MTLD, a Database of Multiple Target Ligands, the Updated Version
Abstract
:1. Introduction
2. Results
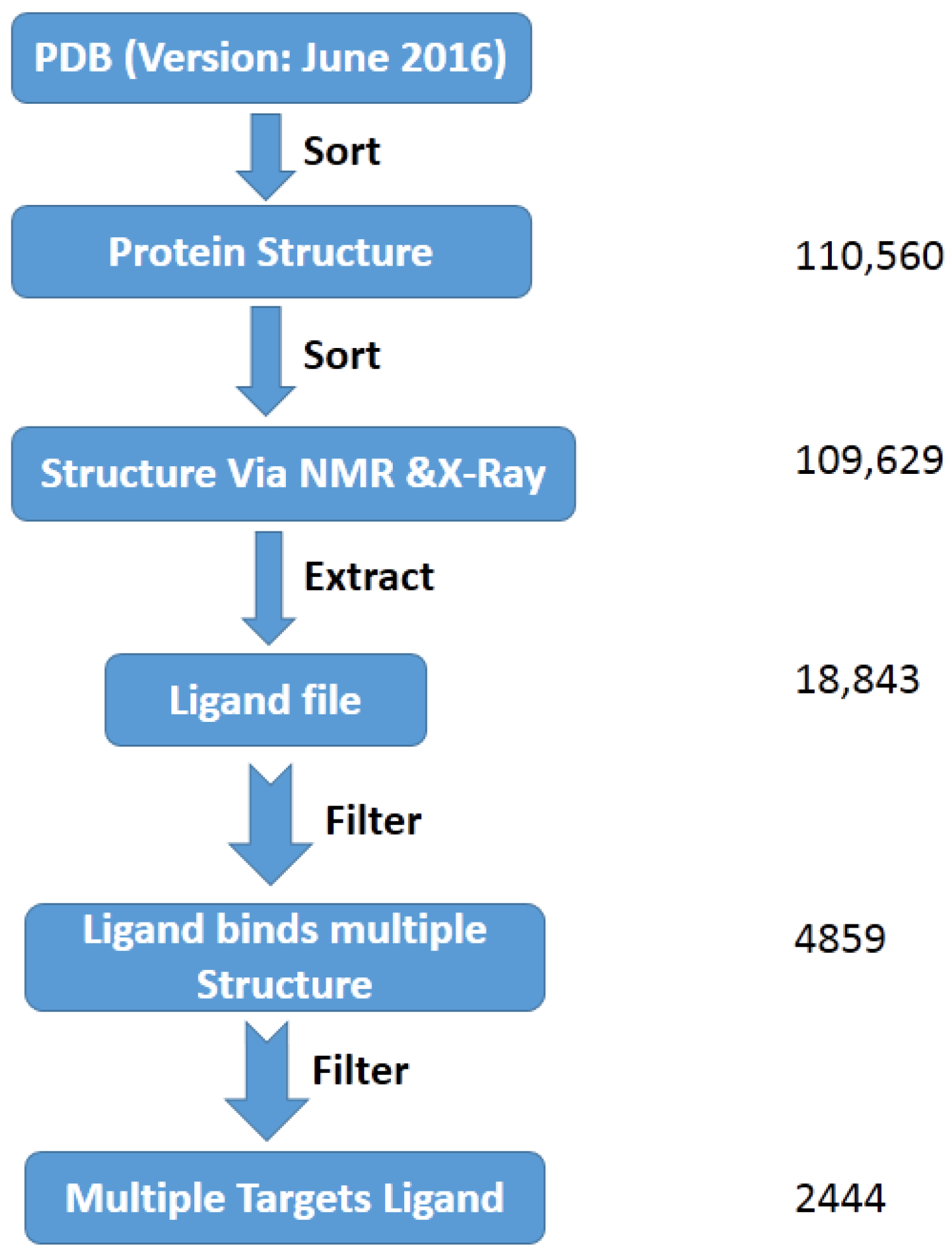
2.1. The Mining of the MTLs from PDB
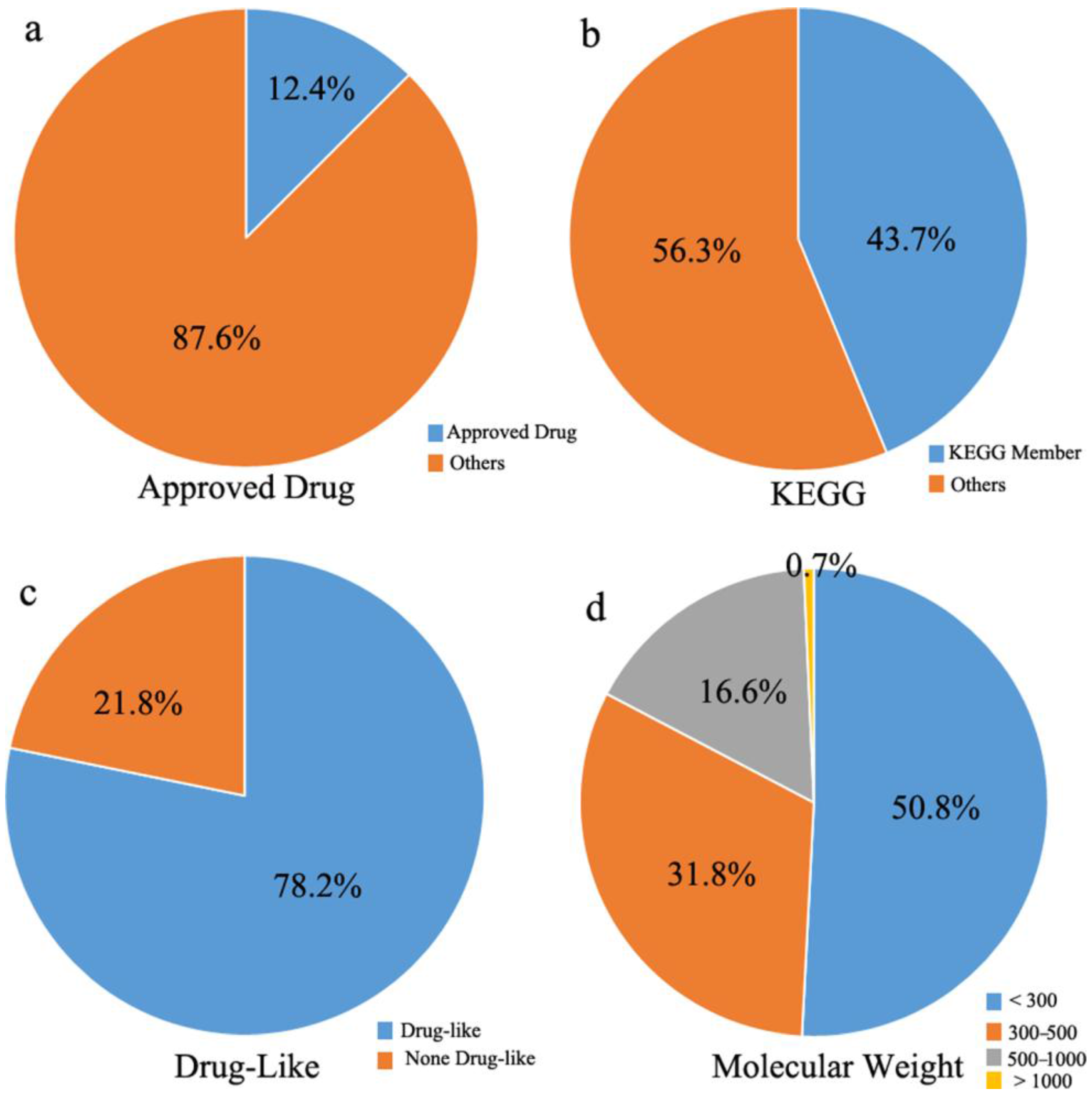
2.2. Statistics for MTLD
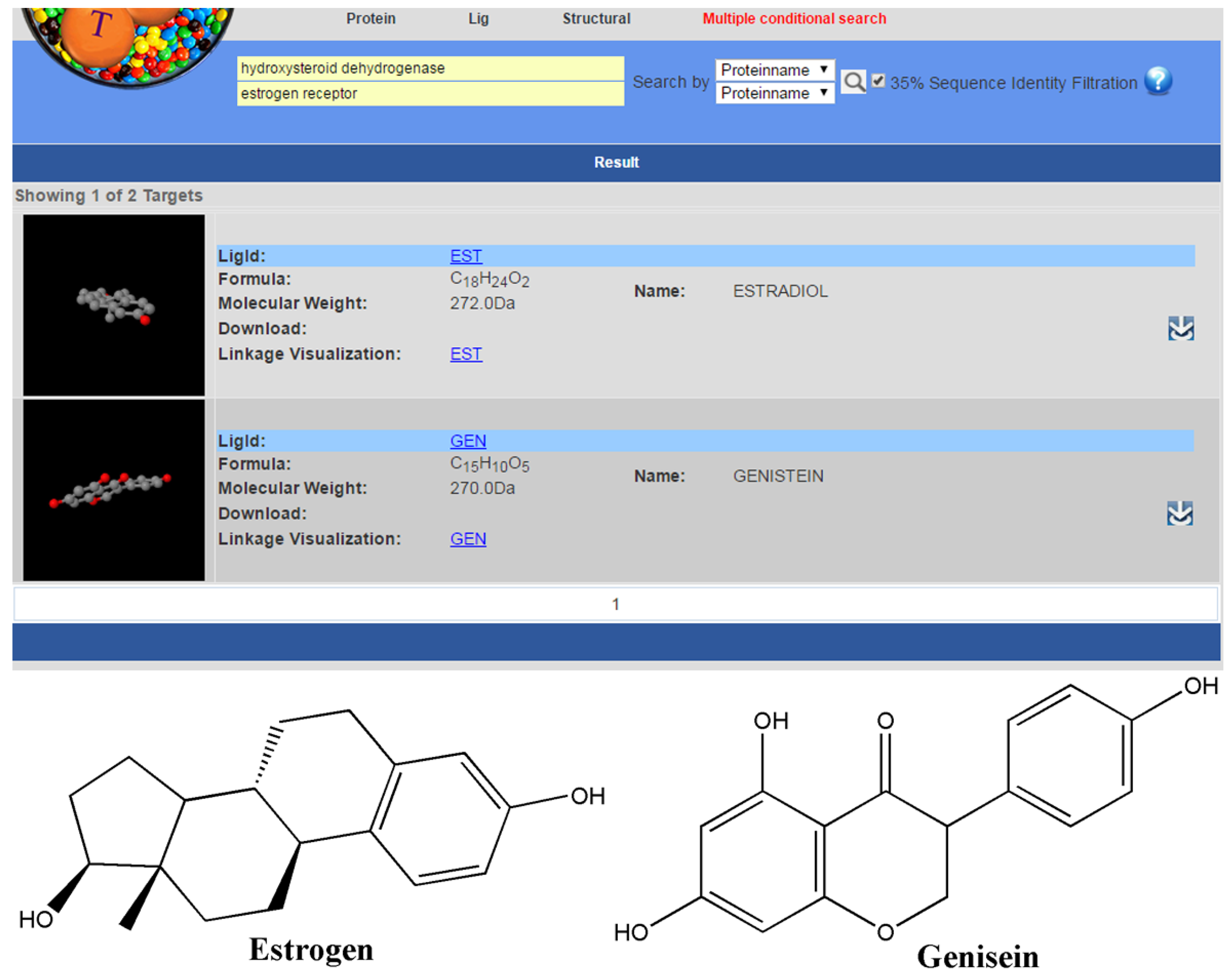
2.3. Multiple Conditional Search
2.4. Linkage Visualization
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Connolly, H.M.; Crary, J.L.; McGoon, M.D.; Hensrud, D.D.; Edwards, B.S.; Edwards, W.D.; Schaff, H.V. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 1997, 337, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Setola, V.; Roth, B.L.; Merryman, W.D. Serotonin receptors and heart valve disease—It was meant 2b. Pharmacol. Ther. 2011, 132, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.B.; Baumann, M.H.; Savage, J.E.; Rauser, L.; McBride, A.; Hufeisen, S.J.; Roth, B.L. Evidence for possible involvement of 5-ht(2b) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 2000, 102, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatr. 1988, 45, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Barlocco, D. Designed multiple ligands: Basic research vs. clinical outcomes. Curr. Med. Chem. 2012, 19, 3353–3387. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.U. Polypharmacology—Foe or friend? J. Med. Chem. 2013, 56, 8955–8971. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.Y.; Wu, C.C.; Chen, B.R.; Yen, L.B.; Wu, K.K. Nonsteroidal anti-inflammatory drugs induced endothelial apoptosis by perturbing peroxisome proliferator-activated receptor-delta transcriptional pathway. Mol. Pharmacol. 2008, 74, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Zhang, J.; Li, Z.; Yin, Y.; Wang, Y.; Marin, T.L.; Gongol, B.; Xiao, H.; Zhang, Y.Y.; Chen, Z.; et al. Cardiovascular protective effect of metformin and telmisartan: Reduction of parp1 activity via the ampk-parp1 cascade. PLoS ONE 2016, 11, e0151845. [Google Scholar] [CrossRef] [PubMed]
- Moehring, A.; Wohlbold, L.; Aulitzky, W.E.; van der Kuip, H. Role of poly(adp-ribose) polymerase activity in imatinib mesylate-induced cell death. Cell Death Differ. 2005, 12, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Erler, J.T.; Linding, R. Network-based drugs and biomarkers. J. Pathol. 2010, 220, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Zhang, S. Polypharmacology: Drug discovery for the future. Expert Rev. Clin. Pharmacol. 2013, 6, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, G.; Pinzi, L. Computational polypharmacology comes of age. Front. Pharmacol. 2015, 6, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Li, Q.; Wu, M.; Chen, C.; Cen, S. Progress in the rational design for polypharmacology drug. Curr. Pharm. Des. 2016, 22, 3182–3189. [Google Scholar] [CrossRef] [PubMed]
- Haupt, V.J.; Daminelli, S.; Schroeder, M. Drug promiscuity in pdb: Protein binding site similarity is key. PLoS ONE 2013, 8, e65894. [Google Scholar] [CrossRef]
- Sturm, N.; Desaphy, J.; Quinn, R.J.; Rognan, D.; Kellenberger, E. Structural insights into the molecular basis of the ligand promiscuity. J. Chem. Inf. Model. 2012, 52, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; He, Y.; Wu, J.; Zhou, J. Creation of a free, internet-accessible database: The multiple target ligand database. J. Cheminform. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Foster, P.A.; Tutill, H.J.; Parsons, M.F.; Newman, S.P.; Chander, S.K.; Allan, G.M.; Lawrence, H.R.; Vicker, N.; Potter, B.V.; et al. 17beta-hydroxysteroid dehydrogenase type 1, and not type 12, is a target for endocrine therapy of hormone-dependent breast cancer. Int. J. Cancer 2008, 122, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Zaslavskiy, M.; Vert, J.P.; Stoven, V. A new protein binding pocket similarity measure based on comparison of clouds of atoms in 3d: Application to ligand prediction. BMC Bioinform. 2010, 11, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sael, L.; Kihara, D. Detecting local ligand-binding site similarity in nonhomologous proteins by surface patch comparison. Proteins 2012, 80, 1177–1195. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Tan, Z.; Zhang, S. Curation and analysis of multitargeting agents for polypharmacological modeling. J. Chem. Inf. Model. 2014, 54, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Medina, T.M.; Lewis, K.D. The evolution of combined molecular targeted therapies to advance the therapeutic efficacy in melanoma: A highlight of vemurafenib and cobimetinib. OncoTargets Ther. 2016, 9, 3739–3752. [Google Scholar]
- Bollag, G.; Hirth, P.; Tsai, J.; Zhang, J.; Ibrahim, P.N.; Cho, H.; Spevak, W.; Zhang, C.; Zhang, Y.; Habets, G.; et al. Clinical efficacy of a raf inhibitor needs broad target blockade in braf-mutant melanoma. Nature 2010, 467, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Mathea, S.; Abdul Azeez, K.R.; Salah, E.; Tallant, C.; Wolfreys, F.; Konietzny, R.; Fischer, R.; Lou, H.J.; Brennan, P.E.; Schnapp, G.; et al. Structure of the human protein kinase zak in complex with vemurafenib. ACS Chem. Biol. 2016, 11, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.M.; Bagby, G.F. Carprofen: A new nonsteroidal antiinflammatory drug. Pharmacology, clinical efficacy and adverse effects. Pharmacotherapy 1987, 7, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bertolacci, L.; Romeo, E.; Veronesi, M.; Magotti, P.; Albani, C.; Dionisi, M.; Lambruschini, C.; Scarpelli, R.; Cavalli, A.; De Vivo, M.; et al. A binding site for nonsteroidal anti-inflammatory drugs in fatty acid amide hydrolase. J. Am. Chem. Soc. 2013, 135, 22–25. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Data-Sets | MTLD | MTLD_Updated | Increase Rate |
|---|---|---|---|
| MTL entries | 1732 | 2444 | 41% |
| PDB structures | 12,759 | 18,231 | 42.8% |
| Approved drugs | 222 | 304 | 36.9% |
| Drug-like | 1334 | 1911 | 43.2% |
| KEGG * | 815 | 1069 | 31.1% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wu, M.; Cen, S.; Wu, J.; Zhou, J. MTLD, a Database of Multiple Target Ligands, the Updated Version. Molecules 2017, 22, 1375. https://doi.org/10.3390/molecules22091375
Chen C, Wu M, Cen S, Wu J, Zhou J. MTLD, a Database of Multiple Target Ligands, the Updated Version. Molecules. 2017; 22(9):1375. https://doi.org/10.3390/molecules22091375
Chicago/Turabian StyleChen, Chao, Meng Wu, Shan Cen, Jianhui Wu, and Jinming Zhou. 2017. "MTLD, a Database of Multiple Target Ligands, the Updated Version" Molecules 22, no. 9: 1375. https://doi.org/10.3390/molecules22091375




