role in the pathogenesis of Alternaria brassicae. Plant Pathol. 1992, 41, 55–63. [Google Scholar] [CrossRef]
- Ayer, W.A.; Pena-Rodriguez, L.M. Metabolites produced by Alternaria brassicae, the black spot pathogen of canola. Part 1, the phytotoxic components. J. Nat. Prod. 1987, 50, 400–407. [Google Scholar] [CrossRef]
- Liebermann, B.; Koelblin, R. A new phytotoxic activity of the cyclic peptides tentoxin and dihydrotentoxin. J. Phytopathol. 1992, 135, 245–250. [Google Scholar] [CrossRef]
- Horiuchi, M.; Akimoto, N.; Ohnishi, K.; Yamashita, M.; Maoka, T. Rapid and simultaneous determination of tetra cyclic peptide phytotoxins, tentoxin, isotentoxin and dihydrotentoxin, from Alternaria porri by LC/MS. Chromatography 2003, 24, 109–116. [Google Scholar]
- Ma, Y.-T.; Qiao, L.-R.; Shi, W.-Q.; Zhang, A.-L.; Gao, J.-M. Metabolites produced by an endophyte Alternaria alternata isolated from Maytenus hookeri. Chem. Nat. Compd. 2010, 46, 504–506. [Google Scholar] [CrossRef]
- Gloer, J.B.; Poch, G.K.; Short, D.M.; McCloskey, D.V. Structure of brassicicolin A: A novel isocyanide antibiotic from the phylloplane fungus Alternaria brassicicola. J. Org. Chem. 1988, 53, 3758–3761. [Google Scholar]
- Pedras, M.S.C.; Chumala, P.B.; Jin, W.; Islam, M.S.; Hauck, D.W. The phytopathogenic fungus Alternaria brassicicola: Phytotoxin production and phytoalexin elicitation. Phytochemistry 2009, 70, 394–402. [Google Scholar]
- Ma, Y.; Feng, C.; Zhang, H.; Zhou, X. Two indole alkaloids produced by endophytic fungus FL25 from Ficus carica. Chem. Res. Appl. 2009, 21, 1173–1175. [Google Scholar]
- Chen, J.; Qiu, X.; Wang, R.; Duan, L.; Chen, S.; Luo, J.; Kong, L. Inhibition of human gastric carcinoma cell growth in vitro and in vivo by cladosporol isolated from the paclitaxel-producing strain Alternaria alternata var. monosporus. Biol. Pharm. Bull. 2009, 32, 2072–2074. [Google Scholar] [CrossRef]
- Seitz, L.M.; Paukstelis, J.V. Metabolites of Alternaria alternata: ergosterol and ergosta-4,6,8(14),22-tetraen-3-one. J. Agric. Food Chem. 1977, 25, 838–841. [Google Scholar] [CrossRef]
- Gu, W. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana. World J. Microbiol. Biotechnol. 2009, 25, 1677–1683. [Google Scholar] [CrossRef]
- Liebermann, B.; Nussbaum, R.-P.; Gunther, W. Bicycloalternarenes produced by the phytopathogenic fungus Alternaria alternata. Phytochemistry 2000, 55, 987–992. [Google Scholar]
- Liebermann, B.; Ellinger, R.; Gunther, W.; Ihn, W.; Gallander, H. Tricycloalternarenes produced by Alternaria alternata related to ACTG-toxins. Phytochemistry 1997, 46, 297–303. [Google Scholar]
- Yuan, L.; Zhao, P.-J.; Ma, J.; Li, G.-H.; Shen, Y.-M. Tricycloalternarenes A-E: Five new mixed terpenoids from the endophytic fungal strain Alternaria alternata Ly83. Helv. Chim. Acta 2008, 91, 1588–1594. [Google Scholar] [CrossRef]
- Nussbaum, R.-P.; Gunther, W.; Heinze, S.; Liebermann, B. New tricycloalternarenes produced by the phytopathogenic fungus Alternaria alternata. Phytochemistry 1999, 52, 593–599. [Google Scholar]
- Qiao, L.-R.; Yuan, L.; Gao, J.-M.; Zhao, P.-J.; Kang, Q.-J.; Shen, Y.-M. Tricycloalternarene derivatives produced by an endophyte Alternaria alternata isolated from Maytenus hookeri. J. Basic Microbiol. 2007, 47, 340–343. [Google Scholar]
- Kono, Y.; Gardner, J.M.; Suzuki, Y.; Kondo, H.; Takeuchi, S. New minor components of host-selective ACTG-toxin and a novel sesquiterpene produced by a pathotype of Alternaria citri causing brown spot disease of mandarins. Nippon Noyaku Gakkaishi 1989, 14, 223–228. [Google Scholar]
- MacKinnon, S.L.; Keifer, P.; Ayer, W.A. Components from the phytotoxic extract of Alternaria brassicicola, a black spot pathogen of canola. Phytochemistry 1999, 51, 215–221. [Google Scholar]
- Dahiya, J.S.; Tewari, J.P.; Woods, D.L. Abscisic acid from Alternaria brassicae. Phytochemistry 1988, 27, 2983–2984. [Google Scholar] [CrossRef]
- Robeson, D.J.; Gray, G.R.; Strobel, G.A. Production of the phytotoxins radicinin and radicinol by Alternaria chrysanthemi. Phytochemitry 1982, 21, 2359–2362. [Google Scholar]
- Sheridan, H.; Canning, A.-M. Novel radicinol derivatives from long-term cultures of Alternaria chrysanthemi. J. Nat. Prod. 1999, 62, 1568–1569. [Google Scholar] [CrossRef]
- Tal, B.; Robeson, D.J.; Burke, B.A.; Aasen, A.J. Phytotoxins from Alternaria helianthi: radicinin and the structures of deoxyradicinol and radianthin. Phytochemistry 1985, 24, 729–731. [Google Scholar]
- Solfrizzo, M.; Vitti, C.; De Girolamo, A.; Visconti, A.; Logrieco, A.; Fanizzi, F.P. Radicinols and radicinin phytotoxins produced by Alternaria radicina on carrots. J. Agric. Food Chem. 2004, 52, 3655–3660. [Google Scholar] [CrossRef]
- Chen, Q.F.; Zhou, M.; Yang, T.; Chen, X.Z.; Wang, C.; Zhang, G.L.; Li, G.Y. Secondary metabolites from fungus Alternaria sp. CIB108. Chin. Chem. Lett. 2011, 22, 1226–1228. [Google Scholar]
- Robeson, D.J.; Strobel, R.A. Deoxyradicinin, a novel phytotoxin from Alternaria helianthi. Phytochemistry 1982, 21, 1821–1823. [Google Scholar]
- Robeson, D.J.; Strobel, G.A. Deoxyradicinin and 3-epideoxyradicinol production by the sunflower pathogen Alternaria helianthi. Ann. Appl. Biol. 1985, 107, 409–415. [Google Scholar] [CrossRef]
- Ivanova, L.; Petersen, D.; Uhlig, S. Phomenins and fatty acids from Alternaria infectoria. Toxicon 2010, 55, 1107–1114. [Google Scholar] [CrossRef]
- Christensen, K.B.; Van Klink, J.W.; Weavers, R.T.; Larsen, T.O.; Andersen, B.; Phipps, R.K. Novel chemotaxonomic markers of the Alternaria infectoria species-group. J. Agric. Food Chem. 2005, 53, 9431–9435. [Google Scholar]
- Larsen, T.O.; Perry, N.B.; Andersen, B. Infectopyrone, a potential mycotoxin from Alternaria infectoria. Tetrahedron Lett. 2003, 44, 4511–4513. [Google Scholar] [CrossRef]
- Patel, S.J.; Subramanian, R.B.; Jha, Y.S. A simple and rapid method for isolation of alternaric acid from Alternaria solani. Curr. Trends Biotechnol. Pharm. 2011, 5, 1098–1103. [Google Scholar]
- Gardner, J.M.; Kono, Y.; Tatum, J.H.; Suzuki, Y.; Takeuchi, S. Plant pathotoxins from Alternaria citri: the major toxin specific for rough lemon plants. Phytochemistry 1985, 24, 2861–2867. [Google Scholar] [CrossRef]
- Kono, Y.; Gardner, J.M.; Suzuki, Y.; Takeuchi, S. Plant pathotoxins from Alternaria citri: the minor ACRL toxins. Phytochemistry 1985, 24, 2869–2874. [Google Scholar] [CrossRef]
- Ichihara, A.; Tazaki, H.; Sakamura, S. Solanapyrones A, B and C, phytotoxic metabolites from the fungus Alternaria solan. Tetrahedron Lett. 1983, 24, 5373–5376. [Google Scholar] [CrossRef]
- Oikawa, H.; Yokota, T.; Sakano, C.; Suzuki, Y.; Naya, A.; Ichiara, A. Solanapyrones, phytotoxins produced by Alternaria solani: Biosynthesis and isolation of minor components. Biosci. Biotechnol. Biochem. 1988, 62, 2016–2022. [Google Scholar]
- Kjer, J.; Wray, V.; Edrada-Ebel, R.A.; Ebel, R.; Pretsch, A.; Lin, W.; Proksch, P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009, 72, 2053–2057. [Google Scholar] [CrossRef]
- Chu, F.S. Isolation of altenuisol and altertoxins I and II, minor mycotoxins elaborated by Alternaria. J. Am. Oil Chem. Soc. 1981, 58, 1006–1008. [Google Scholar] [CrossRef]
- Pero, R.W.; Harvan, D.; Blois, M.C. Isolation of the toxin, altenuisol, from the fungus, Alternaria tenuis Auct. Tetrahedron Lett. 1973, 12, 945–948. [Google Scholar] [CrossRef]
- Thomas, R. Biosyntesis of fungal metabolites. IV. Alternariol monomethyl ether and its relation to other phenolic metabolites of Alternaria tenuis. Biochem. J. 1961, 80, 234–240. [Google Scholar]
- Maeda, N.; Kokai, Y.; Ohtani, S.; Sahara, H.; Kuriyama, I.; Kamisuki, S.; Takahashi, S.; Sakaguchi, K.; Sugawara, F.; Yoshida, H. Anti-tumor effects of dehydroaltenusin, a specific inhibitor of mammalian DNA polymerase α. Biochem. Biophys. Res. Commun. 2007, 352, 390–396. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Indriani, I.D.; Wray, V.; Muller, W.E.G.; Totzke, F.; Zirrgiebel, U.; Schachtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar] [CrossRef]
- Watanabe, I.; Kakishima, M.; Adachi, Y.; Nakajima, H. Potential mycotoxin productivity of Alternaria alternata isolated from garden trees. Mycotoxins 2007, 57, 3–9. [Google Scholar] [CrossRef]
- Jeon, Y.-T.; Ryu, K.-H.; Kang, M.-K.; Park, S.-H.; Yun, H.; QT, P.; Kim, S.-U. Alternariol monomethyl ether and α,β-dehydrocurvularin from endophytic fungi Alternaria spp. inhibit appressorium formation of Magnaporthe grisea. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 39–42. [Google Scholar]
- Pero, R.W.; Main, C.E. Chlorosis of tobacco induced by alternariol monomethyl ether produced by Alternaria tenuis. Phytopathology 1970, 60, 1570–1573. [Google Scholar] [CrossRef]
- Visconti, A.; Bottalico, A.; Solfrizzo, M.; Palmisano, F. Isolation and structure elucidation of isoaltenuene, a new metabolite of Alternaria alternata. Mycotoxin Res. 1989, 5, 69–76. [Google Scholar] [CrossRef]
- Bradburn, N.; Coker, R.D.; Blunden, G.; Turner, C.H.; Crabb, T.A. 5'-Epialtenuene and neoaltenuene, dibenzo-α-pyrones from Alternaria alternata cultured on rice. Phytochemistry 1994, 35, 665–669. [Google Scholar]
- Zheng, C.-J.; Shao, C.-L.; Guo, Z.-Y.; Chen, J.-F.; Deng, D.-S.; Yang, K.-L.; Chen, Y.-Y.; Fu, X.-M.; She, Z.-G.; Lin, Y.-C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Stoessl, A.; Unwin, C.H.; Stothers, J.B. Metabolites of Alternaria solani. Part V. Biosynthesis of altersolanol A and incorporation of carbon-13 labeled altersolanol A into altersolanol B and macrosporin. Tetrahedron Lett. 1979, 27, 2481–2484. [Google Scholar] [CrossRef]
- Stoessl, A.; Stothers, J.B. Metabolites of Alternaria solani. Part VIII. Tetrahydroaltersolanol B, a hexahydroanthronol from Alternaria solani. Can. J. Chem. 1983, 61, 378–382. [Google Scholar] [CrossRef]
- Charudattan, R.; Rao, K.V. Bostrycin and 4-deoxybostrycin: Two nonspecific phytotoxins produced by Alternaria eichhorniae. Appl. Environ. Microbiol. 1982, 43, 846–849. [Google Scholar]
- Okamura, N.; Haraguchi, H.; Hashimoto, K.; Yagi, A. Altersolanol-related antimicrobial compounds from a strain of Alternaria solani. Phytochemistry 1993, 34, 1005–1009. [Google Scholar] [CrossRef]
- Suemitsu, R.; Yamada, Y.; Sano, T.; Yamashita, K. Studies of the metabolic products of Alternaria porri (Ellis) Ciferri. Part XII. Phytotoxic activities of altersolanol A, B and dactylariol, and activities of altersolanol A against some microorganisms. Agric. Biol. Chem. 1984, 48, 2383–2384. [Google Scholar] [CrossRef]
- Okamura, N.; Yagi, A.; Haraguchi, H.; Hashimoto, K. Simultaneous high-performance liquid chromatographic determination of altersolanol A, B, C, D, E and F. J. Chromatogr. 1992, 630, 418–422. [Google Scholar]
- Yagi, A.; Okamura, N.; Haraguchi, H.; Abo, T.; Hashimoto, K. Antimicrobial tetrahydroanthraquinones from a strain of Alternaria solani. Phytochemistry 1993, 33, 87–91. [Google Scholar]
- Suemitsu, R.; Nakamura, A.; Isono, F.; Sano, T. Studies on the metabolic products of Alternaria porri (Ellis) Ciferri. Part XI. Isolation and identification of dactylariol from the culture liquid Alternaria porri (Ellis) Ciferri. Agric. Biol. Chem. 1982, 46, 1693–1694. [Google Scholar] [CrossRef]
- Suemitsu, R.; Horiuchi, K.; Kubota, M.; Okamatsu, T. Production of alterporriols, altersolanols and marosporin by Alternaria porri and A. solani. Phytochemistry 1990, 29, 1509–1511. [Google Scholar]
- Huang, C.-H.; Pan, J.-H.; Chen, B.; Yu, M.; Huang, H.-B.; Zhu, X.; Lu, Y.-J.; She, Z.-G.; Lin, Y.-C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9–6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef]
- Okuno, T.; Natsume, I.; Sawai, K.; Sawamura, K.; Furusaki, A.; Matsumoto, T. Structure of antifungal and phytotoxic pigments produced by Alternaria species. Tetrahedron Lett. 1983, 24, 5653–5656. [Google Scholar]
- Zhang, S.-Y.; Li, Z.-L.; Bai, J.; Wang, Y.; Zhang, L.-M.; Wu, X.; Hua, H.-M. A new perylenequinone from a halotolerant fungus, Alternaria sp. M6. Chin. J. Nat. Med. 2012, 10, 68–71. [Google Scholar] [CrossRef]
- Hradil, C.M.; Hallock, Y.F.; Clardy, J.; Kenfield, D.S.; Strobel, G. Phytotoxins from Alternaria cassiae. Phytochemistry 1989, 28, 73–75. [Google Scholar]
- Stack, M.E.; Mazzola, E.P.; Page, S.W.; Pohland, A.E.; Highet, R.J.; Tempesta, M.S.; Corley, D.G. Mutagenic perylenequinone metabolites of Alternaria alternata: altertoxins I, II and III. J. Nat. Prod. 1986, 49, 866–871. [Google Scholar] [CrossRef]
- Gao, S.-S.; Li, X.-M.; Wang, B.-G. Perylene derivatives produced by Alternaria alternata, an endophytic fungus isolated from Laurencia species. Nat. Prod. Commun. 2009, 4, 1477–1480. [Google Scholar]
- Hu, D.; Liu, M.; Xia, X.; Chen, D.; Zhao, F.; Ge, M. Preparative isolation and purification of altertoxin I from an Alternaria sp. by HSCCC. Chromatographia 2008, 67, 863–867. [Google Scholar] [CrossRef]
- Visconti, A.; Sibilia, A.; Sabia, C. Alternaria alternata from oilseed rape: mycotoxin production, and toxicity to Artemia salina larvae and rape seedlings. Mycotoxin Res. 1992, 8, 9–16. [Google Scholar] [CrossRef]
- Robeson, D.; Strobel, G.; Matusumoto, G.K.; Fisher, E.L.; Chen, M.H.; Clardy, J. Alteichin: An unusual phytotoxin from Alternaria eichorniae, a fungal pathogen of water hyacinth. Experientia 1984, 40, 1248–1250. [Google Scholar] [CrossRef]
- Gamboa-Angulo, M.M.; Garcia-Sosa, K.; Alejos-Gonzalez, F.; Escalante-Erosa, F.; Delgado-Lamas, G.; Pena-Rodriguez, L.M. Tagetolone and tagetenolone: two phytotoxic polyketides from Alternaria tagetica. J. Agric. Food Chem. 2001, 49, 1228–1232. [Google Scholar] [CrossRef]
- Xia, X.; Qi, J.; Wei, F.; Jia, A.; Yuan, W.; Meng, X.; Zhang, M.; Liu, C.; Wang, C. Isolation and characterization of a new benzofuran from the fungus Alternaria sp. (HS-3) associated with a sea cucumber. Nat. Prod. Commun. 2011, 6, 1913–1914. [Google Scholar]
- Kaul, S.; Wani, M.; Dhar, K.L.; Dhar, M.K. Production and GC-MS trace analysis of methyl eugenol form endophytic isolate of Alternaria from rose. Ann. Microbiol. 2008, 58, 443–445. [Google Scholar] [CrossRef]
- Tietjen, K.G.; Schaller, E.; Matern, U. Phytotoxins from Alternaria carthami Chowdhury: Structural identification and physiological significance. Physiol. Plant Pathol. 1983, 23, 387–400. [Google Scholar] [CrossRef]
- Cotty, P.J.; Misaghi, I.J. Zinniol production by Alternaria species. Phytopathology 1984, 74, 785–788. [Google Scholar] [CrossRef]
- Berestetskii, A.O.; Yuzikhin, O.S.; Katkova, A.S.; Dobrodumov, A.V.; Sivogrivov, D.E.; Kolombet, L.V. Isolation, Identification, and characteristics of the phytotoxin produced by the fungus Alternaria cirsinoxia. Appl. Biochem. Microbiol. 2010, 46, 75–79. [Google Scholar] [CrossRef]
- Barasch, I.; Mor, H.; Netzer, D.; Kashman, Y. Production of zinniol by Alternaria dauci and its phytotoxic effect on carrot. Physiol. Plant Pathol. 1981, 19, 7–16. [Google Scholar]
- Gamboa-Angulo, M.M.; Escalante-Erosa, F.; Garcia-Sosa, K.; Alejos-Gonzalez, F.; Delgado-Lamas, G.; Pena-Rodriguez, L.M. Natural zinniol derivatives from Alternaria tagetica. Isolation, synthesis, and structure-activity correlation. J. Agric. Food Chem. 2002, 50, 1053–1058. [Google Scholar] [CrossRef]
- Gamboa-Angulo, M.M.; Alejos-Gonzalez, F.; Pena-Rodriguez, L.M. Homozinniol, a new phytotoxic metabolite from Alternaria solani. J. Agric. Food Chem. 1997, 45, 282–285. [Google Scholar]
- Moreno-Escobar, J.; Puc-Carrillo, A.; Ceres-Farfan, M.C.A.; Pena-Rodriguez, L.M.; Gamboa-Angulo, M.M. Two new zinniol-related phytotoxins from Alternaria solani. Nat. Prod. Res. 2005, 19, 603–607. [Google Scholar] [CrossRef]
- Cotty, P.J.; Mishagi, I.; Hine, R. Produciton of zinniol by Alternaria tagetica and its phytotoxic effect on Tagetes erecta. Phytopathology 1983, 73, 1326–1328. [Google Scholar] [CrossRef]
- Starratt, A.N. Zinniol: a major metabolite of Alternaria zinniae. Can. J. Chem. 1968, 46, 767–770. [Google Scholar] [CrossRef]
- Gamboa-Angulo, M.M.; Alejos-Gonzalez, F.; Escalante-Erosa, F.; Garcia-Sosa, K.; Delgado-Lamas, G.; Pena-Rodriguez, L.M. Novel dimeric metabolites from Alternaria tagetica. J. Nat. Prod. 2000, 63, 1117–1120. [Google Scholar] [CrossRef]
- Cota, B.B.; Rosa, L.H.; Caligiorne, R.B.; Rabello, A.L.T.; Alves, T.M.A.; Rosa, C.A.; Zani, C.L. Altenusin, a biphenyl isolated from the endophytic fungus Alternaria sp., inhibits trypanothione reductase from Trypanosoma cruzi. FEMS Microbiol. Lett. 2008, 285, 177–182. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, S.; Wei, J.; Fang, N.; Yin, L.; Sun, J. Porric acid D from marine-derived fungus Alternaria sp. isoltated from Bohai Sea. Chem. Nat. Compd. 2012, 47, 893–895. [Google Scholar] [CrossRef]
- Chadwick, D.J.; Easton, I.W.; Johnstone, R.A.W. Fungal metabolites. Part 9. Isolation and x-ray structure determination of alternarian acid from Alternaria mali Sp. Tetrahedron 1984, 40, 2451–2455. [Google Scholar] [CrossRef]
- Evidente, A.; Punzo, B.; Andolfi, A.; Berestetskiy, A.; Motta, A. Alternethanoxins A and B, polycyclic ethanones produced by Alternaria sonchi, potential mycoherbicides for Sonchus arvensis biocontrol. J. Agric. Food Chem. 2009, 57, 6656–6660. [Google Scholar]
- Robeson, D.J.; Strobel, G.A. αβ-Dehydrocurvularin and curvularin from Alternaria cineraiae. Z. Naturforsch., C:. J. Biosci. 1981, 36C, 1081–1083. [Google Scholar]
- Hyeon, S.-B.; Ozaki, A.; Suzuki, A.; Tamura, S. Isolation of αβ-dehydrocurvularin and β-hydroxycurvularin from Alternaria tomato as sporulation-suppressing factors. Agric. Biol. Chem. 1976, 40, 1663–1664. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Vederas, J.C. Biosynthetic incorporation of advanced precursors into dehydrocurvularin, a polyketide phytotoxin from Alternaria cinerariae. Tetrahedron 1998, 54, 15937–15958. [Google Scholar] [CrossRef]
- Vurro, M.; Evidente, A.; Andolfi, A.; Zonno, M.C.; Giordano, F.; Motta, A. Brefeldin A and α,β-dehydrocurvularin, two phytotoxins from Alternaria zinniae, a biocontrol agent of Xanthium occidental. Plant Sci. 1998, 138, 67–79. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. [Google Scholar] [CrossRef]
- Suemitsu, R.; Ohnishi, K.; Morikawa, Y.; Ideguchi, I.; Uno, H. Porritoxinol, a phytotoxin of Alternaria porri. Phytochemistry 1994, 35, 603–605. [Google Scholar]
- Matsumoto, M.; Matsutani, S.; Shigeru, S.; Kenji, Y.; Hiroshi, H.; Hayashi, F.; Terui, Y.; Nakai, H.; Uotani, N.; Kawamura, Y. Depudecin: a novel compound inducing the flat phenotype of NIH3T3 cells doubly transformed by ras- and src- oncogene, produced by Alternaria brassicicola. J. Antibiot. 1992, 45, 879–885. [Google Scholar] [CrossRef]
- Sugiyama, N.; Kashima, C.; Yamamoto, M.; Mohri, R. Altenin, a new phytopathologically toxic metabolite from Alternaria kikuchiana. Bull. Chem. Soc. Jpn. 1965, 38, 2028. [Google Scholar] [CrossRef]
- Maekawa, N.; Nishimura, S.; Kohmoto, K.; Watanabe, Y. Isolation of the host-specific toxins produced by Alternaria alternata, Strawberry pathotype. Ann. Phytopathol. Soc. Jpn. 1979, 45, 536–537. [Google Scholar]
- Hayashi, N.; Tanabe, K.; Tsuge, T.; Nishimura, S.; Kohmoto, K.; Otani, H. Determination of host-selective toxin production during spore germination of Alternaria alternata by high-performance liquid chromatography. Phytopathology 1990, 80, 1088–1091. [Google Scholar] [CrossRef]
- Abbas, H.K.; Tanaka, T.; Duke, S.O. Pathogenicity of Alternaria alternata and Fusarium moniliforme and phytotoxicity of AAL-toxin and fumonisin B1 on tomato cultivars. J. Phytopathol. 1995, 143, 329–334. [Google Scholar] [CrossRef]
- Yamagishi, D.; Akamatsu, H.; Otani, H.; Kodama, M. Pathological evaluation of host-specific AAL-toxins and fumonisin mycotoxins produced by Alternaria and Fusarium species. J. Gen. Plant Pathol. 2006, 72, 323–327. [Google Scholar] [CrossRef]
- Liu, J.Y.; Song, Y.C.; Zhang, Z.; Wang, L.; Guo, Z.J.; Zou, W.X.; Tan, R.X. Aspergillus fumigatus CY018, an endophytic fungus in Cynodon dactylon as a versatile producer of new and bioactive metabolites. J. Biotechnol. 2004, 114, 279–287. [Google Scholar] [CrossRef]
- Zhao, J.; Mou, Y.; Shan, T.; Li, Y.; Lu, S.; Zhou, L. Preparative separation of helvolic acid from the endophytic fungus Pichia guilliermondii Ppf9 by high-speed counter-current chromatography. World J. Microbiol. Biotechnol. 2012, 28, 835–840. [Google Scholar] [CrossRef]
- Van Rozendaal, E.L.M.; Kurstjens, S.J.L.; Van Beek, T.A.; Van Den Berg, R.G. Chemotaxonomy of Taxus. Phytochemistry 1999, 52, 427–433. [Google Scholar]
- Brisdelli, F.; D’Andrea, G.; Bozzi, A. Resveratrol: a natural polyphenol with multiple chemopreventive properties (review). Curr. Drug MeTable 2009, 10, 530–546. [Google Scholar] [CrossRef]
- Zhou, L.G.; Wu, J.Y. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat. Prod. Rep. 2006, 23, 789–810. [Google Scholar] [CrossRef]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: a fungal pathway for life on land. Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef]
- Robison, S.H.; Barr, D.B. Use of biomonitoring data to evaluate methyl eugenol exposure. Environ. Health Persp. 2006, 114, 1797–1801. [Google Scholar]
- Valenzano, D.R.; Terzibasi, E.; Genade, T.; Cattaneo, A.; Domenici, L.; Cellerino, A. Resveratrol prolongs life span and retards the onset of age-related markers in a shortlived vertebrate. Curr. Biol. 2006, 16, 296–300. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef]
- Graniti, A. Phytotoxins and their involvement in plant diseases. Experientia 1991, 47, 751–755. [Google Scholar]
- Pedras, M.S.C.; Biesenthal, C.J.; Zaharia, I.L. Comparison of the phytotoxic activity of the phytotoxin destruxin B and four natural analogs. Plant Sci. 2000, 156, 185–192. [Google Scholar] [CrossRef]
- Howlett, B.J. Secondary metabolite toxins and nutrition of plant pathogenic fungi. Curr. Opin. Plant Biol. 2006, 9, 371–375. [Google Scholar] [CrossRef]
- Johnson, L.J.; Johnson, R.D.; Akamatsu, H.; Salamiah, A.; Otani, H.; Kohmoto, K.; Kodama, M. Spontaneous loss of a conditionally dispensable chromosome from the Alternaria alternata apple pathotype leads to loss of toxin production and pathogenicity. Curr. Genet. 2001, 40, 65–72. [Google Scholar] [CrossRef]
- Winter, C.K.; Gilchrist, D.G.; Dickman, M.B.; Jones, C. Chemistry and biological activity of AAL toxins. Adv. Exp. Med. Biol. 1996, 392, 307–316. [Google Scholar] [CrossRef]
- Brandwagt, B.F.; Mesbah, L.A.; Takken, F.L.W.; Laurent, P.L.; Kneppers, T.J.A.; Hille, J.; Nijkamp, H.J. A longevity assurance gene homolog of tomato mediates resistance to Alternaria alternata f.sp. lycopersici toxins and fumonisin B1. Proc. Natl. Acad. Sci. USA 2000, 97, 4961–4966. [Google Scholar] [CrossRef]
- Liu, B.-L.; Tzeng, Y.-M. Development and application of destruxins: a reviw. Biotechnol. Adv. 2012, 30, 1242–1254. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Yaya, E.E.; Glawischnig, E. The phytoalexins from cultivated and wild crucifers: chemistry and biology. Nat. Prod. Rep. 2011, 28, 1381–1405. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Zaharia, I.L.; Gai, Y.; Smith, K.C.; Ward, D.E. Metabolism of the host-selective toxins destruxin B and homodestruxin B: probing a plant disease resistance trait. Org. Lett. 1999, 1, 1655–1658. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Montaut, S.; Zaharia, I.L.; Gai, Y.; Ward, D.E. Transformation of the host-selective toxin destruxin B by wild crucifers: probing a detoxification pathway. Phytochemistry 2003, 64, 957–963. [Google Scholar] [CrossRef]
- Halloin, J.M.; De Zoeten, G.A.; Gaard, G.R.; Walker, J.C. Effects of tentoxin on chlorophyll synthesis and plastid structure in cucumber and cabbage. Plant Physiol. 1970, 45, 310–314. [Google Scholar] [CrossRef]
- Avni, A.; Anderson, J.D.; Hollan, N.; Rochaix, J.D.; Gromet-Elhanan, Z.; Edelman, M. Tentoxin sensitivity of chloroplasts determined by codon 83 of β subunit of proton ATPase. Science 1992, 257, 1245–1247. [Google Scholar]
- Liu, Y.-X.; Xu, X.-M.; Dai, X.-B.; Qiang, S. Alternaria alternata crofton-weed toxin: a natural inhibitor of photosystem II in Chlamydomonas reinhardtii thylakoids. J. Agric. Food Chem. 2007, 55, 5180–5185. [Google Scholar] [CrossRef]
- Haraguchi, H.; Abo, T.; Fukuda, A.; Okamura, N.; Yagi, A. Mode of phytotoxic action of altersolanols. Phytochemistry 1996, 43, 989–992. [Google Scholar] [CrossRef]
- Robeson, D.J.; Strobel, G.A. Zinniol induces chlorophyll retention in barley leaves: The selective action of a non-specific phytotoxin. Phytochemistry 1984, 23, 1597–1599. [Google Scholar] [CrossRef]
- Qui, J.A.; Castro-Concha, L.A.; Garcia-Sosa, K.; Miranda-Ham, M.L.; Pena-Rodriguez, L.M. Is zinniol a true phytotoxin? Evaluation of its activity at the cellular level against Tagetes erecta. J. Gen. Plant Pathol. 2010, 76, 94–101. [Google Scholar] [CrossRef]
- Kenfield, D.; Bunkers, G.; Strobel, G.A.; Sugawara, F. Potential new herbicides—phytotoxins from plant pathogens. Weed Technol. 1988, 2, 519–524. [Google Scholar]
- Abbas, H.K.; Duke, S.O. Phytotoxins from plant pathogens as potential herbicides. Toxin Rev. 1995, 14, 523–543. [Google Scholar] [CrossRef]
- Mallik, M.A.B. Selective isolation and screening of soil microorganisms for metabolites with herbicidal potential. J. Crop Prot. 2001, 4, 219–236. [Google Scholar] [CrossRef]
- Abbas, H.K.; Hamed, K.; Vesonder, R.F.; Boyette, C.D.; Peterson, S.W. Phytotoxicity of AAL-toxin and other compounds produced by Alternaria alternata to jimson weed (Datura stramonium). Can. J. Bot. 1993, 71, 155–160. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: an overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Bensassi, F.; Gallerne, C.; Sharaf El Dein, O.; Hajlaoui, M.R.; Bacha, H.; Lemaire, C. Cell death induced by the Alternaria mycotoxin alternariol. Toxicol. In Vitro 2012, 26, 915–923. [Google Scholar] [CrossRef]
- Schreck, I.; Deigendesch, U.; Burkhardt, B.; Marko, D.; Weiss, C. The Alternaria mycotoxins alternariol and alternariol methyl ether induce cytochrome P450 1A1 and apoptosis in murine hepatoma cells dependent on the aryl hydrocarbon receptor. Arch. Toxicol. 2012, 86, 625–632. [Google Scholar] [CrossRef]
- Mizushina, Y.; Maeda, N.; Kuriyama, I.; Yoshida, H. Dehydroalternusin is a specific inhibitor of mammalian DNA polymerase α. Expert Opin. Inv. Drug. 2011, 20, 1523–1534. [Google Scholar] [CrossRef]
- Huang, C.; Jin, H.; Song, B.; Zhu, X.; Zhao, H.; Cai, J.; Lu, Y.; Chen, B.; Lin, Y. The cytotoxicity and anticancer mechanisms of alterporriol L, a marine bianthraquinone, against MCF-7 human breast cancer cells. Appl. Microbiol. Biotechnol. 2012, 93, 777–785. [Google Scholar] [CrossRef]
- Bury, M.; Punzo, B.; Berestetskiy, A.; Lallemand, B.; Dubois, J.; Lefranc, F.; Mathieu, V.; Andolfi, A.; Kiss, R.; Evidente, A. Evaluation of the anticancer activities of two fungal polycyclic ethanones, alternethanoxins A and B, and two of their derivatives. Int. J. Oncol. 2011, 38, 227–232. [Google Scholar]
- Moreno, M.A.P.; Alonso, I.G.; Martin de Santos, R.; Lacarra, T.G. The role of the genus Alternaria in mycotoxin production and human deseases. Nutricion Hospitalaria 2012, 27, 1772–1781. [Google Scholar]
- Zajkowski, P.; Grabarkiewicz-Szcesna, J.; Schmidt, R. Toxicity of mycotoxins produced by four Alternaria species to Artemia salina larvae. Mycotoxin Res. 1991, 7, 11–15. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dallin, S. Toxicity of the Alternaria spp metabolites, tenuazonic acid, alternariol, altertoxin-I, and alternariol monomethyl ether to brine shrimp (Artemia salina L.) larvae. J. Sci. Food Agric. 1994, 66, 493–496. [Google Scholar] [CrossRef]
- Berto, P.; Belingheri, L.; Dehorter, B. Production and purification of a novel extracellular lipase from Alternaria brassicicola. Biotechnol. Lett. 1997, 19, 533–536. [Google Scholar] [CrossRef]
- Isshiki, A.; Akimitsu, K.; Nishio, K.; Tsukamoto, M.; Yamamoto, H. Purification and characterization of an endopolygalacturonase from the rough lemon pathotype of Alternaria alternata, the cause of citrus brown spot disease. Physiol. Mol. Plant Pathol. 1997, 51, 155–167. [Google Scholar] [CrossRef]
- Kulye, M.; Liu, H.; Zhang, Y.L.; Zeng, H.M.; Yang, X.F.; Qiu, D.W. Hrip1, a novel protein elicitor from necrotrophic fungus, Alternaria tenuissima, elicits cell death, expression of defence-related genes and systemic acquired resistance in tobacco. Plant Cell Envrion. 2012, 35, 2104–2120. [Google Scholar] [CrossRef]
- Saha, D.; Fetzner, R.; Burkhardt, B.; Podlech, J.; Metzler, M.; Dang, H.; Lawrence, C.; Fischer, R. Identification of a polyketide synthase required for alternariol (AOH) and alternariol-9-methyl ether (AME) formation in Alternaria alternata. PLoS ONE 2012, 7, e40456. [Google Scholar]
- Shinya, T.; Menard, R.; Kozone, I.; Matsuoka, H.; Shibuya, N.; Kauffmann, S.; Matsuoka, K.; Saito, M. Novel beta-1,3-, 1,6-oligoglucan elicitor from Alternaria alternata 102 for defense responses in tobacco. FEBS J. 2006, 273, 2421–2423. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, D.; Zeng, H.; Yuan, J.; Mao, J. Purification and characterization of a glycoprotein elicitor from Alternaria tenuissima. World J. Microbiol. Biotechnol. 2009, 25, 2035–2042. [Google Scholar] [CrossRef]
- Oikawa, H.; Yamawaki, D.; Kagawa, T.; Ichihara, A. Total synthesis of AAL-toxin TA1. Tetrahedron Lett. 1999, 40, 6621–6625. [Google Scholar]
- Bobylev, M.M.; Bobyleva, L.I.; Strobel, G.A. Synthesis and bioactivity of analogs of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa). J. Agric. Food Chem. 1996, 44, 3960–3964. [Google Scholar]
- Lee, S.; Aoyagi, H.; Shimohigashi, Y.; Izumiya, N.; Ueno, T.; Fukami, H. Syntheses of cyclotretradepsipeptides, AM-toxin I and its analogs. Tetrahedron Lett. 1976, 17, 843–846. [Google Scholar]
- Koch, K.; Podlech, J.; Pfeiffer, E.; Metzler, M. Total synthesis of alternariol. J. Org. Chem. 2005, 70, 3275–3276. [Google Scholar]
- Altemoller, M.; Podlech, J.; Fenske, D. Total synthesis of altenuene and isoaltenuene. Eur. J. Org. Chem. 2006, 2006, 1678–1684. [Google Scholar] [CrossRef]
- Altenoeller, M.; Podlech, J. Total synthesis of neoaltenuene. Eur. J. Org. Chem. 2009, 2009, 2275–2282. [Google Scholar]
- Geiseler, O.; Mueller, M.; Podlech, J. Synthesis of the altertoxin III framework. Tetrahedron 2013, 69, 3683–3689. [Google Scholar] [CrossRef]
- Martin, J.A.; Vogel, E. The synthesis of zinniol. Tetrahedron 1980, 36, 791–794. [Google Scholar] [CrossRef]
- Cudaj, J.; Podlech, J. Total synthesis of altenusin and alterlactone. Synlett 2012, 23, 371–374. [Google Scholar] [CrossRef]
- Beresteskiy, A.O. A review of fungal phytotoxins: from basic studies to practical use. Appl. Biochem. Microbiol. 2008, 44, 453–465. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria Fungi and Their Bioactivities. Molecules 2013, 18, 5891-5935. https://doi.org/10.3390/molecules18055891
Lou J, Fu L, Peng Y, Zhou L. Metabolites from Alternaria Fungi and Their Bioactivities. Molecules. 2013; 18(5):5891-5935. https://doi.org/10.3390/molecules18055891
Chicago/Turabian StyleLou, Jingfeng, Linyun Fu, Youliang Peng, and Ligang Zhou. 2013. "Metabolites from Alternaria Fungi and Their Bioactivities" Molecules 18, no. 5: 5891-5935. https://doi.org/10.3390/molecules18055891
APA StyleLou, J., Fu, L., Peng, Y., & Zhou, L. (2013). Metabolites from Alternaria Fungi and Their Bioactivities. Molecules, 18(5), 5891-5935. https://doi.org/10.3390/molecules18055891






