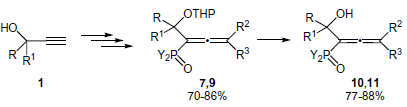
Bifunctionalized Allenes. Part XIII. A Convenient and Efficient Method for Regioselective Synthesis of Phosphorylated α-Hydroxyallenes with Protected and Unprotected Hydroxy Group
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Phosphorylated α-Hydroxyallenes with Protected Hydroxy Group
2.1.1. Synthesis of (Tetrahydro-2H-pyran-2-yloxy)-alkynols

| Entry | Alcohol | R | R1 | R2 | R3 | Yield a, % |
|---|---|---|---|---|---|---|
| 1 | 5a | H | H | Me | Et | 61 |
| 2 | 5b | H | H | Me | Bu | 59 |
| 3 | 5c | H | H | -(CH2)5- | 58 | |
| 4 | 5d | H | Me | Me | Et | 57 |
| 5 | 5e | H | Me | Me | Bu | 56 |
| 6 | 5f | H | Me | -(CH2)5- | 56 | |
| 7 | 5g | Me | Me | Me | Et | 54 |
| 8 | 5h | Me | Me | Me | Bu | 53 |
2.1.2. Synthesis of Dimethyl 1-(Tetrahydro-2H-pyran-2-yloxy)-1,2-dienephosphonates

| Entry | Allene | R | R1 | R2 | R3 | Yield a, % |
|---|---|---|---|---|---|---|
| 1 | 7a | H | H | Me | Et | 78 |
| 2 | 7b | H | H | Me | Bu | 75 |
| 3 | 7c | H | H | -(CH2)5- | 73 | |
| 4 | 7d | H | Me | Me | Et | 74 |
| 5 | 7e | H | Me | Me | Bu | 72 |
| 6 | 7f | H | Me | -(CH2)5- | 75 | |
| 7 | 7g | Me | Me | Me | Et | 71 |
| 8 | 7h | Me | Me | Me | Bu | 70 |
2.1.3. Synthesis of 2-[2-(Diphenylphosphinoyl-2,3-dienyloxy)]-tetrahydro-2H-pyrans
| Entry | Allene | R | R1 | R2 | R3 | Yield a, % |
|---|---|---|---|---|---|---|
| 1 | 9a | H | H | Me | Et | 86 |
| 2 | 9b | H | H | Me | Bu | 84 |
| 3 | 9c | H | H | -(CH2)5- | 81 | |
| 4 | 9d | H | Me | Me | Et | 83 |
| 5 | 9e | H | Me | Me | Bu | 82 |
| 6 | 9f | H | Me | -(CH2)5- | 80 | |
| 7 | 9g | Me | Me | Me | Et | 80 |
| 8 | 9h | Me | Me | Me | Bu | 78 |

2.2. Synthesis of Phosphorylated α-Hydroxyallenes with Unprotected Hydroxy Group

| Entry | Allene | R | R1 | R2 | R3 | Yield a, % |
|---|---|---|---|---|---|---|
| 1 | 10a | H | H | Me | Et | 80 |
| 2 | 10b | H | H | Me | Bu | 78 |
| 3 | 10c | H | H | -(CH2)5- | 77 | |
| 4 | 10d | H | Me | Me | Et | 80 |
| 5 | 10e | H | Me | Me | Bu | 79 |
| 6 | 10f | H | Me | -(CH2)5- | 81 | |
| 7 | 10g | Me | Me | Me | Et | 79 |
| 8 | 10h | Me | Me | Me | Bu | 78 |
| 9 | 11a | H | H | Me | Et | 86 |
| 10 | 11b | H | H | Me | Bu | 83 |
| 11 | 11c | H | H | -(CH2)5- | 81 | |
| 12 | 11d | H | Me | Me | Et | 87 |
| 13 | 11e | H | Me | Me | Bu | 85 |
| 14 | 11f | H | Me | -(CH2)5- | 88 | |
| 15 | 11g | Me | Me | Me | Et | 84 |
| 16 | 11h | Me | Me | Me | Bu | 83 |
3. Experimental Section
3.1. General Information
3.2. General Procedure [80,81,82,83] for Synthesis of the Alkynyloxy-tetrahydro-2H-pyrans 2
3.3. General Procedure for Synthesis of (Tetrahydro-2H-pyran-2-yloxy)-alkynols 5
3.4. General Procedure for Synthesis of the Dimethyl 1-(Tetrahydro-2H-pyran-2-yloxy)-1,2-dienephosphonates 7
3.5. General Procedure for Synthesis of the 2-(2-Diphenylphosphinoyl-2,3-dienyloxy)-tetrahydro-2H-pyrans 9
3.6. General Procedure for Synthesis of the 1-Hydroxyalkyl-1,2-dienephosphonates 10, the 3-Diphenylphosphinoyl-2,3-dien-1-ols 11a–c and the 3-Diphenylphosphinoyl-3,4-dien-2-ols 11d–h
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patai, S. (Ed.) The Chemistry of Ketenes, Allenes and Related Compounds; John Wiley & Sons: New York, NY, USA, 1980.
- Landor, S.R. (Ed.) The Chemistry of the Allenes; Academic Press: London, UK, 1982; Volume 1–3.
- Pasto, D.J. Recent developments in allene chemistry. Tetrahedron 1984, 40, 2805–2827. [Google Scholar] [CrossRef]
- Schuster, H.F.; Coppola, G.M. Allenes in Organic Synthesis; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Zimmer, R. Alkoxyallenes—Building blocks in organic synthesis. Synthesis 1993, 1993, 165–178. [Google Scholar] [CrossRef]
- Elsevier, C.J. Methods of Organic Chemistry (Houben-Weyl); Helmchen, R.W., Mulzer, J., Schaumann, E., Eds.; Thieme: Stuttgart, Gremany, 1995; Volume E21a, pp. 537–566. [Google Scholar]
- Krause, N.; Hashmi, A.S.K. (Eds.) Modern Allene Chemistry; Wiley-VCH: Weinheim, Gremany, 2004; Volume 1–2.
- Brummond, K.M.; DeForrest, J.E. Synthesizing allenes today (1982–2006). Synthesis 2007, 2007, 795–818. [Google Scholar] [CrossRef]
- Bates, R.W.; Satcharoen, V. Nucleophilic transition metal based cyclization of allenes. Chem. Soc. Rev. 2002, 31, 12–21. [Google Scholar] [CrossRef]
- Ma, S. Recent advances in the chemistry of allenes. Aldrichim. Acta 2007, 40, 91–102. [Google Scholar]
- Hassan, H.H.A.M. Recent progress in the chemistry of allenes. Curr. Org. Synth. 2007, 4, 413–439. [Google Scholar] [CrossRef]
- Pinho e Melo, T.M.V.D. Allenes as dipolarophiles and 1,3-dipole precursors: Synthesis of carbocyclic and heterocyclic compounds. Curr. Org. Chem. 2009, 13, 1406–1431. [Google Scholar] [CrossRef]
- Back, T.G.; Clary, K.N.; Gao, D. Cycloadditions and cyclizations of acetylenic, allenic, and conjugated dienyl sulfones. Chem. Rev. 2010, 110, 4498–4553. [Google Scholar] [CrossRef]
- Enomoto, M.; Katsuki, T.; Yamaguchi, M. Highly regioselective isomerization of acetylenes to allenes. Tetrahedron Lett. 1986, 27, 4599–4600. [Google Scholar] [CrossRef]
- Oroshnik, W.; Mebane, A.; Karmas, G. Synthesis of polyenes. III. Prototropic rearrangements in β-ionols and related compounds. J. Am. Chem. Soc. 1953, 75, 1050–1058. [Google Scholar] [CrossRef]
- Phadtare, S.; Zemlicka, J. Nucleic acid derived allenols: Unusual analogues of nucleosides with antiretroviral activity. J. Am. Chem. Soc. 1989, 111, 5925–5931. [Google Scholar] [CrossRef]
- Crabbé, P.; Fillion, H.; André, D.; Luche, J.-L. Efficient homologation of acetylenes to allenes. J. Chem. Soc. Chem. Commun. 1979, 859–860. [Google Scholar]
- Ma, S.; Hou, H.; Zhao, S.; Wang, G. Efficient synthesis of optically active 2,3-allenols via the simple CuBr-mediated reaction of optically active propargylic alcohols with paraformaldehyde. Synthesis 2002, 2002, 1643–1645. [Google Scholar] [CrossRef]
- Ye, J.; Li, S.; Chen, B.; Fan, W.; Kuang, J.; Liu, J.; Liu, Y.; Miao, B.; Wan, B.; Wang, Y.; et al. Catalytic asymmetric synthesis of optically active allenes from terminal alkynes. Org. Lett. 2012, 14, 1346–1349. [Google Scholar] [CrossRef]
- Boldrini, G.P.; Lodi, L.; Tagliavini, E.; Tarasco, C.; TrombinI, C.; Umanl-Ronchi, A. Synthesis of enantiomerically enriched homoallylic alcohols and of 1,2-dien-1-ols using chiral tin(IV) complexes containing diethyl tartrate as an auxiliary ligand. J. Org. Chem. 1987, 52, 5447–5452. [Google Scholar] [CrossRef]
- Hoffman, R.W.; Weldmann, U. Stereoselective synthesis of alcohols, XIX. The sense of asymmetric induction on addition to α-chiral aldehydes. Chem. Ber. 1985, 118, 3966–3979. [Google Scholar] [CrossRef]
- Corey, E.J.; Imwinkelried, R.; Pikul, S.; Xiang, Y.B. Practical enantioselective Diels-Alder and aldol reactions using a new chiral controller system. J. Am. Chem. Soc. 1989, 111, 5493–5495. [Google Scholar] [CrossRef]
- Corey, E.J.; Yu, C.-M.; Lee, D.-H. Copper catalyzed asymmetric propargylation of aldehydes. J. Am. Chem. Soc. 1990, 112, 878–879. [Google Scholar] [CrossRef]
- Corey, E.J.; Jones, G.B. Enantioselective route to α-hydroxy aldehyde and acid derivatives. Tetrahedron Lett. 1991, 32, 5713–5716. [Google Scholar] [CrossRef]
- Li, J.; Kong, W.; Fu, C.; Ma, S. An efficient CuCN-catalyzed synthesis of optically active 2,3-allenols from optically active 1-substituted 4-chloro-2-butyn-1-ols. J. Org. Chem. 2009, 74, 5104–5106. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Fu, C.; Ma, S. Studies on Cu(I)-catalyzed synthesis of simple 3-substituted 1,2-allenes and optically active 2-substituted secondary 2,3-allenols. Tetrahedron 2009, 65, 3695–3703. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, X.; Ma, S. Studies on Highly stereoselective addition-elimination reactions of 3-(methoxycarbonyl)-1,2-allen-4-ols with MX. An efficient synthesis of 3-(methoxycarbonyl)-2-halo-1,3(Z)-dienes. J. Org. Chem. 2007, 72, 5901–5904. [Google Scholar] [CrossRef]
- Alexakis, A.; Marek, I.; Mangeney, P.; Normant, J.F. Diastereoselective synthesis of α-allenic alcohols from propargylic epoxides. Tetrahedron Lett. 1989, 30, 2387–2390. [Google Scholar] [CrossRef]
- Alexakis, A.; Marek, I.; Mangeney, P.; Normant, J.F. Diastereoselective syn or anti opening of propargylic epoxides. Synthesis of α-allenic alcohols. Tetrahedron 1991, 47, 1677–1696. [Google Scholar] [CrossRef]
- Marshall, J.A.; Pinney, K.G. Stereoselective synthesis of 2,5-dihydrofurans by sequential SN2’ cleavage of alkynyloxiranes and silver(I)-catalyzed cyclization of the allenylcarbinol products. J. Org. Chem. 1993, 58, 7180–7184. [Google Scholar] [CrossRef]
- Krause, N.; Hoffmann-Röder, A.; Canisius, J. From amino acids to dihydrofurans: Functionalized allenes in modern organic synthesis. Synthesis 2002, 12, 1759–1774. [Google Scholar]
- Krause, N.; Hoffmann-Röder, A. Synthesis of allenes with organometallic reagents. Tetrahedron 2004, 60, 11671–11694. [Google Scholar] [CrossRef]
- Deutsch, C.; Lipshutz, B.H.; Krause, N. Small but effective: Copper hydride catalyzed synthesis of α-hydroxyallenes. Angew. Chem. Int. Ed. 2007, 46, 1650–1653. [Google Scholar] [CrossRef]
- Aksin-Artok, Ö.; Krause, N. Combined Rhodium/Gold catalysis: From propargyloxiranes to 2,5-dihydrofurans in one pot. Adv. Synth. Catal. 2011, 353, 385–391. [Google Scholar] [CrossRef]
- Poonoth, M.; Krause, N. Cycloisomerization of bifunctionalized Allenes: Synthesis of 3(2H)-furanones in water. J. Org. Chem. 2011, 76, 1934–1936. [Google Scholar] [CrossRef]
- Aurrecoechea, J.M.; Solay, M. Stereoselective samarium diiodide-promoted intermolecular coupling of alkynyloxiranes with ketones. Synthesis of 2,3-pentadiene-1,5-diols. Tetrahedron Lett. 1995, 36, 2501–2504. [Google Scholar] [CrossRef]
- Aurrecoechea, J.M.; Alonso, E.; Solay, M. Synthesis of allenic diols by samarium diiodide-promoted coupling between alkynyloxiranes and ketones. Tetrahedron 1998, 54, 3833–3850. [Google Scholar] [CrossRef]
- Cowie, J.S.; Landor, P.D.; Landor, S.R. A new method for the preparation of allenic alcohols. J. Chem. Soc. Chem. Commun. 1969. [Google Scholar] [CrossRef]
- Cowie, J.S.; Landor, P.D.; Landor, S.R. Allenes. Part XXIV. Preparation of α-allenic alcohols from the mono-O-tetrahydropyran-2-yl derivatives of butyne-1,4-diols. J. Chem. Soc. Perkin Trans. 1 1973. [Google Scholar] [CrossRef]
- Nakano, M.; Furuichi, N.; Mori, H.; Katsumura, S. Novel synthesis of the allene moiety of carotenoids via biomimetic photosensitized oxygenation. Tetrahedron Lett. 2001, 42, 7307–7310. [Google Scholar]
- Darcel, C.; Bruneau, C.; Dixneuf, P.H. Palladium(0), copper(I) catalysed synthesis of conjugated alkynyl α-allenols from alkynyl cyclic carbonates and terminal alkynes. J. Chem. Soc. Chem. Commun. 1994, 1845–1846. [Google Scholar] [CrossRef]
- Darcel, C.; Bartsch, S.; Bruneau, C.; Dixneuf, P.H. Selective catalytic transformations of alkynyl cyclic carbonates into either homopropargylic or α-allenyl alcohols. Synlett 1994, 1994, 457–458. [Google Scholar] [CrossRef]
- Hoff, S.; Brandsma, L.; Arens, J.F. Preparation, metallation and alkylation of allenyl ethers. Rec. Trav. Chim. Pays-Bas 1968, 87, 916–924. [Google Scholar]
- Hoff, S.; Brandsma, L.; Arens, J.F. Some conversions of allenyl ethers. Rec.Trav. Chim. Pays-Bas 1968, 87, 1179–1184. [Google Scholar] [CrossRef]
- Hormuth, S.; Reissig, H.-U. Diastereoselective additions of lithiated methoxyallene towards chiral Aaldehydes. Synlett 1991, 1994, 179–180. [Google Scholar] [CrossRef]
- Hormuth, S.; Reissig, H.-U.; Dorsch, D. Stereoselective synthesis of (2R,3S)-norstatine derivatives by addition of lithiated methoxyallene to amino aldehydes and subsequent ozonolysis. Liebigs Ann. Chem. 1994, 1994, 121–127. [Google Scholar] [CrossRef]
- Hormuth, S.; Reissig, H.-U. Stereoselective synthesis of 3(2H)-dihydrofuranones by addition of lithiated methoxyallene to chiral aldehydes. J. Org. Chem. 1994, 59, 67–73. [Google Scholar] [CrossRef]
- Marshall, J.A.; Tang, Y. Allene-directed diastereoselection. Additions to chiral allenyl aldehydes and ketones. J. Org. Chem. 1993, 58, 3233–3234. [Google Scholar] [CrossRef]
- Krause, N.; Aksin-Artok, Ö.; Asikainen, M.; Breker, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Minkler, S.; Poonoth, M.; et al. Combined coinage metal catalysis for the synthesis of bioactive molecules. J. Organomet. Chem. 2012, 704, 1–8. [Google Scholar]
- Mark, V. The Uncatalyzed Rearrangements of Tervalent Phosphorus Esters in Selective Organic Transformations; Thyagarajan, B.S., Ed.; John Wiley & Sons: New York, NY, USA, 1970; pp. 319–437. [Google Scholar]
- Landor, P.D. The Chemistry of the Allenes; Landor, S.R., Ed.; Academic Press: New York, NY, USA, 1982; Volume 1, pp. 174–178. [Google Scholar]
- Saalfrank, R.W.; Lurz, C.-J. Methoden der Organischen Chemie (Houben Weyl); Kropf, H., Scheumann, E., Eds.; Thieme: Stuttgart, Gremany, 1993; pp. 2959–3102. (In German) [Google Scholar]
- Hashmi, A.S.K. Synthesis of Allenes in Modern Allene Chemistry; Krause, N., Hashmi, A.S.K., Eds.; Wiley-VCH: Weinheim, Gremany, 2004; Volume 1, pp. 3–50. [Google Scholar]
- Macomber, R.S. Phosphorus-containing products from propargyl alcohols and phosphorus trihalides. 5. A stereochemical investigation of the formation and cyclization of allenic phosphonic acids. Preparation of 4-substituted 1,2-oxaphosphol-3-enes. J. Am. Chem. Soc. 1977, 99, 3072–3075. [Google Scholar] [CrossRef]
- Denmark, S.E.; Marlin, J.E. Carbanion-accelerated Claisen rearrangements. 7. Phosphine oxide and phosphonate anion stabilizing groups. J. Org. Chem. 1991, 56, 1003–1013. [Google Scholar] [CrossRef]
- Cai, B.; Blackburn, G.M. The syntheses and reactions of 3,4-bisphosphono-1,2,4,5-tetraenes. Synth. Commun. 1997, 27, 3943–3949. [Google Scholar] [CrossRef]
- Saalfrank, R.W.; Haubner, M.; Deutscher, C.; Bauer, U. Yne-allenes from 1-bromoallenes and trimethylstannylacetylenes via palladium-mediated C–C coupling reactions. Eur. J. Org. Chem. 1999, 1999, 2367–2372. [Google Scholar] [CrossRef]
- Bhuvan Kumar, N.N.; Kumara Swamy, K.C. The reaction of allenes with phosphorus(III) compounds bearing a P-NH-(t-Bu) group: Isolation of both enantiomers in crystalline form from an achiral system. Tetrahedron Lett. 2008, 49, 7135–7138. [Google Scholar] [CrossRef]
- Kumar, B.; Chakravarty, M.; Kumar, S.; Sajna, K.; Swamy, K. Allenylphosphonates with a 1,3,2-dioxaphosphorinane ring: Synthesis, structures, stability and utility. J. Chem. Sci. 2009, 121, 23–36. [Google Scholar] [CrossRef]
- Boiselle, A.P.; Meinhardt, N.A. Acetylene-allene rearrangements reactions of trivalent phosphorus chlorides with α-acetylenic acohols and glycols. J. Org. Chem. 1962, 27, 1828–1833. [Google Scholar] [CrossRef]
- Mark, V. A facile SNi’ rearrangement: The formation of 1,2-alkadienylphosphonates from 2-alkynyl phosphites. Tetrahedron Lett. 1962, 3, 281–284. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Maligres, P.; Shin, J.; de Leon, E.; Rideout, D. DNA-cleavage and antitumor activity of designed molecules with conjugated phosphine oxide-allene-ene-yne functionalities. J. Am. Chem. Soc. 1990, 112, 7825–7826. [Google Scholar] [CrossRef]
- Curfin, M.L.; Okamura, W.H. Synthetic and kinetic studies of the intramolecular Diels-Alder reactions of cycloalkenylallenylphosphine oxides. J. Org. Chem. 1990, 55, 5278–5287. [Google Scholar] [CrossRef]
- Grissom, J.W.; Huang, D. Low-temperature tandem enyne allene radical cyclizations: Efficient synthesis of 2,3-dihydroindenes from simple enediynes. Angew. Chem. Int. Ed. 1995, 34, 2037–2039. [Google Scholar] [CrossRef]
- Darcel, C.; Bruneau, C.; Dixneuf, P.H. Straightforward syntheses of dienyl- and diallenylphosphine oxides from α-allenols. Synthesis 1996, 1996, 711–714. [Google Scholar] [CrossRef]
- De Frutos, O.; Echavarren, A.M. An approach to the synthesis of the benzo[b]fluorene core of the kinamycins by an arylalkyne-allene cycloaddition. Tetrahedron Lett. 1997, 38, 7941–7943. [Google Scholar] [CrossRef]
- Schmittel, M.; Steffen, J.-P.; Maywald, M.; Engels, B.; Helten, H.; Musch, P. Ring size effects in the C2–C6 biradical cyclisation of enyne–allenes and the relevance for neocarzinostatin. J. Chem. Soc. Perkin Trans. 2 2001. [Google Scholar] [CrossRef]
- Guo, H.; Qian, R.; Guo, Y.; Ma, S. Neighboring group participation of phosphine oxide functionality in the highly regio- and stereoselective iodohydroxylation of 1,2-allenylic diphenyl phosphine oxides. J. Org. Chem. 2008, 73, 7934–7938. [Google Scholar] [CrossRef]
- Srinivas, V.; Sajna, K.V.; Kumara Swamy, K.C. Zn(OTf)2 catalyzed addition-cyclization reaction of allenylphosphine oxides with propargyl alcohol-unexpected formation of 2,5-dimethylenetetrahydrofurans and 2-substituted furans. Tetrahedron Lett. 2011, 52, 5323–5326. [Google Scholar]
- Brel, V.K. Synthesis and cyclization of diethylphosphono-substituted α-allenic alcohols to 4-(diethylphosphono)-2,5-dihydrofurans. Synthesis 1999, 1999, 463–466. [Google Scholar] [CrossRef]
- Brel, V.K.; Abramkin, E.V. Cyclization of allenyl phosphonates to 3-chloro-4-(diethylphosphono)-2,5-dihydrofurans induced by CuCl2. Mendeleev Commun. 2002, 12, 64–65. [Google Scholar] [CrossRef]
- Metcalf, B.W.; Wright, C.L.; Burkhart, J.P.; Johnston, J.O. Substrate-induced inactivation of aromatase by allenic and acetylenic steroids. J. Am. Chem. Soc. 1981, 103, 3221–3222. [Google Scholar] [CrossRef]
- Muller, M.; Mann, A.; Taddei, M. A new method for the preparation of (2R)-2-amino-5-phosphonopentanoic acid. Tetrahedron Lett. 1993, 34, 3289–3290. [Google Scholar] [CrossRef]
- Brel, V.K.; Stang, P.J. A new approach to phosphonate analogues of phosphatidyl derivatives. Eur. J. Org. Chem. 2003, 2003, 224–229. [Google Scholar] [CrossRef]
- Brel, V.K. Synthesis and intramolecular cyclization of diethylphosphono-substituted allenic glycols. Synthesis 2001, 2001, 1539–1545. [Google Scholar] [CrossRef]
- Brel, V.K.; Belsky, V.K.; Stash, A.I.; Zvodnik, V.E.; Stang, P.J. Synthesis and molecular structure of new acyclic analogues of nucleotides with a 1,2-alkadienic skeleton. Org. Biomol. Chem. 2003, 1, 4220–4226. [Google Scholar] [CrossRef]
- Brel, V.K.; Belsky, V.K.; Stash, A.I.; Zvodnik, V.E.; Stang, P.J. Synthesis and molecular structure of new unsaturated analogues of nucleotides containing six-membered rings. Eur. J. Org. Chem. 2005, 2005, 512–521. [Google Scholar] [CrossRef]
- Ivanov, I.K.; Parushev, I.D.; Christov, V.Ch. Bifunctionalized allenes, Part IX: An efficient method for regioselective synthesis of 4-heteroatom-functionalized allenecarboxylates. Heteroat. Chem. 2013, 24, 322–331. [Google Scholar] [CrossRef]
- Ivanov, I.K.; Parushev, I.D.; Christov, V.Ch. Bifunctionalized allenes, Part XI: Competitive electrophilic cyclization and addition reactions of 4-phosphorylated allenecarboxylates. Heteroat. Chem. 2014, 25, 60–71. [Google Scholar] [CrossRef]
- Robertson, D.N. Adducts of tert-alcohols containing an ethynyl group with dihydropyran. Potentially useful intermediates. J. Org. Chem. 1960, 25, 931–932. [Google Scholar] [CrossRef]
- Miyashita, M.; Yoshikoshi, A.; Griecolb, P.A. Pyridinium p-toluenesulfonate. A mild and efficient catalyst for the tetrahydropyranylation of alcohols. J. Org. Chem. 1977, 42, 3772–3774. [Google Scholar] [CrossRef]
- Joshi, M.C.; Joshi, P.; Rawat, D.S. Microwave assisted synthesis of symmetrically and asymmetrically substituted acyclic enediynes. ARKIVOC 2006, xvi, 65–74. [Google Scholar]
- Partha, B.; Pimkov, I. Composition, Synthesis, and Use of New Substituted Pyran and Pterin Compounds. US Patent 8378123 B2, 25 March 2011. [Google Scholar]
- Sample Availability: Samples of the compounds 7, 9, 10 and 11 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ismailov, I.E.; Ivanov, I.K.; Christov, V.C. Bifunctionalized Allenes. Part XIII. A Convenient and Efficient Method for Regioselective Synthesis of Phosphorylated α-Hydroxyallenes with Protected and Unprotected Hydroxy Group. Molecules 2014, 19, 6309-6329. https://doi.org/10.3390/molecules19056309
Ismailov IE, Ivanov IK, Christov VC. Bifunctionalized Allenes. Part XIII. A Convenient and Efficient Method for Regioselective Synthesis of Phosphorylated α-Hydroxyallenes with Protected and Unprotected Hydroxy Group. Molecules. 2014; 19(5):6309-6329. https://doi.org/10.3390/molecules19056309
Chicago/Turabian StyleIsmailov, Ismail E., Ivaylo K. Ivanov, and Valerij Ch. Christov. 2014. "Bifunctionalized Allenes. Part XIII. A Convenient and Efficient Method for Regioselective Synthesis of Phosphorylated α-Hydroxyallenes with Protected and Unprotected Hydroxy Group" Molecules 19, no. 5: 6309-6329. https://doi.org/10.3390/molecules19056309
APA StyleIsmailov, I. E., Ivanov, I. K., & Christov, V. C. (2014). Bifunctionalized Allenes. Part XIII. A Convenient and Efficient Method for Regioselective Synthesis of Phosphorylated α-Hydroxyallenes with Protected and Unprotected Hydroxy Group. Molecules, 19(5), 6309-6329. https://doi.org/10.3390/molecules19056309