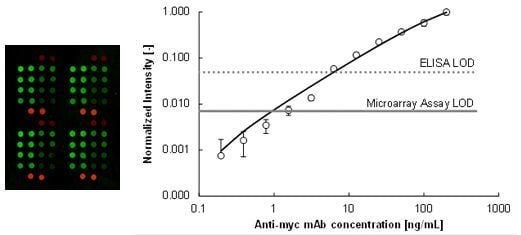
ADIBO-Based “Click” Chemistry for Diagnostic Peptide Micro-Array Fabrication: Physicochemical and Assay Characteristics
Abstract
:1. Introduction
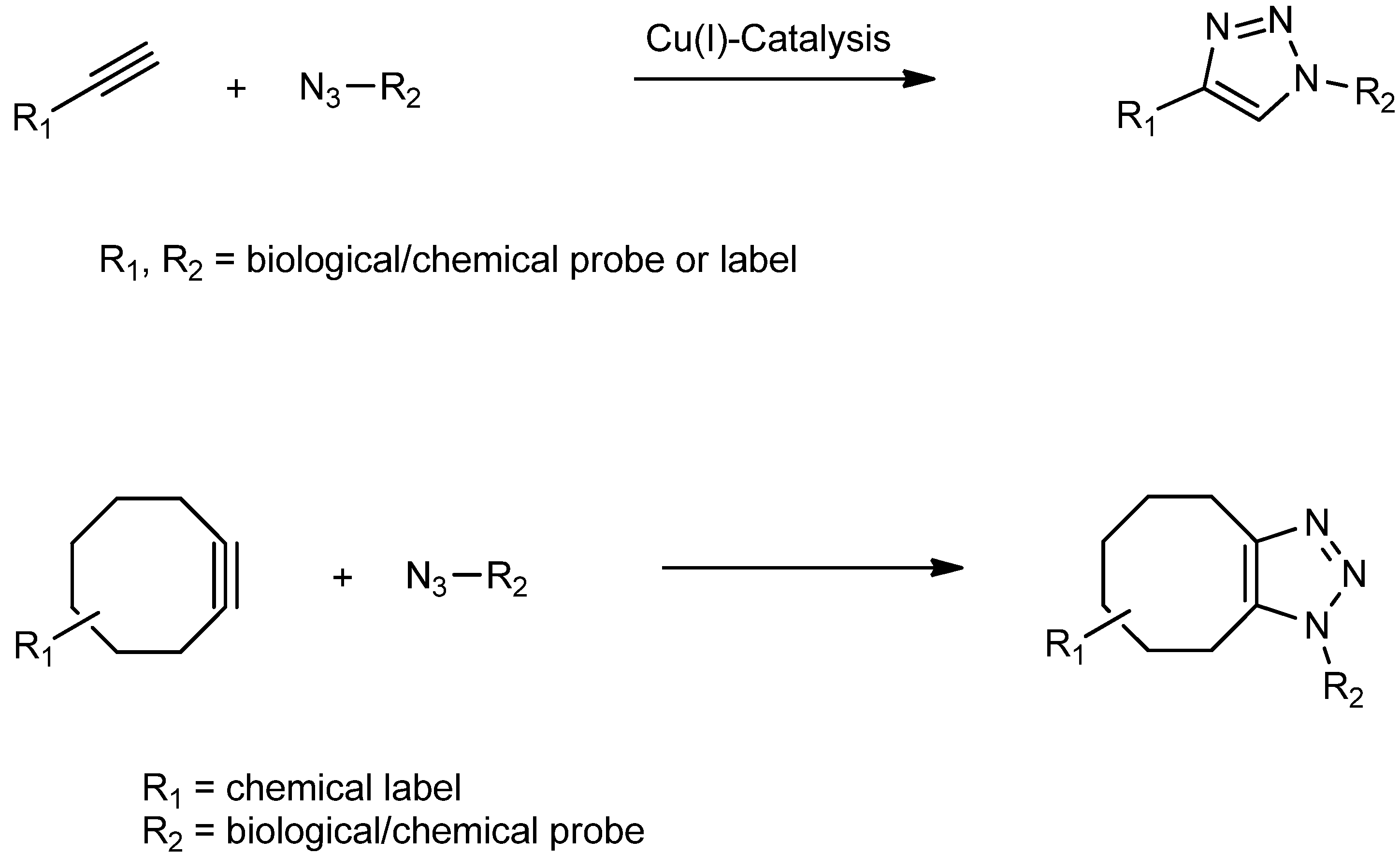

2. Results and Discussion
2.1. Kinetics of Click Immobilization of Small Molecules and Peptides
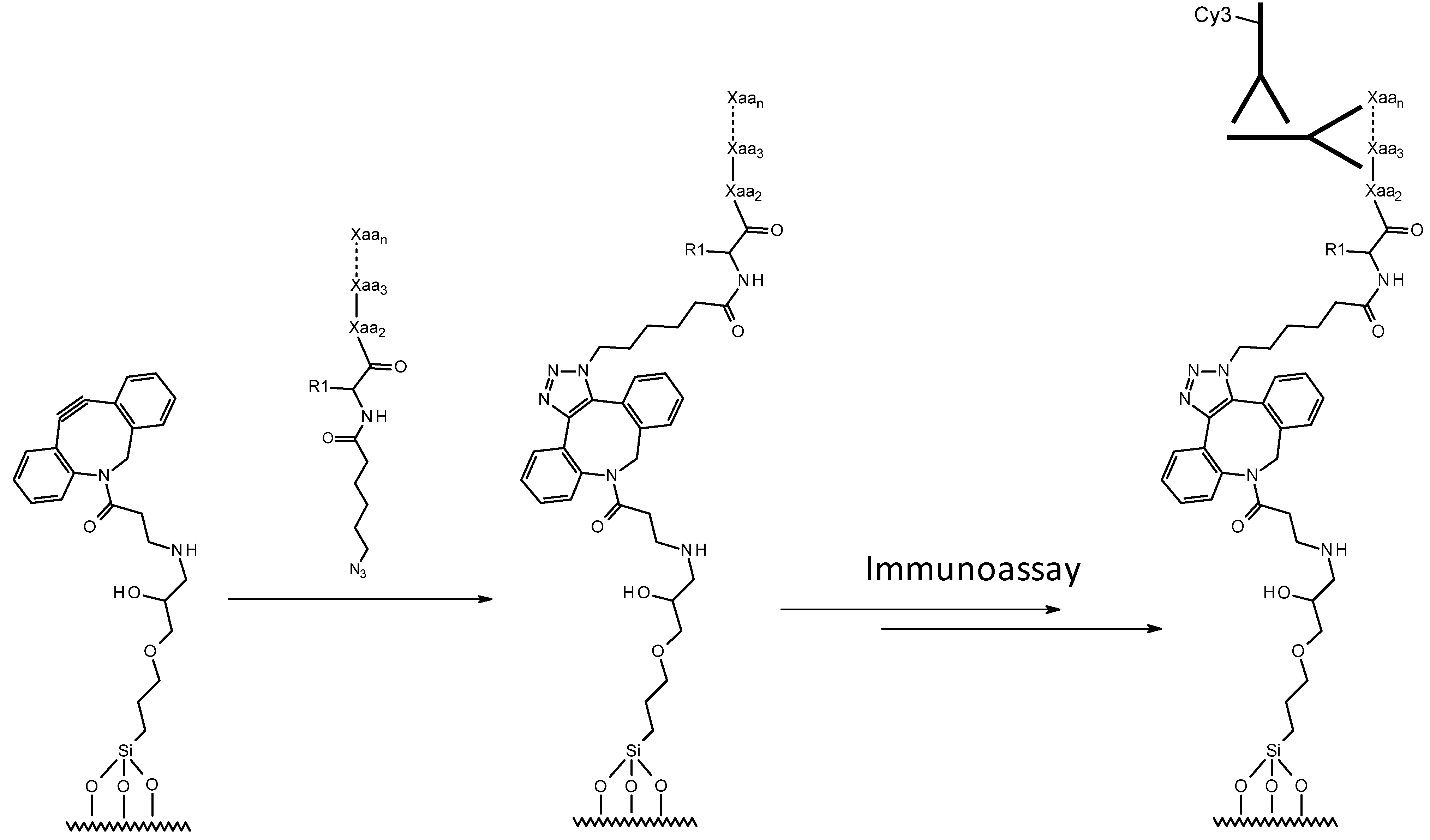
| Name | Sequence (amino- to carboxy-terminus) | Comment |
|---|---|---|
| N3-Cy5-myc | N3-  -K(Cy5)- EQKLISEEDL-OH -K(Cy5)- EQKLISEEDL-OH | Labeled assay development model |
| N3-myc | N3-  -EQKLISEEDL-OH -EQKLISEEDL-OH | Assay development model |
| N3-P1 | N3-  -DTQLKSRDPSKIPV-NH2 -DTQLKSRDPSKIPV-NH2 | NC biomarker peptide |
| Cy5-P1 | K(Cy5)- DTQLKSRDPSKIPV-NH2 | Labeled NC biomarker peptide for characterization of NSB |
| N3-Cy5-P1 | N3-  -K(Cy5)-DTQLKSRDPSKIPV-NH2 -K(Cy5)-DTQLKSRDPSKIPV-NH2 | Labeled NC biomarker peptide |
| N3-P2 | N3-  -Undisclosed sequence -Undisclosed sequence | Putative cancer biomarker peptide |
| N3-Cy5-P2 | N3-  -K(Cy5)-Undisclosed sequence -K(Cy5)-Undisclosed sequence | Labeled putative cancer biomarker peptide |
 - corresponds to N3-(CH2)5-(CO)-, NC = negative control.
- corresponds to N3-(CH2)5-(CO)-, NC = negative control.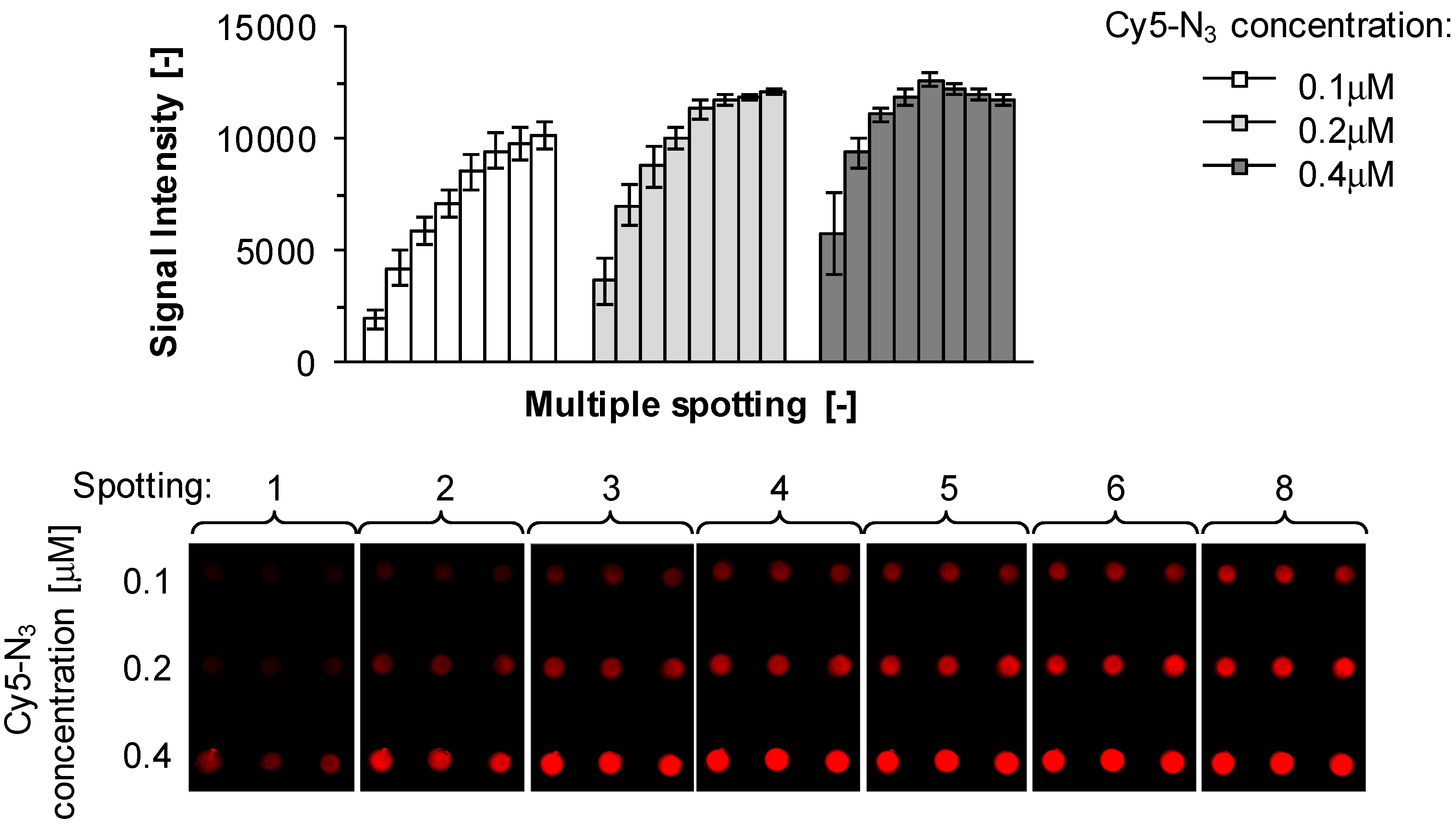
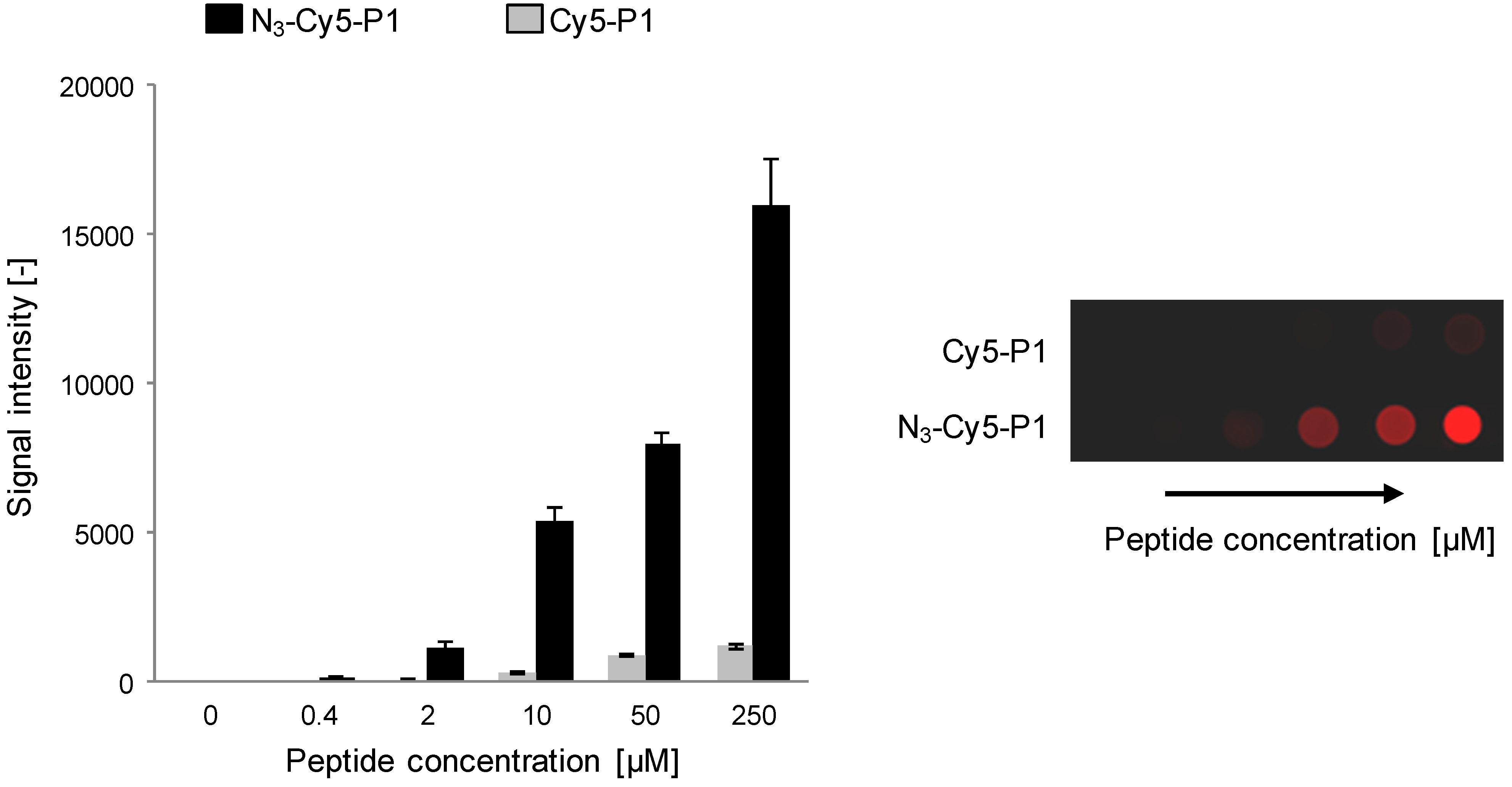
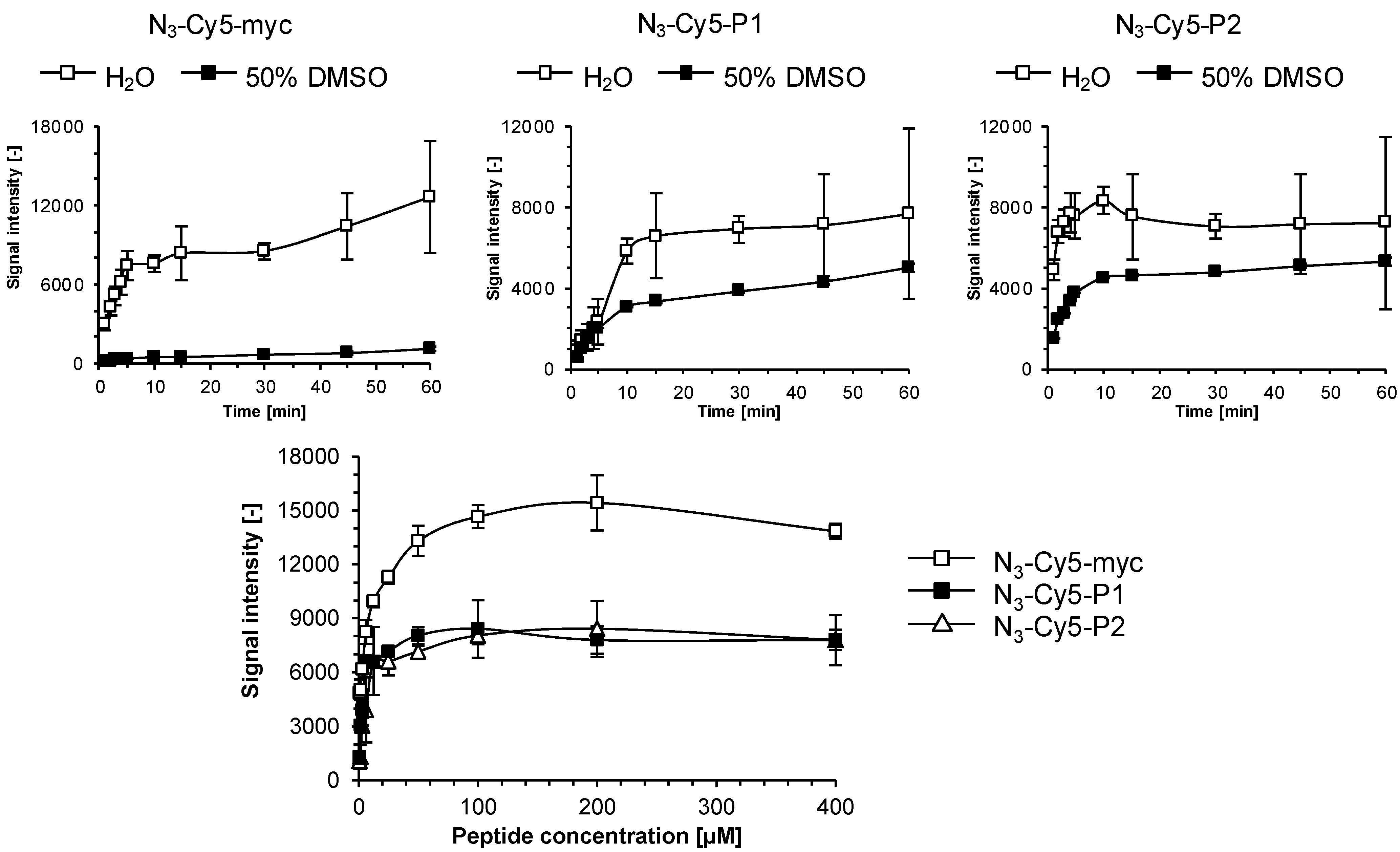
2.2. Immunoassay Performance Comparison
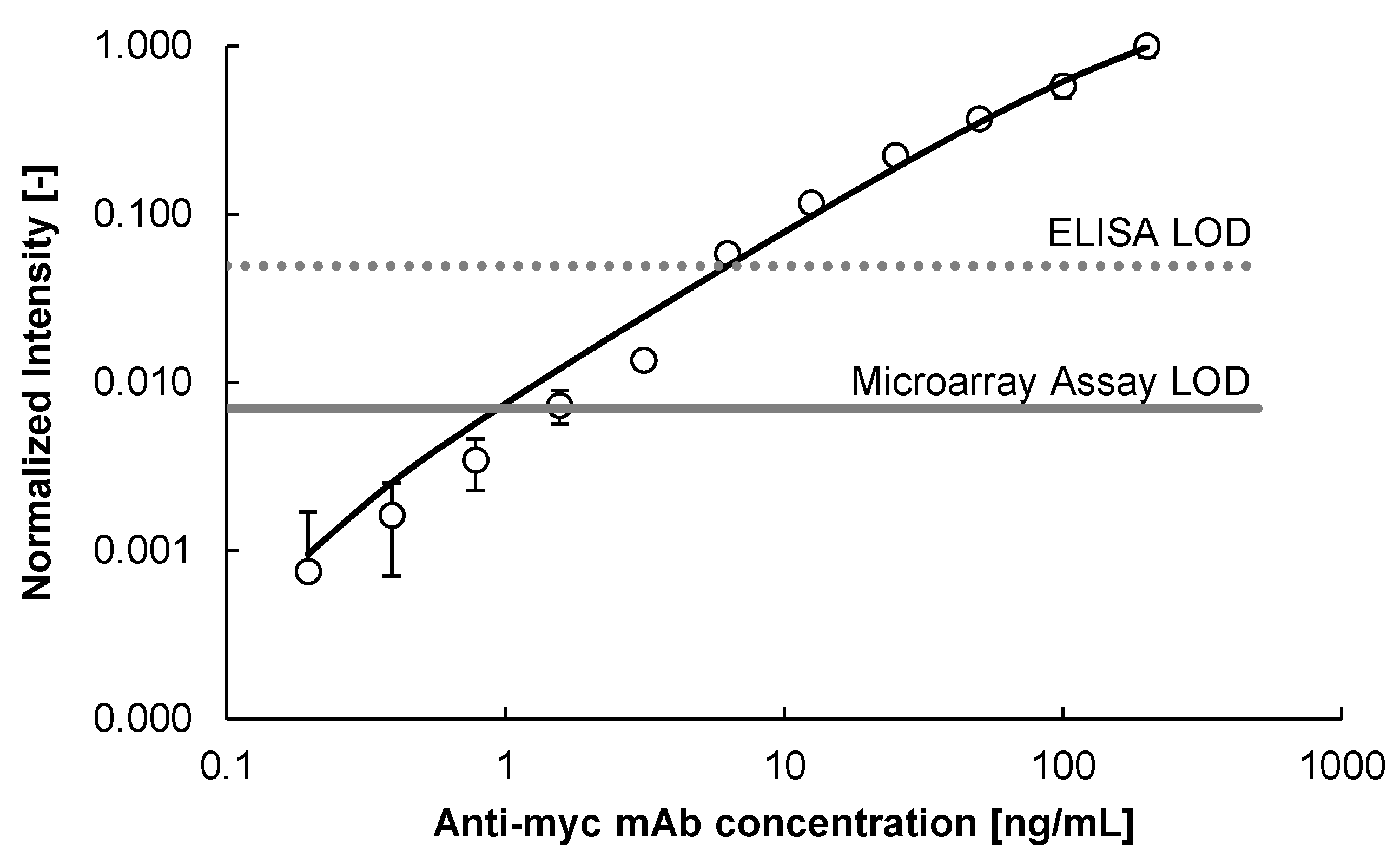
2.3. Blocking Reagents to Reduce Non-Specific Binding (NSB)
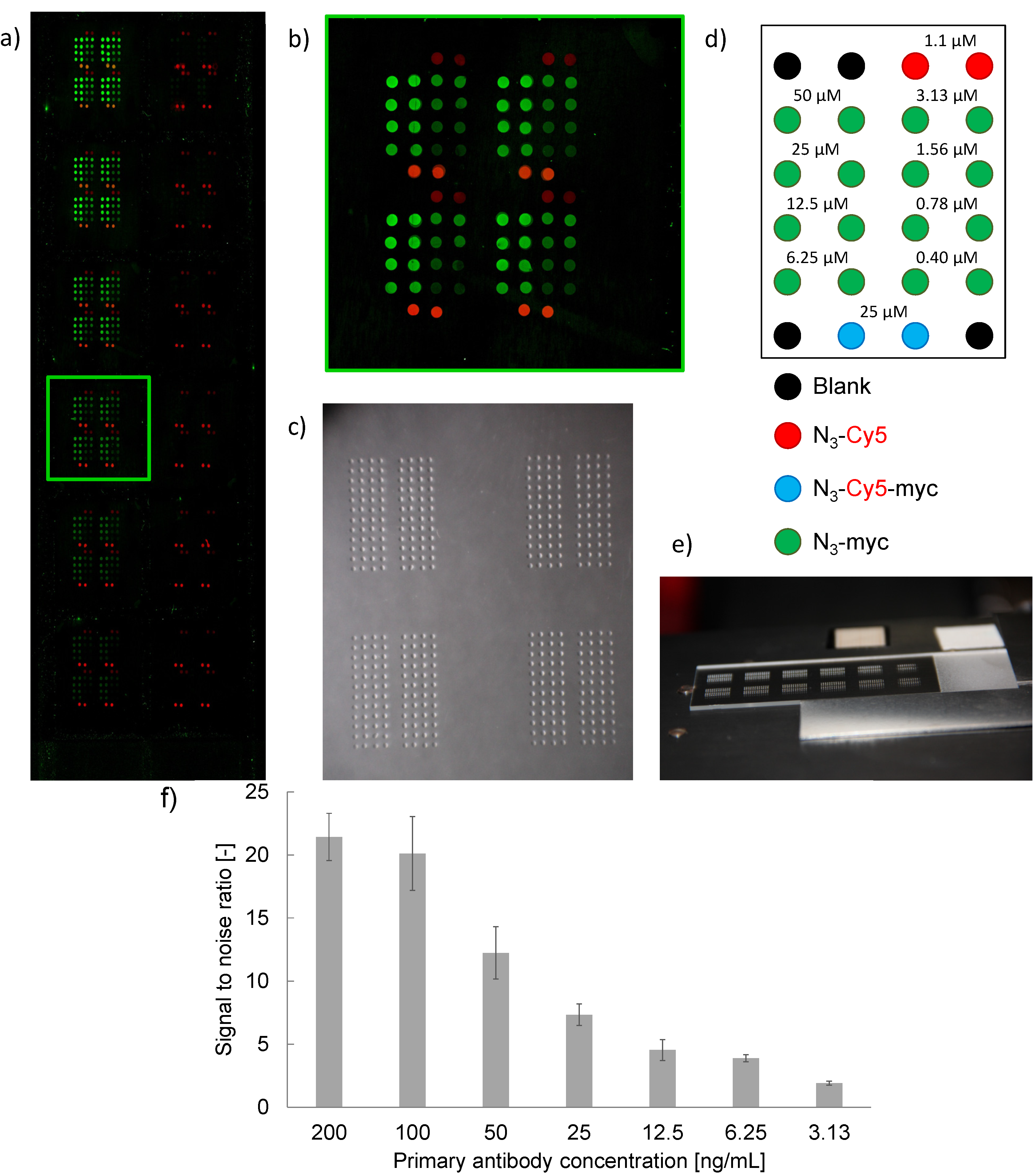
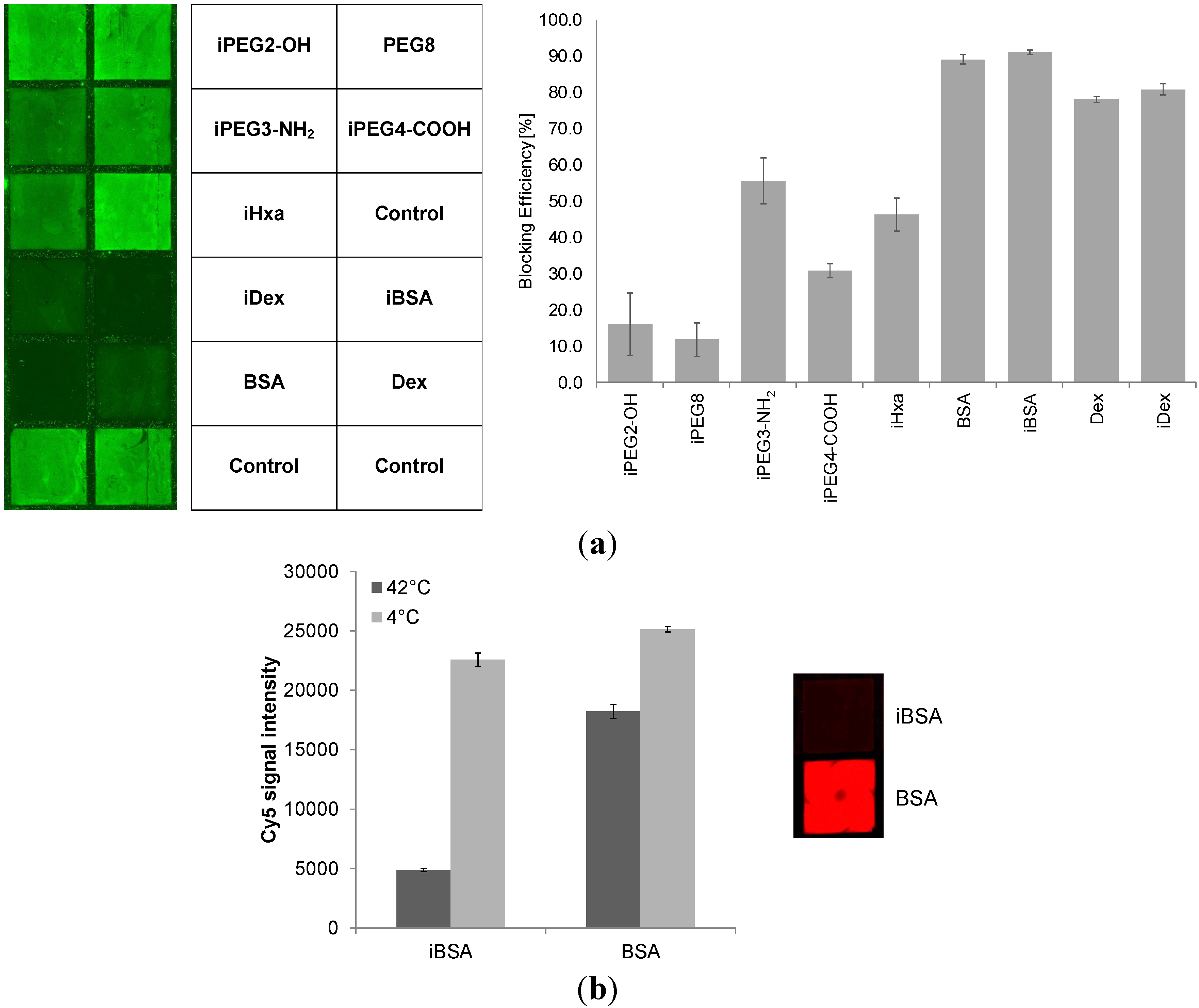
 |
3. Experimental
3.1. General information, Peptide Synthesis and Modifications
3.2. Blocking Reagents
3.3. Sequential Microarray Spotting
3.4. Blocking Efficiency
3.5. Fluorescence Immunoassay
3.6. Streptavidin-Biotin ELISA
3.7. Automated Microarray Pattern Printing
3.8. Microarray Spot Fluorescence Measurements
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sobek, J.; Bartscherer, K.; Jacob, A.; Hoheisel, J.D.; Angenendt, P. Microarray technology as a universal tool for high-throughput analysis of biological systems. Comb. Chem. High Throughput Screening 2006, 9, 365–380. [Google Scholar] [CrossRef]
- Min, D.H.; Mrksich, M. Peptide arrays: Towards routine implementation. Curr. Opin. Chem. Biol. 2004, 8, 554–558. [Google Scholar] [CrossRef]
- Fodor, S.P.A.; Read, J.L.; Pirrung, M.C.; Stryer, L.; Lu, A.T.; Solas, D. Light-directed, spatially addressable parallel chemical synthesis. Science 1991, 251, 767–773. [Google Scholar]
- Foong, Y.M.; Fu, J.; Yao, S.Q.; Uttamchandani, M. Current advances in peptide and small molecule microarray technologies. Curr. Opin. Chem. Biol. 2012, 16, 234–242. [Google Scholar] [CrossRef]
- Linnebacher, M.; Lorenz, P.; Koy, C.; Jahnke, A.; Born, N.; Steinbeck, F.; Wollbold, J.; Latzkow, T.; Thiesen, H.J.; Glocker, M.O. Clonality characterization of natural epitope-specific antibodies against the tumor-related antigen topoisomerase IIa by peptide chip and proteome analysis: A pilot study with colorectal carcinoma patient samples. Anal. Bioanal. Chem. 2012, 403, 227–238. [Google Scholar] [CrossRef]
- Lin, J.; Bardina, L.; Shreffler, W.G.; Andreae, D.A.; Ge, Y.; Wang, J.; Bruni, F.M.; Fu, Z.; Han, Y.; Sampson, H.A. Development of a novel peptide microarray for large scale epitope mapping of food allergens. J. Allergy Clin. Immunol. 2009, 124, 315–322. [Google Scholar] [CrossRef]
- Hecker, M.; Lorenz, P.; Steinbeck, F.; Hong, L.; Riemekasten, G.; Li, Y.; Zettl, U.; Thiesen, H.J. Computational analysis of high-density peptide microarray data with application from systemic sclerosis to multiple sclerosis. Autoimmun. Rev. 2012, 11, 180–190. [Google Scholar] [CrossRef]
- Duburcq, X.; Olivier, C.; Malingue, F.; Desmet, R.; Bouzidi, A.; Zhou, F.; Auriault, F.; Gras-Masse, H.; Melnyk, O. Peptide-protein microarrays for the simultaneous detection of pathogen infections. Bioconjug. Chem. 2004, 15, 307–316. [Google Scholar] [CrossRef]
- Reimer, U.; Reineke, U.; Schneider-Mergener, J. Peptide arrays: from macro to micro. Curr. Opin. Biotechnol. 2002, 13, 315–320. [Google Scholar]
- Volkmer, R.; Tapia, V.; Landgraf, C. Synthetic peptide arrays for investigating protein interaction domains. FEBS Lett. 2012, 586, 2780–2786. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Shin, D.S.; Kim, D.H.; Chung, W.J.; Lee, Y.S. Combinatorial solid phase peptide synthesis and bioassays. J. Biochem. Mol. Biol. 2005, 38, 517–525. [Google Scholar] [CrossRef]
- Köhn, M. Immobilization strategies for small molecule, peptide and protein microarrays. J. Pept. Sci. 2009, 15, 393–397. [Google Scholar] [CrossRef]
- Lahaan, J. Click Chemistry for Biotechnology and Materials Science; Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Kuzmin, A.; Poloukhtine, A.; Wolfert, M.A.; Popik, V.V. Surface functionalization using catalyst-free azide-alkyne cycloaddition. Bioconjug. Chem. 2010, 21, 2076–2085. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Lee, I.; Song, Y.; Qin, X.; Zaera, F.; Liao, J. Chemoselective fabrication of high density peptide microarray by hetero-bifunctional tetra (ethylene glycol) linker for click chemistry conjugation. J. Biomed. Mater. Res. 2012, 100A, 103–110. [Google Scholar] [CrossRef]
- Köhn, M.; Wacker, R.; Peters, C.; Schröder, H.; Soulère, L.; Breinbauer, R.; Niemeyer, C.M.; Waldmann, H. Staudinger ligation: A new immobilization strategy for the preparation of small-molecule arrays. Angew. Chem. Int. Ed. Engl. 2003, 42, 5830–5834. [Google Scholar] [CrossRef]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar]
- Debets, M.F.; van der Doelen, C.W.J.; Rutjes, F.P.J.T.; van Delft, F.L. Azide: A unique dipole for metal-free bioorthogonal ligations. ChemBioChem 2010, 11, 1168–1184. [Google Scholar]
- Cosandey, V.; Debrot, F.; Kaeser, J.; Marti, R.; Passeraub, P.; Pétremand, J.; Prim, D.; Pfeifer, M.E. Construction of a peptide microarray for auto-antibody detection. Chimia 2012, 66, 803–806. [Google Scholar] [CrossRef]
- Prim, D.; Pfeifer, M.E.; University of Applied Sciences Western Switzerland, Sion, Switzerland. Personal communication, 2011.
- Cosandey, V.; Pfeifer, M.E.; University of Applied Sciences Western Switzerland, Sion, Switzerland. Unpublished work. 2013.
- O’Connell, M.A.; Belanger, B.A.; Haaland, P.D. Calibration and assay development using the four-parameter logistic model. Chemom. Intell. Lab. Syst. 1993, 20, 97–114. [Google Scholar] [CrossRef]
- Yang, Z.; Chevolot, Y.; Géhin, T.; Solassol, J.; Mange, A.; Souteyrand, E.; Laurenceau, E. Improvement of protein immobilization for the elaboration of tumor-associated antigen microarrays: Application to the sensitive and specific detection of tumor markers from breast cancer sera. Biosens. Bioelectron. 2013, 40, 385–392. [Google Scholar] [CrossRef]
- Piehler, J.; Brecht, A.; Valiokas, R.; Liedberg, B.; Gauglitz, G. A high-density poly (ethylene glycol) polymer brush for immobilization on glass-type surfaces. Biosens. Bioelectron. 2000, 15, 473–481. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Adsorption of proteins onto surfaces containing end-attached oligo (ethylene oxide): A model system using self-assembled monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Kannan, B.; Castelino, K.; Chen, F.F.; Majumdar, A. Lithographic techniques and surface chemistries for the fabrication of PEG-passivated protein microarrays. Biosens. Bioelectron. 2006, 21, 1960–1967. [Google Scholar] [CrossRef]
- Kingshott, P.; Thissen, H.; Griesser, H.J. Effects of cloud-point grafting, chain length, and density of PEG layers on competitive adsorption of ocular proteins. Biomaterials 2002, 23, 2043–2056. [Google Scholar] [CrossRef]
- Wolter, A.; Niessner, R.; Seidel, M. Preparation and characterization of functional poly (ethylene glycol) surfaces for the use of antibody microarrays. Anal. Chem. 2007, 79, 4529–4537. [Google Scholar] [CrossRef]
- Ferrer, M.C.C.; Yang, S.; Eckmann, D.M.; Composto, R.J. Creating biomimetic polymeric surfaces by photochemical attachment and patterning of dextran. Langmuir 2010, 26, 14126–14134. [Google Scholar] [CrossRef]
- Kröger, K.; Bauer, J.; Fleckenstein, B.; Rademann, J.; Jung, G.; Gauglitz, G. Epitope-mapping of transglutaminase with parallel label-free optical detection. Biosens. Bioelectron. 2002, 17, 937–944. [Google Scholar] [CrossRef]
- Masson, J.F.; Battaglia, T.M.; Davidson, M.J.; Kim, Y.C.; Prakash, A.M.C.; Beaudoin, S.; Booksh, K.S. Biocompatible polymers for antibody support on gold surfaces. Talanta 2005, 67, 918–925. [Google Scholar] [CrossRef]
- Grandjean, C.; Boutonnier, A.; Guerreiro, C.; Fournier, J.M.; Mulard, L.A. On the preparation of carbohydrate—Protein conjugates using the traceless staudinger ligation. J. Org. Chem. 2005, 70, 7123–7132. [Google Scholar] [CrossRef]
- Annessi-Ramseyer, I.; Passeraub, P.; Turck, N.; Borradori, L.; Favre, B.; Hochstrasser, D.F.; Fontao, L.; Zimmermann-Ivol, C.G. Multiple autoantibodies detection for bullous pemphigoid diagnosis by protein microarray. In Proceedings of HUPO 10th Annual World Conference, Geneva, Switzerland, 4–7 September 2011.
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prim, D.; Rebeaud, F.; Cosandey, V.; Marti, R.; Passeraub, P.; Pfeifer, M.E. ADIBO-Based “Click” Chemistry for Diagnostic Peptide Micro-Array Fabrication: Physicochemical and Assay Characteristics. Molecules 2013, 18, 9833-9849. https://doi.org/10.3390/molecules18089833
Prim D, Rebeaud F, Cosandey V, Marti R, Passeraub P, Pfeifer ME. ADIBO-Based “Click” Chemistry for Diagnostic Peptide Micro-Array Fabrication: Physicochemical and Assay Characteristics. Molecules. 2013; 18(8):9833-9849. https://doi.org/10.3390/molecules18089833
Chicago/Turabian StylePrim, Denis, Fabien Rebeaud, Vincent Cosandey, Roger Marti, Philippe Passeraub, and Marc E. Pfeifer. 2013. "ADIBO-Based “Click” Chemistry for Diagnostic Peptide Micro-Array Fabrication: Physicochemical and Assay Characteristics" Molecules 18, no. 8: 9833-9849. https://doi.org/10.3390/molecules18089833
APA StylePrim, D., Rebeaud, F., Cosandey, V., Marti, R., Passeraub, P., & Pfeifer, M. E. (2013). ADIBO-Based “Click” Chemistry for Diagnostic Peptide Micro-Array Fabrication: Physicochemical and Assay Characteristics. Molecules, 18(8), 9833-9849. https://doi.org/10.3390/molecules18089833