Identification of Compounds from the Water Soluble Extract of Cinnamomum cassia Barks and Their Inhibitory Effects against High-Glucose-Induced Mesangial Cells
Abstract
:1. Introduction
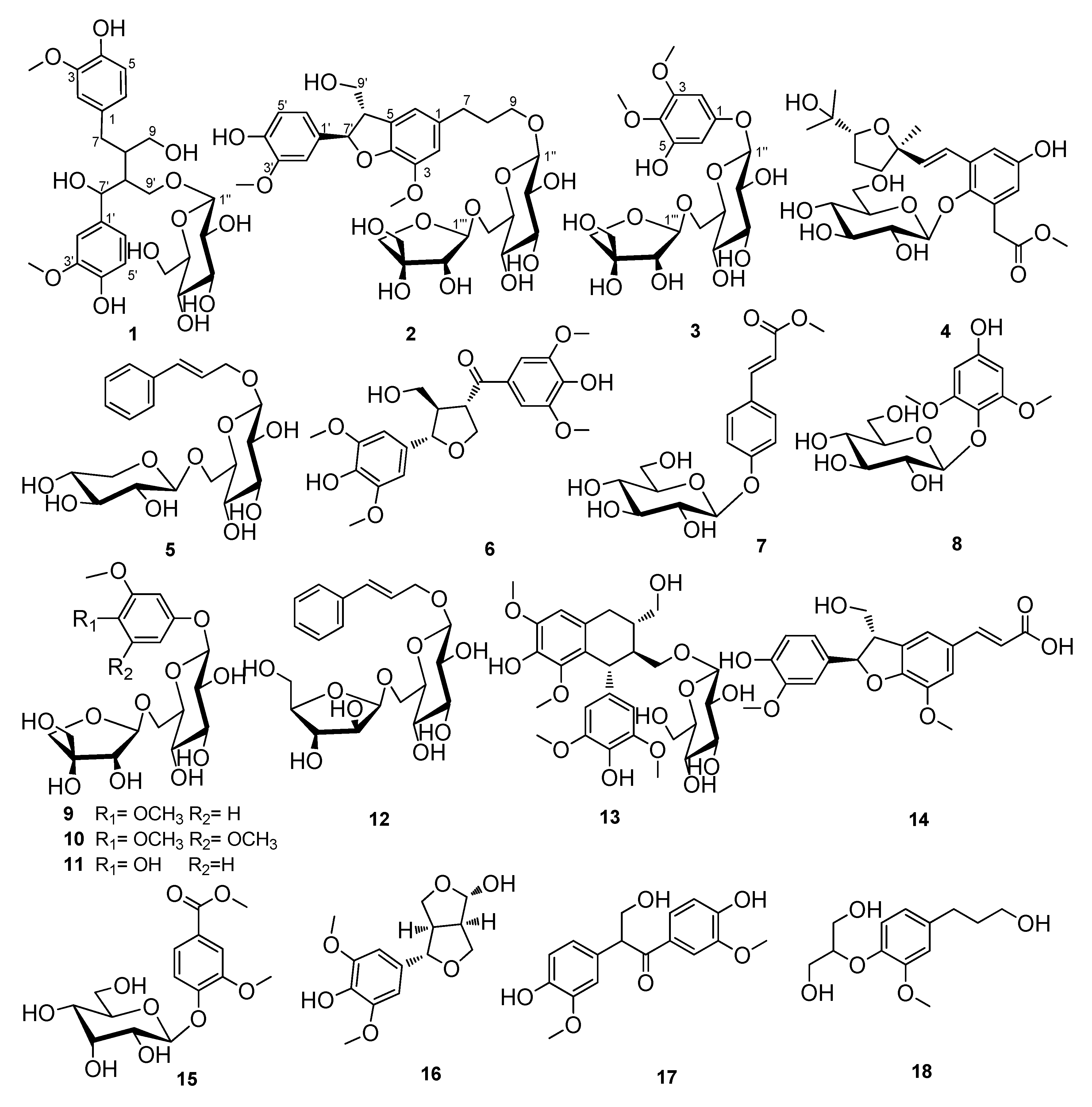
2. Results and Discussion
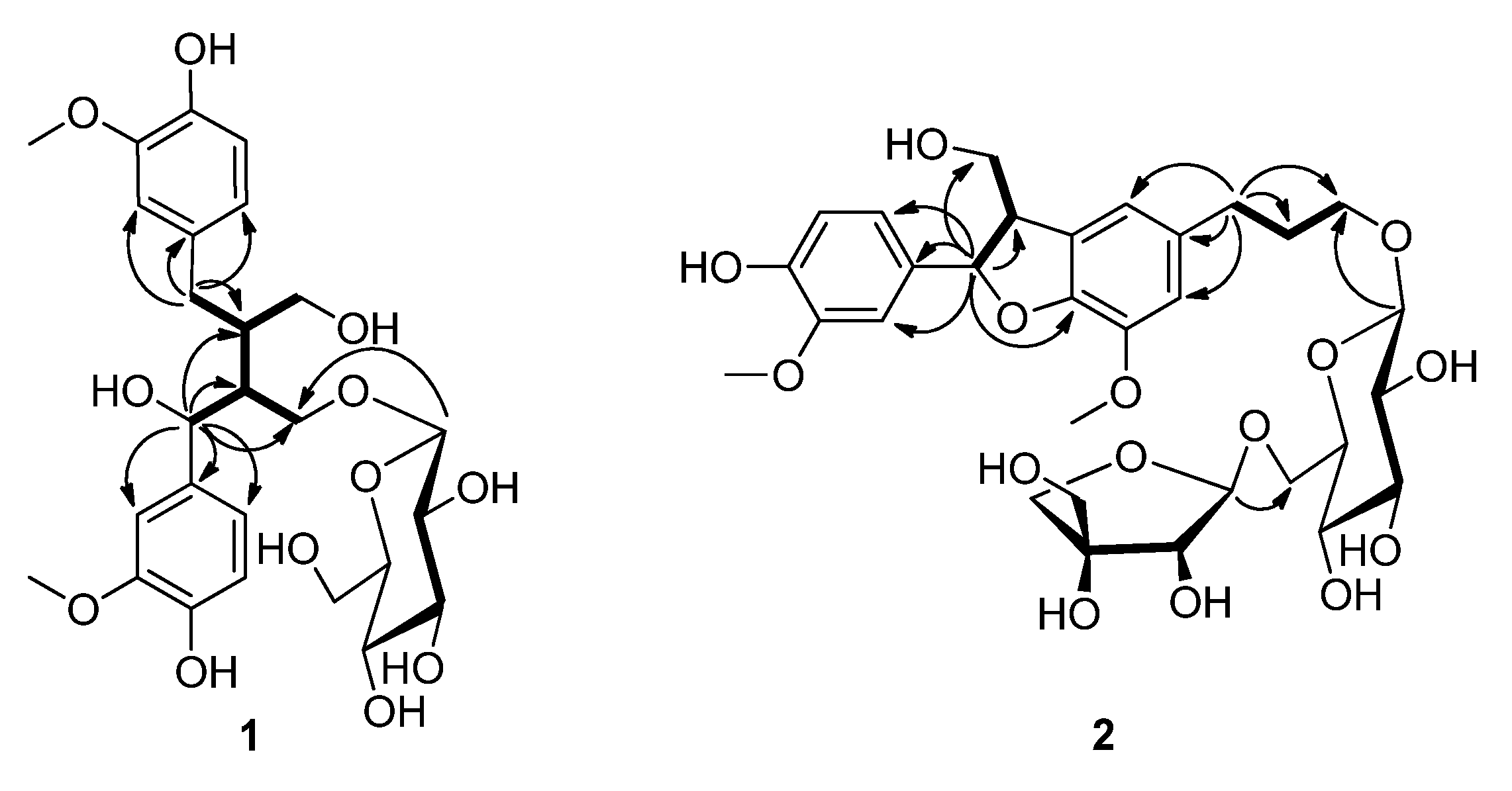
2.1. Structural Identification
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, mult | δH (J in Hz) | δC, mult | δH (J in Hz) | δC, mult | |
| 1 | 131.9, qC | 137.1, qC | 155.8, qC | |||
| 2 | 6.74, d, 1.5 | 112.7, CH2 | 6.76, s | 114.2, CH | 6.30, s | 95.2, CH |
| 3 | 147.5, qC | 145.3, qC | 154.8, qC | |||
| 4 | 144.6, qC | 147.6, qC | 133.2, qC | |||
| 5 | 6.66, d, 8.0 | 115.4, CH | 129.9, qC | 152.0, qC | ||
| 6 | 6.57, dd, 8.0, 1.5 | 120.6, CH | 6.75, s | 118.1, qC | 6.30, s | 99.1, CH |
| 7 | 2.83, dd, 13.5, 4.4 | 32.2, CH2 | 2.69, dd, 13.8, 6.6 | 33.1, CH2 | ||
| 2.41, m | ||||||
| 8 | 2.57, m | 42.1, CH | 1.91, m | 33.2, CH2 | ||
| 9 | 3.85, t, 7.4 | 72.0, CH2 | 3.90, m | 70.3, CH2 | ||
| 3.57, t, 7.4 | 3.54, m | |||||
| 1′ | 134.5, qC | 134.9, qC | ||||
| 2′ | 6.84, d, 1.5 | 110.0, CH2 | 6.95, d, 2.0 | 110.6, CH | ||
| 3′ | 147.5, qC | 149.2, qC | ||||
| 4′ | 145.6, qC | 147.6, qC | ||||
| 5′ | 6.70, d, 8.0 | 115.1, CH | 6.77, d, 8.0 | 116.2, CH | ||
| 6′ | 6.70, d, 8.0 | 118.3, CH | 6.82, dd, 8.0, 2.0 | 119.8, CH | ||
| 7′ | 4.72, d, 6.0 | 81.9, CH | 5.50, d, 6.3 | 89.2, CH | ||
| 8′ | 2.30, m | 50.0, CH | 3.48, m | 55.6, CH | ||
| 9′ | 4.05, dd, 9.0, 7.8 | 66.8, CH2 | 3.86, m, | 65.1, CH2 | ||
| 3.43, m | 3.76, m | |||||
| 1′′ | 4.17, d, 7.8 | 103.2, CH | 4.24, d, 7.8 | 104.7, CH | 4.75, d, 7.5 | 103.0, CH |
| 2′′ | 2.79, m | 73.6, CH | 3.19, m | 75.3, CH | 3.75, d, 9.8 | 74.9, CH |
| 3′′ | 3.10, m | 76.9, CH | 3.40, m | 77.0, CH | 3.92, m | 78.0, CH |
| 4′′ | 3.05, m | 70.1, CH | 3.28, m | 71.8, CH | 3.32, m | 71.7, CH |
| 5′′ | 3.12, m | 77.0, CH | 3.90, m | 78.2, CH | 3.56, m | 77.1, CH |
| 6′′ | 3.66, d,10.3 | 61.2, CH2 | 3.98, dd, 11.2, 1.8 | 68.8, CH2 | 4.03, d, 10.7 | 68.7, CH2 |
| 3.43, m | 3.61, m | 3.60, m | ||||
| 1′′′ | 5.01, d, 2.4 | 111.1, CH2 | 4.98, d, 2.5 | 111.0, CH | ||
| 2′′′ | 3.33, m | 78.1, CH2 | 3.42, d, 8.6 | 78.0, CH | ||
| 3′′′ | 80.7, qC | 80.5, CH | ||||
| 4′′′ | 3.96, d, 9.7 | 75.1, CH2 | 3.98, d, 9.6 | 75.0, CH2 | ||
| 3.75, m | 3.76, d, 7.4 | |||||
| 5′′′ | 3.56, m | 65.6, CH2 | 3.58, 2H, m | 65.7, CH2 | ||
| 3-OCH3 | 3.74, 3H, s | 55.6, CH3 | 3.82, s | 56.5, CH3 | 3.80, s | 56.5, CH3 |
| 3′-OCH3 | 3.73, 3H, s | 55.6, CH3 | 3.86, s | 56.9, CH3 | ||
| 4-OCH3 | 3.72, s | 61.1, CH3 | ||||
2.2. Inhibition of Fibronectin, Collagen IV, and IL-6 Secretion
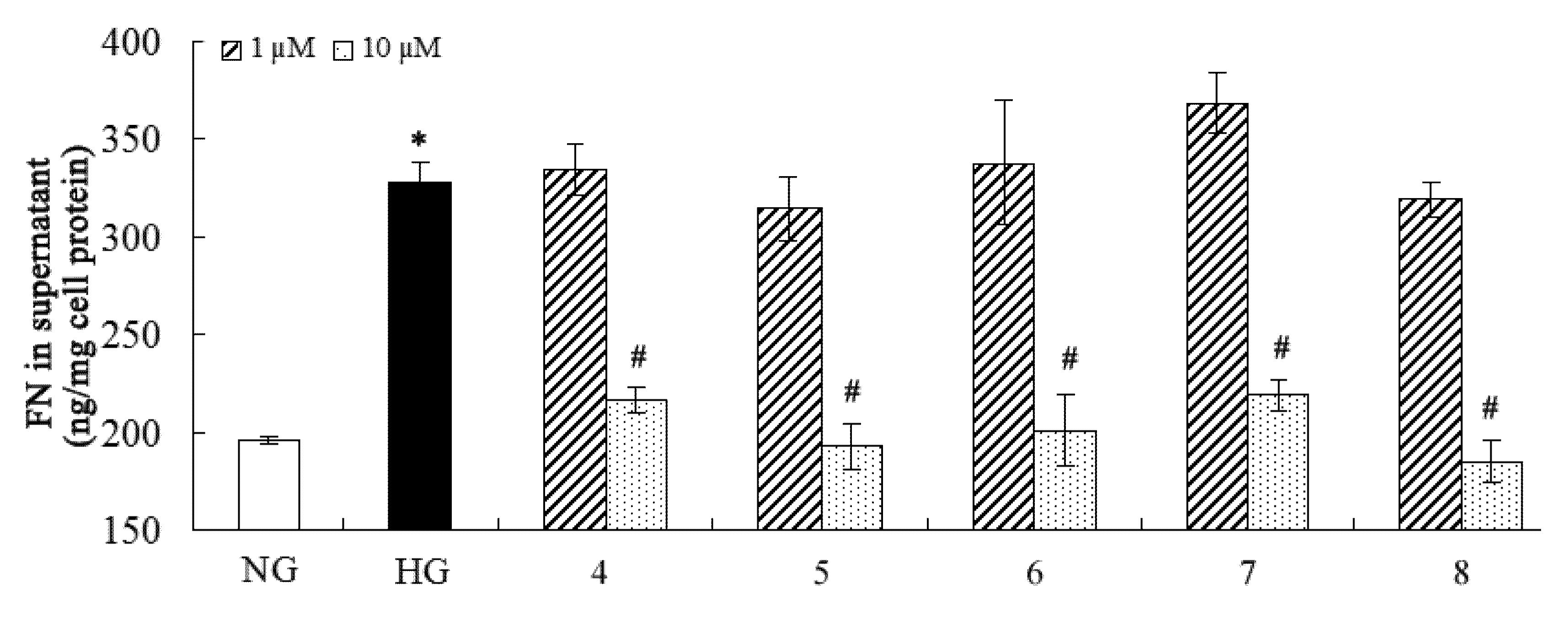
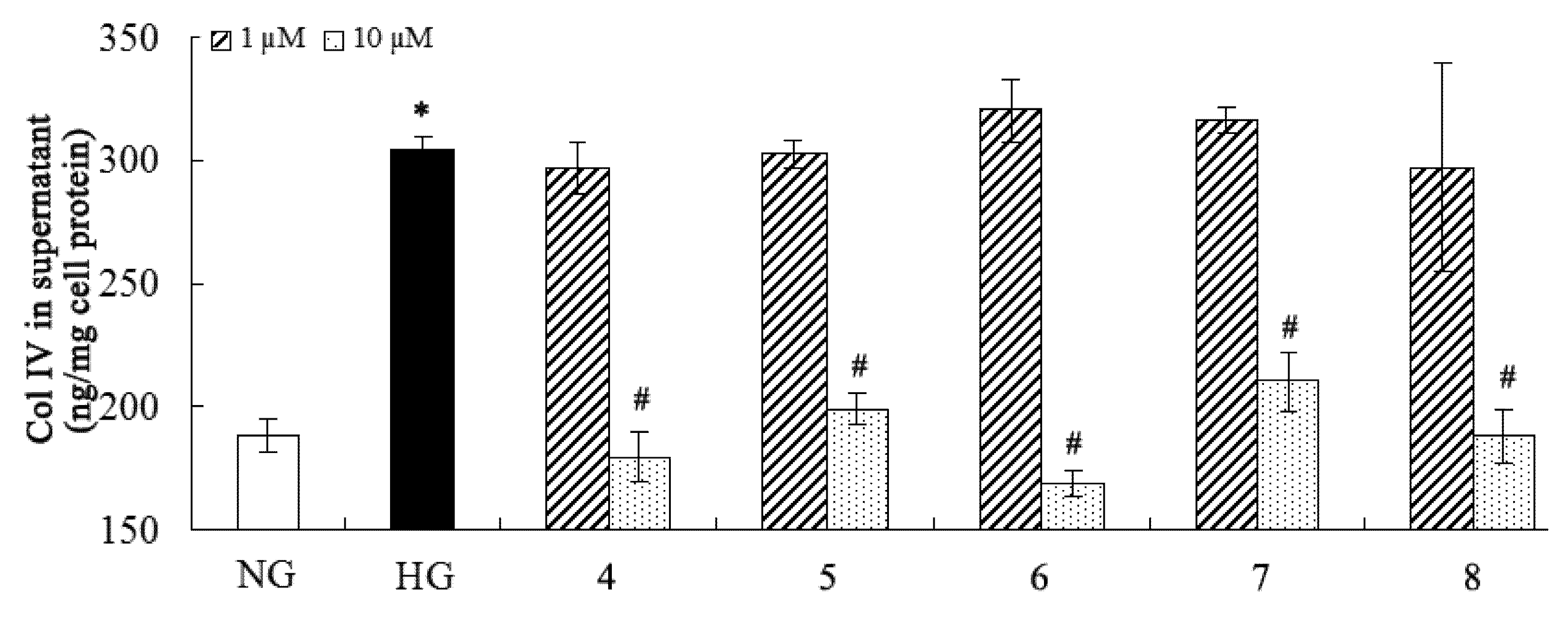
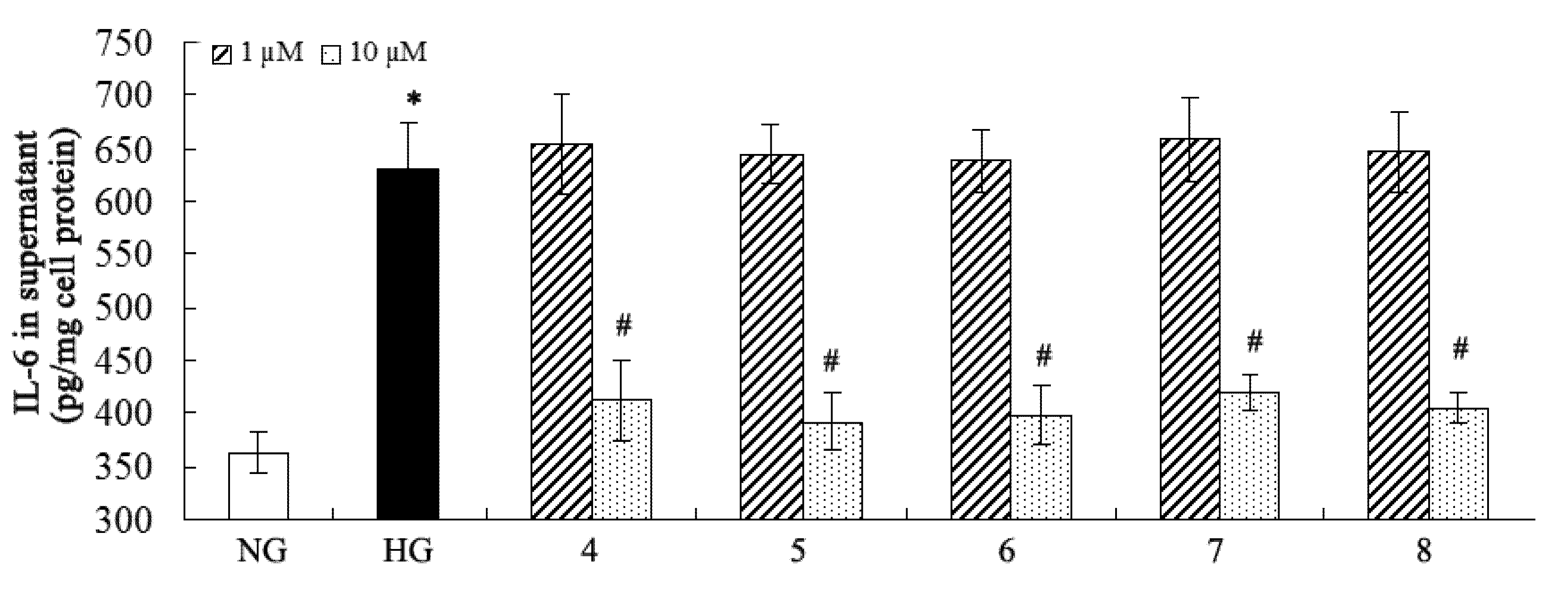
3. Experimental
3.1. Equipment
3.2. Plant Material
3.3. Extraction and Isolation
 −31.9 (c 0.2, MeOH); UV (MeOH) λmax (log ε): 203 (4.65), 226 (4.06), 281 (3.64) nm;1H (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) data, see Table 1; EI-MS: m/z 540 [M]+; HREIMS: m/z 540.2180 [M]+ (calcd for C26H36O12, 540.2207).
−31.9 (c 0.2, MeOH); UV (MeOH) λmax (log ε): 203 (4.65), 226 (4.06), 281 (3.64) nm;1H (DMSO-d6, 600 MHz) and 13C-NMR (DMSO-d6, 150 MHz) data, see Table 1; EI-MS: m/z 540 [M]+; HREIMS: m/z 540.2180 [M]+ (calcd for C26H36O12, 540.2207). −38.0 (c 0.2, MeOH); UV (MeOH) λmax (log ε): 205 (4.74), 283 (3.72) nm;1H (CD3OD, 600 MHz) and 13C-NMR (CD3OD, 150 MHz) data, see Table 1; EIMS: m/z 654 [M]+; HREIMS: m/z 654.2521 [M]+, calcd for C31H42O15, 654.2524).
−38.0 (c 0.2, MeOH); UV (MeOH) λmax (log ε): 205 (4.74), 283 (3.72) nm;1H (CD3OD, 600 MHz) and 13C-NMR (CD3OD, 150 MHz) data, see Table 1; EIMS: m/z 654 [M]+; HREIMS: m/z 654.2521 [M]+, calcd for C31H42O15, 654.2524). −82.6 (c 0.2, MeOH); UV (MeOH) λmax (log ε): 204 (4.54), 271 (3.01) nm;1H (CD3OD, 600 MHz) and 13C-NMR (CD3OD, 125 MHz) data, see Table 1; EI-MS: m/z 464 [M]+; HREIMS: m/z 464.1519 [M]+ (calcd for C19H28O13, 464.1530).
−82.6 (c 0.2, MeOH); UV (MeOH) λmax (log ε): 204 (4.54), 271 (3.01) nm;1H (CD3OD, 600 MHz) and 13C-NMR (CD3OD, 125 MHz) data, see Table 1; EI-MS: m/z 464 [M]+; HREIMS: m/z 464.1519 [M]+ (calcd for C19H28O13, 464.1530).3.4. Acid Hydrolysis of 1-3
 +37 (c 0.06, H2O); apiose: EtOAC–MeOH–H2O (4:1:0.1), Rf 0.5,
+37 (c 0.06, H2O); apiose: EtOAC–MeOH–H2O (4:1:0.1), Rf 0.5,  +5(c 0.05, H2O)].
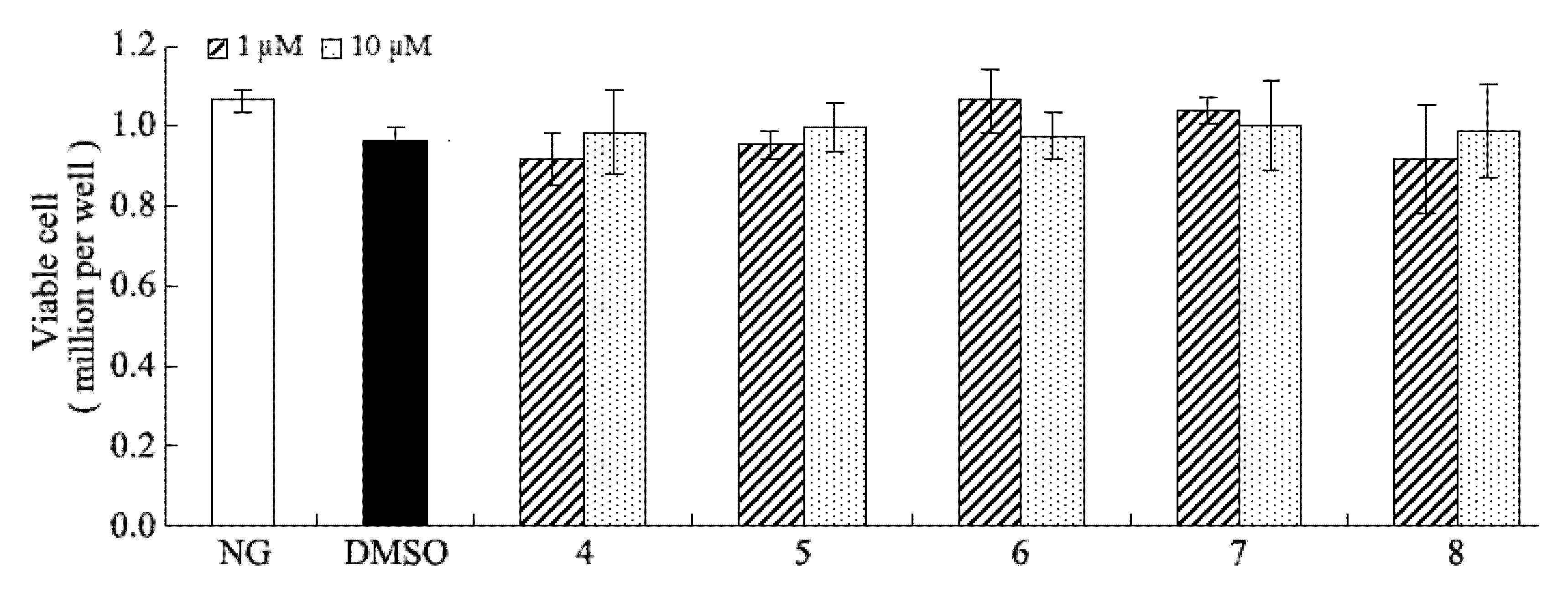
+5(c 0.05, H2O)].3.5. Inhibition of Fibronectin, Collagen IV, and IL-6 Secretion
3.6. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References and Notes
- Ha, H.; Hwang, I.A.; Park, J.H.; Lee, H.B. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res. Clin. Pract. 2008, 82s, s42–s45. [Google Scholar]
- Navarro-González, J.F.; Mora-Fernández, C. The role of inflammatory cytokines in diabetic nephropathy. J. Amer. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef]
- Sriniva Navarro, J.F.; Mora, C. Role of inflammation in diabetic complications. Nephrol. Dialysis Transplant. 2005, 20, 2601–2604. [Google Scholar] [CrossRef]
- San, K. Role of spices beyond food flavoring: Nutraceuticals with multiple health effects. Food Rev. Int. 2005, 21, 167–188. [Google Scholar] [CrossRef]
- Woehrlin, F.; Fry, H.; Abraham, K.; Preiss-Weigert, A. Quantification of flavoring constituents in Cinnamon: High variation of coumarin in cassia bark from the German retail market and in authentic samples from Indonesia. J. Agric. Food Chem. 2010, 58, 10568–10575. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Nirmal Babu, K. Introduction. In Cinnamon and Cassia: The Genus Cinnamomum; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–13. [Google Scholar]
- Chen, L.; Sun, P.; Wang, T.; Chen, K.X.; Jia, Q.; Wang, H.Y.; Li, Y.M. Diverse mechanisms of antidiabetic effects of the different procyanidin oligomer types of two different cinnamon species on db/db mice. J. Agric. Food Chem. 2012, 60, 9144–9150. [Google Scholar]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Lila, M.A.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef]
- Khan, A.; Safdar, M.; Khan, M.H.A.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef]
- Li, X.W.; Li, J.; Werff, H. “Cinnamomum cassia”. Flora of China; Missouri Botanical Garden: St. Louis, MO, USA; Harvard University Herbaria: Cambridge, MA, USA, 2013. [Google Scholar]
- U.S. Food and Drug Administration. Inspections, Compliance, Enforcement, and 347 Criminal Investigations: CPG Sec. 525.750 Spices – Definitions: Cinnamon (Cassia). 348 1980.
- Nohara, T.; Kashiwada, Y.; Nishioka, I.; Cinncassiol, E. A diterpene from the bark of Cinnamomum cassia. Phytochemistry 1985, 24, 1849–1850. [Google Scholar]
- Nohara, T.; Kashiwada, Y.; Tomimatsu, T.; Nishioka, I. Two novel diterpenes from bark of Cinnamomum cassia. Phytochemistry 1982, 21, 2130–2132. [Google Scholar]
- Lin, R.J.; Cheng, M.J.; Huang, J.C.; Lo, W.L.; Yeh, Y.T.; Yen, C.M.; Lu, C.M.; Chen, C.Y. Cytotoxic compounds from the stems of Cinnamomum tenuifolium. J. Nat. Prod. 2009, 72, 1816–1824. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Lee, I.; Ha, D.T.; Kim, H.; Min, B.; Bae, K. Tyrosinase-inhibitory constituents from the twigs of Cinnamomum cassia. J. Nat. Prod. 2009, 72, 1205–1208. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Dong, X.; Jiang, G.; Zhang, H.; Xie, H.; Jiang, Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov. Food Sci. Emerg. Technol. 2009, 10, 627–632. [Google Scholar] [CrossRef]
- Miyamura, M.; Nohara, T.; Tomimatsu, T.; Nishiokat, I. Seven aromatic compounds from bark of Cinnamomum cassia. Phytochemistry 1983, 22, 215–218. [Google Scholar]
- Ooi, L.S.; Li, Y.; Kam, S.L.; Wang, H.; Wong, E.Y.; Ooi, V.E. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006, 34, 511–522. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, S.J.; Chang, C.H.; Ng, L.T. Antioxidant activity of Cinnamomum cassia. Phytother. Res. 2003, 17, 726–730. [Google Scholar] [CrossRef]
- Shiraga, Y.; Okano, K.; Akira, T.; Fukaya, C.; Yokoyama, K.; Tanaka, S.; Fukui, H.; Tabata, M. Structures of potent antiulcerogenic compounds from Cinnamomum cassia. Tetrahedron 1988, 44, 4703–4711. [Google Scholar] [CrossRef]
- Verspohl, E.J.; Bauer, K.; Neddermann, E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother. Res. 2005, 19, 203–206. [Google Scholar] [CrossRef]
- Nagai, H.; Shimazawa, T.; Matsuura, N.; Koda, A. Immunopharmacological studies of the aqueous extract of Cinnamomum cassia (CCAq). I. Anti-allergic action. Jpn. J. Pharmacol. 1982, 32, 813. [Google Scholar] [CrossRef]
- Zheng, H.T.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.Y.; Zhang, D.D. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.D.; Wu, X.D. New lignan glucosides with tyrosinase inhibitory activities from exocarp of Castanea henryi. Carbohyd. Res. 2012, 335, 45–49. [Google Scholar]
- Jiang, J.; Feng, Z.; Wang, Y.; Zhang, P. New phenolics from the roots of Symplocos caudata Wall. Chem. Pharm. Bull. 2005, 53, 110–113. [Google Scholar] [CrossRef]
- Fang, J.M.; Lee, C.K.; Cheng, Y.S. Lignans from leaves of Juniperus chinensis. Phytochemistry 1992, 31, 3659–3661. [Google Scholar] [CrossRef]
- Liu, J.F.; Jiang, Z.Y.; Geng, C.A.; Zhang, Q.; Shi, Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Two new lignans and anti-HBV constituents from Illicium henryi. Chem. Biodivers. 2011, 8, 692–698. [Google Scholar] [CrossRef]
- Liao, S.G.; Yuan, T.; Zhang, C.; Yang, S.P.; Wu, Y.; Yue, J.M. Cinnacassides A-E, five geranylphenylacetate glycosides from Cinnamomum cassia. Tetrahedron 2009, 65, 883–887. [Google Scholar] [CrossRef]
- Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2003, 51, 467–470. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, C.; Li, Y.; Tian, Y.; Lin, S.; Yuan, S.; Hu, J.; Hou, Q.; Chen, N.; Yang, Y. Lignans and neolignans from Sinocalamus affinis and their absolute configurations. J. Nat. Prod. 2011, 74, 1188–1200. [Google Scholar] [CrossRef]
- Luyengi, L.; Pezzuto, J.M.; Waller, D.P.; Beecher, C.W.; Fong, H.H.; Che, C.T.; Bowen, P.E. Linusitamarin, a new phenylpropanoid glucoside from Linum usitatissimum. J. Nat. Prod. 1993, 56, 2012–2015. [Google Scholar] [CrossRef]
- Luecha, P.; Umehara, K.; Miyase, T.; Noguchi, H. Antiestrogenic constituents of the Thai medicinal plants Capparis flavicans and Vitex glabrata. J. Nat. Prod. 2009, 72, 1954–1959. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic diglycosides from Canthium berberidifolium. Phytochemistry 2002, 61, 461–464. [Google Scholar] [CrossRef]
- Achenbach, H.; Löwel, M.; Waibel, R.; Gupta, M.; Solis, P. New lignan glucosides from Stemmadenia minima. Planta Med. 2007, 58, 270–272. [Google Scholar]
- Zheng, J.; Chen, G.T.; Gao, H.Y.; Wu, B.; Wu, L.J. Two new lignans from Mentha spicata L. J. Asian Nat. Prod. Res. 2007, 9, 431–435. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Gong, Y.; Yu, B. Synthesis of oligomeric 4-(glycosyloxy) benzoate macrocyclic glycosides. J. Org. Chem. 2011, 76, 3654–3663. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, Q.; Fu, J.J.; Kou, Z.J.; Chang, R.J.; Jin, H.Z.; Zhang, W.D. Chemical constituents from Tsoongiodendron odorum Chun. Biochem. Syst. Ecol. 2011, 39, 209–212. [Google Scholar] [CrossRef]
- Wu, T.S.; Yeh, J.H.; Wu, P.L. The heartwood constituents of tetradium glabrifolium. Phytochemistry 1995, 40, 121–124. [Google Scholar]
- Kouno, I.; Yukari, Y.G.; Satomi, S.M.; Miki, S.; Yang, C.S. Phenylpropanoids from the Barks of Illicium difengpi. Chem. Pharm. Bull. 1992, 40, 2461–2464. [Google Scholar] [CrossRef]
- Kolset, S.O.; Reinholt, F.P.; Jenssen, T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Dubishchev, A.V.; Ezhkov, V.N.; Titova, I.N.; Avdeeva, E.V. Antidepressant activity of some phytopharmaceuticals and phenylpropanoids. Pharm. Chem. J. 2006, 40, 614–619. [Google Scholar] [CrossRef]
- Sokolov, S.Y.; Ivashin, V.M.; Zapesochnaya, G.G.; Kurkin, V.A.; Shchavlinskii, A.N. Studies of neurotropic activity of newcompounds isolated from Rhodiola rosea. Clin. Pharm. J. 1985, 19, 1367–1371. [Google Scholar]
- Ewa, S.R.; Malgorzata, H.; Andrzej, K.S.; Aleksander, W.; Ewa, S.; Michal, M.; Janusz, B.; Henryk, S. The influence of Rhodiola rosea extracts and rosavin on cutaneous angiogenesis induced in mice after grafting of syngeneic tumor cells. Cent. Eur. J. Immunol. 2008, 33, 102–107. [Google Scholar]
- Kurkin, V.A. Phenylpropanoids from medicinal plants: Distribution, classification, structural analysis, and Biological activity. Chem. Nat. Compd. 2003, 39, 123–153. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Dubishchev, A.V.; Titova, I.N.; Volotsueva, A.V.; Petrova, E.S.; Zhestkova, N.V.; Klimova, I.Y. Neurotropic properties of certain plant extracts containing phenylpropanoids. Rastit. Resur. 2003, 39, 115–122. [Google Scholar]
- Min, D.; Lyons, J.G.; Bonner, J.; Twigg, S.M.; Yue, D.K.; McLennan, S.V. Mesangial cell-derived factors alter monocyte activation and function through inflammatory pathways: Possible pathogenic role in diabetic nephropathy. Amer. J. Physiol-Renal Physiol. 2009, 297, F1229–F1237. [Google Scholar] [CrossRef]
- Xia, L.; Wang, H.; Goldberg, H.; Munk, S.; Fantus, I.; Whiteside, C. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. Amer. J. Physiol. Renal. Physiol. 2006, 290, F345–F356. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1, 3, 9, and 10 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luo, Q.; Wang, S.-M.; Lu, Q.; Luo, J.; Cheng, Y.-X. Identification of Compounds from the Water Soluble Extract of Cinnamomum cassia Barks and Their Inhibitory Effects against High-Glucose-Induced Mesangial Cells. Molecules 2013, 18, 10930-10943. https://doi.org/10.3390/molecules180910930
Luo Q, Wang S-M, Lu Q, Luo J, Cheng Y-X. Identification of Compounds from the Water Soluble Extract of Cinnamomum cassia Barks and Their Inhibitory Effects against High-Glucose-Induced Mesangial Cells. Molecules. 2013; 18(9):10930-10943. https://doi.org/10.3390/molecules180910930
Chicago/Turabian StyleLuo, Qi, Shu-Mei Wang, Qing Lu, Jie Luo, and Yong-Xian Cheng. 2013. "Identification of Compounds from the Water Soluble Extract of Cinnamomum cassia Barks and Their Inhibitory Effects against High-Glucose-Induced Mesangial Cells" Molecules 18, no. 9: 10930-10943. https://doi.org/10.3390/molecules180910930




