Bis(6-Diphenylphosphinoacenaphth-5-yl)Telluride as a Ligand toward Manganese and Rhenium Carbonyls
Abstract
:1. Introduction
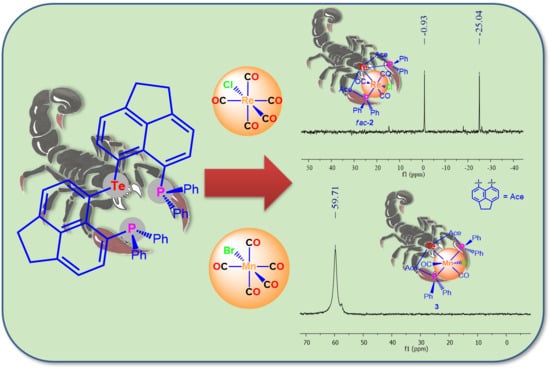
2. Results and Discussion
3. Conclusions
4. Experimental Section
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Murray, S.G.; Hartley, F.R. Coordination chemistry of thioethers, selenoethers, and telluroethers in transition-metal complexes. Chem. Rev. 1981, 81, 365–414. [Google Scholar] [CrossRef]
- Gysling, H.J. The ligand chemistry of tellurium. Coord. Chem. Rev. 1982, 42, 133–244. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, V. The Chemistry of Multidentate Organotellurium Ligands. J. Coord. Chem. 1992, 27, 237–253. [Google Scholar] [CrossRef]
- Hope, E.G.; Levason, W. Recent developments in the coordination chemistry of selenoether and telluroether ligands. Coord. Chem. Rev. 1993, 122, 109–170. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, S. Recent developments in the ligand chemistry of tellurium. Coord. Chem. Rev. 2000, 209, 49–98. [Google Scholar] [CrossRef]
- Levason, W.; Orchard, S.D.; Reid, G. Recent developments in the chemistry of selenoethers and telluroethers. Coord. Chem. Rev. 2002, 225, 159–199. [Google Scholar] [CrossRef]
- Torubaev, Y.; Pasynskii, A.; Mathur, P. Organotellurium halides: New ligands for transition metal complexes. Coord. Chem. Rev. 2012, 256, 709–721. [Google Scholar] [CrossRef]
- Jain, V.K.; Chauhan, R.S. New vistas in the chemistry of platinum group metals with tellurium ligands. Coord. Chem. Rev. 2016, 306, 270–301. [Google Scholar] [CrossRef]
- Gysling, H.J.; Luss, H.R. Synthesis and properties of the hybrid tellurium-phosphorus ligand phenyl o-(diphenylphosphino)phenyl telluride. X-ray structure of [Pt[PhTe(o-(PPh2C6H4)]2][Pt(SCN)4]·2DMF. Organometallics 1984, 3, 596–598. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, H.B.; Butcher, R.J. Syntheses and Characterization of Hybrid Bi- and Multidentate Tellurium Ligands Derived from N,N-Dimethylbenzylamine: Coordination Behavior of Bis[2-((dimethylamino)methyl)phenyl] Telluride with Chromium Pentacarbonyl. Organometallics 1995, 14, 4755–4763. [Google Scholar] [CrossRef]
- Lin, T.P.; Gabbaï, F.P. Two-Electron Redox Chemistry at the Dinuclear Core of a TePt Platform: Chlorine Photoreductive Elimination and Isolation of a TeVPtI Complex. J. Am. Chem. Soc. 2012, 134, 12230–12238. [Google Scholar] [CrossRef] [PubMed]
- Do, T.G.; Hupf, E.; Nordheider, A.; Lork, E.; Slawin, A.M.Z.; Makarov, S.G.; Ketkov, S.Y.; Mebs, S.; Woollins, J.D.; Beckmann, J. Intramolecularly Group 15 Stabilized Aryltellurenyl Halides and Triflates. Organometallics 2015, 34, 5341–5360. [Google Scholar] [CrossRef]
- Kabir, S.E.; Ahmed, F.; Ghosh, S.; Hassan, M.R.; Islam, M.S.; Sharmin, A.; Tocher, D.A.; Haworth, D.T.; Lindeman, S.V.; Siddiquee, T.A.; et al. Reactions of rhenium and manganese carbonyl complexes with 1,8-bis(diphenylphosphino)naphthalene: Ligand chelation, C–H and C–P bond-cleavage reactions. J. Organomet. Chem. 2008, 693, 2657–2665. [Google Scholar] [CrossRef]
- Hoyano, J.; Peterso, L.K. [LM(CO)3,4X] complexes of manganese(I) and rhenium(I) with phosphino- and thiophosphinatopyrazole ligands. Can. J. Chem. 1976, 54, 2697–2705. [Google Scholar] [CrossRef]
- Hieber, W.; Kruck, T. Tellurorganyl-haltige Metallcarbonyle. Chem. Ber. 1962, 95, 2027–2041. [Google Scholar] [CrossRef]
- Liaw, W.F.; Chiou, S.J.; Lee, W.Z.; Gene-Hsiang, P.; Peng, S.M. Syntheses and Characterization of Manganese-Tellurolate Complexes by Using Chelating Metalloligand cis-[Mn(Co)4(TePh)2]− and Potential Electrophilic TeMe+ Reagent. J. Chin. Chem. Soc. 1996, 43, 29–35. [Google Scholar] [CrossRef]
- Sadekov, I.D.; Uraev, A.I.; Garnovskii, A.D. Synthesis, reactions and structures of complexes of metal carbonyls and cyclopentadienyl carbonyls with organotellurium ligands. Russ. Chem. Rev. 1999, 68, 415–433. [Google Scholar] [CrossRef]
- Lau, P.; Huttner, G.; Zsolnai, L. Trigonal-planar koordiniertes Selen bzw. Tellur in [Cp′(CO)2Mn]2EMes+ (E = Se, Te): Verbindungen vom “Iniden”-Typ. J. Organometal. Chem. 1992, 440, 41–46. [Google Scholar] [CrossRef]
- Tondreau, A.M.; Boncella, J.M. The synthesis of PNP-supported low-spin nitro manganese(I) carbonyl complexes. Polyhedron 2016, 116, 96–104. [Google Scholar] [CrossRef]
- Chen, X.; Femia, F.J.; Babich, J.W.; Zubieta, J. Synthesis, crystal structures and magnetic properties of transition metal–azide complexes coordinated with pyridyl nitronyl nitroxides. Inorg. Chim Acta 2001, 315, 147–152. [Google Scholar] [CrossRef]
- Horikawa, H.; Horn, E.; Urushiyama, A.; Miyamoto, K. Crystal structure of tricarbonylbis [1,2-(diphenylphosphino)benzene]-manganese(I) bromide, Mn(CO)3(C30H24P2)Br. Z. Kristallogr. New Cryst. Struct. 2010, 225, 687–688. [Google Scholar] [CrossRef]
- Anderson, P.A.; Keene, F.R.; Horn, E.; Tiekink, E.R.T. Ambidentate coordination of the tripyridyl ligands 2,2′:6′,2″-terpyridyl, tris(2-pyridyl)-amine, tris(2-pyridyl)methane and tris(2-pyridyl)phosphine to carbonylrhenium centres: Structural and spectroscopic studies. Appl. Organomet. Chem. 1990, 4, 523–533. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Spek, A. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. DIAMOND V3.1d; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
Sample Availability: Samples of fac-1, fac-2, and 3 are available from the authors. |







| fac-1 (M = Re) | fac-2 (M = Mn) | 3 (M = Mn) | |
|---|---|---|---|
| Bond Lengths and Angles | |||
| M(1)–Te(1) | 2.733(1) | 2.599(1) | 2.546(1) |
| M(1)–P(1) | 2.466(1) | 2.343(1) | 2.293(1) |
| M(1)–P(2) | - | - | 2.277(1) |
| M(1)–X(1) | 2.487(1) | 2.498(1) | 2.540(1) |
| M(1)–C(5) | 1.930(2) | 1.819(5) | 1.800(4) |
| M(1)–C(6) | 1.930(2) | 1.817(6) | 1.811(4) |
| M(1)–C(7) | 1.951(2) | 1.915(7) | - |
| C(5)–O(5) | 1.142(2) | 1.127(7) | 1.146(6) |
| C(6)–O(6) | 1.121(2) | 1.108(7) | 1.037(5) |
| C(7)–O(7) | 1.141(2) | 0.993(8) a | - |
| P(1)–M(1)–P(2) | - | - | 170.8(1) |
| P(1)–M(1)–C(7) | 171.9(1) | 171.2(2) | - |
| X(1)–M(1)–C(6) | 176.2(1) | 173.9(2) | 176.3(1) |
| Te(1)–M(1)–C(5) | 174.0(1) | 175.9(2) | 175.6(1) |
| peri Region distances | |||
| P(1)···Te(1) | 3.363(1) | 3.466(1) | 3.247(2) |
| P(1)···Te(2) | 3.111(1) | 3.141(1) | 3.429(1) |
| peri Region bond Angles | |||
| P(1)–C(18)–C(19)/P(2)–C(48)–C(49) | 122.9(1)/119.5(1) | 124.6(3)/118.8(3) | 124.3(3)/121.0(3) |
| C(10)–C(19)–C(18)/C(40)–C(49)–C(48) | 130.2(1)/129.0(1) | 129.8(4)/129.9(4) | 130.3(4)/129.8(4) |
| Te(1)–C(10)–C(19)/Te(1)–C(40)–C(49) | 127.6(1)/122.0(1) | 129.6(3)/124.1(3) | 123.2(3)/126.1(3) |
| ∑ of bay angles | 380.7(3)/370.5(3) | 380.0(10)/372.8(1) | 377.8(1)/376.9(1) |
| Splay angle b | 20.7(3)/10.5(3) | 20(10)/12.8(1) | 17.8(1)/17.4(1) |
| Out-of-Plane Displacement | |||
| Te(1) c | 0.198(1)/−0.626(1) | −0.191(1)/−0.390(1) | 0.0899(3)/−0.666(1) |
| P(1) | −0.203(1) | 0.097(1) | −0.160(1) |
| P(2) | 0.278(1) | 0.263(1) | 0.543(1) |
| Central Acenaphthene Ring Torsion Angles | |||
| C:(13)–(14)–(19)–(18)/(43)–(44)–(49)–(48) | 175.5(1)/177.9(1) | −179.3(6)/−173.3(5) | 179.4(4)/169.4(5) |
| C:(15)–(14)–(19)–(10)/(45)–(44)–(49)–(40) | 178.7(1)/176.2(1) | 178.5(5)/−177.5(5) | 179.4(4)/178.6(5) |
| fac-1 | fac-2·CH2Cl2 | 3·2 CH2Cl2 | |
|---|---|---|---|
| Formula | C51H36ClO3P2ReTe | C52H38BrCl2MnO3P2Te | C52H40BrCl4MnO2P2Te |
| Formula weight, g mol−1 | 1107.99 | 1106.11 | 1163.03 |
| Crystal system | Triclinic | Monoclinic | Triclinic |
| Crystal size, mm | 0.09 × 0.08 × 0.06 | 0.10 × 0.08 × 0.05 | 0.09 × 0.08 × 0.08 |
| Space group | P | P21/n | P |
| a, Å | 12.2258(3) | 10.2073(2) | 12.542(5) |
| b, Å | 14.3531(3) | 22.4724(5) | 12.738(5) |
| c, Å | 14.8486(3) | 19.7629(5) | 15.516(5) |
| α, ° | 78.509(1) | 90 | 100.490(5) |
| β, ° | 77.874(1) | 101.421(1) | 100.078(5) |
| γ, ° | 89.216(1) | 90 | 100.930(5) |
| V, Å3 | 2495.33(9) | 4443.5(2) | 2336(2) |
| Z | 2 | 4 | 2 |
| ρcalcd, Mg m−3 | 1.475 | 1.653 | 1.653 |
| μ (Mo Kα), mm−1 | 3.163 | 2.077 | 2.089 |
| F(000) | 1080 | 2200 | 1156 |
| θ range, deg | 2.31 to 29.57 | 2.29 to 27.56 | 2.38 to 30.12 |
| Index ranges | −16 ≤ h ≤ 16 | −13 ≤ h ≤ 12 | −17 ≤ h ≤ 17 |
| −19 ≤ k ≤ 19 | −29 ≤ k ≤ 29 | −17 ≤ k ≤ 17 | |
| −20 ≤ l ≤ 20 | −25 ≤ l ≤ 25 | −21 ≤ l ≤ 21 | |
| No. of reflns collected | 275,626 | 120,248 | 202,873 |
| Completeness to θmax | 99.8% | 99.8% | 99.8% |
| No. indep. Reflns | 13,983 | 10,262 | 13,719 |
| No. obsd reflns with (I > 2σ(I)) | 13,702 | 8891 | 11,512 |
| No. refined params | 532 | 553 | 556 |
| GooF (F2) | 1.074 | 1.042 | 1.054 |
| R1 (F) (I > 2σ(I)) | 0.0148 | 0.0559 | 0.0553 |
| wR2 (F2) (all data) | 0.0385 | 0.1690 | 0.1529 |
| Largest diff peak/hole, e Å−3 | 0.844/−0.846 | 1.724/−1.561 | 1.955/−1.665 |
| CCDC number | 1861602 | 1861603 | 1861604 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.G.; Hupf, E.; Lork, E.; Beckmann, J. Bis(6-Diphenylphosphinoacenaphth-5-yl)Telluride as a Ligand toward Manganese and Rhenium Carbonyls. Molecules 2018, 23, 2805. https://doi.org/10.3390/molecules23112805
Do TG, Hupf E, Lork E, Beckmann J. Bis(6-Diphenylphosphinoacenaphth-5-yl)Telluride as a Ligand toward Manganese and Rhenium Carbonyls. Molecules. 2018; 23(11):2805. https://doi.org/10.3390/molecules23112805
Chicago/Turabian StyleDo, Truong Giang, Emanuel Hupf, Enno Lork, and Jens Beckmann. 2018. "Bis(6-Diphenylphosphinoacenaphth-5-yl)Telluride as a Ligand toward Manganese and Rhenium Carbonyls" Molecules 23, no. 11: 2805. https://doi.org/10.3390/molecules23112805