Aluminates with Fluorinated Schiff Bases: Influence of the Alkali Metal–Fluorine Interactions in Structure Stabilization
Abstract
:1. Introduction
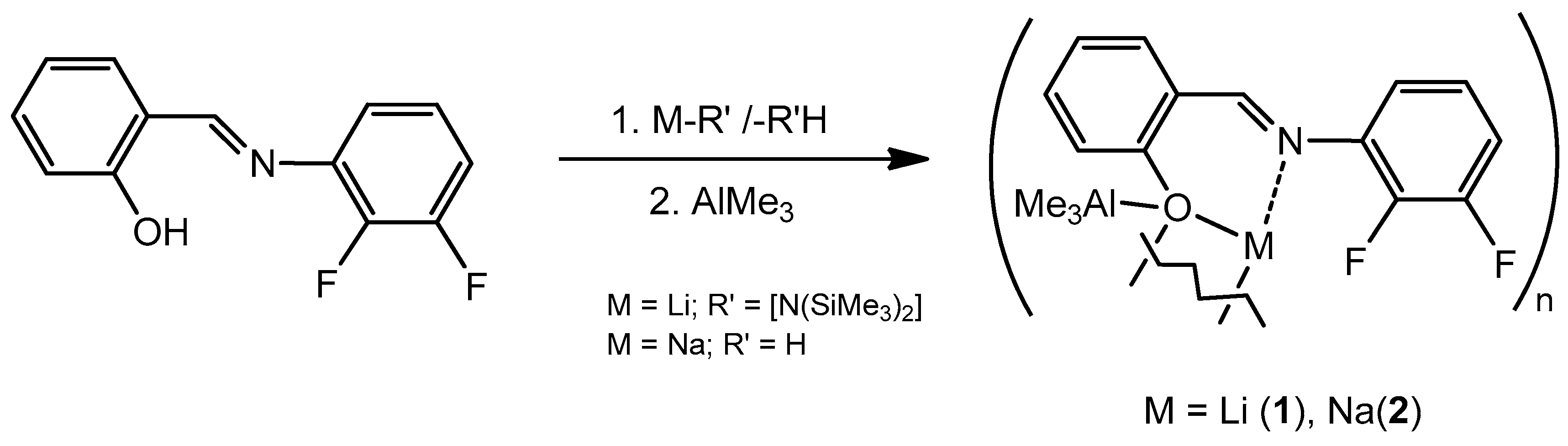
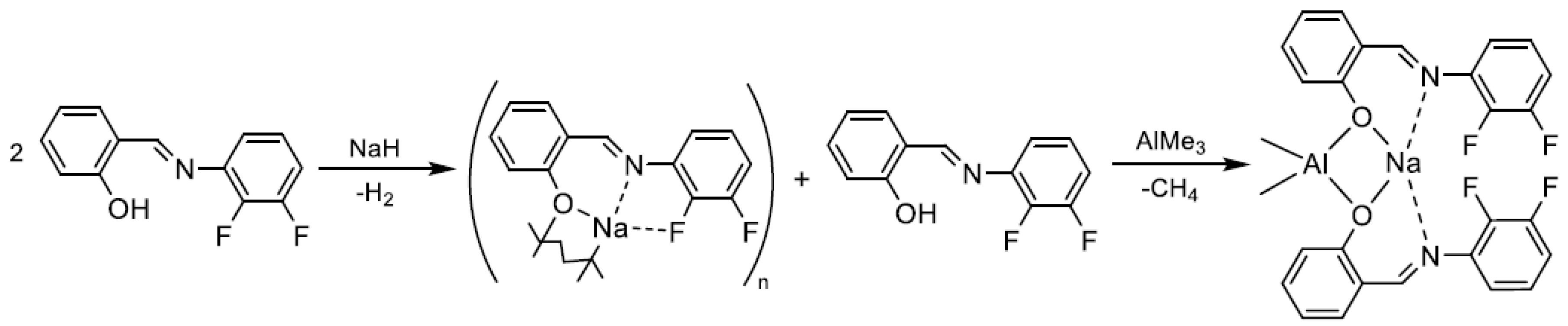
2. Results
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of Complex [LiAlMe3(O-2-{2,3-C6H3F2N=CH}C6H4)], [LiAlMe3La] (1)
3.3. Synthesis of Complex [NaAlMe3{(O-2-(2,3-C6H3F2N=CH)C6H4)}], [NaAlMe3(La)] (2)
3.4. Synthesis of Complex [NaAlMe2{(O-2-(2,3-C6H3F2N=CH)C6H4)}2], [NaAlMe2(La)2] (3)
3.5. Single-crystal X-Ray Structure Determination for (1·2C6D6) and 2 (Table 1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- García-Alvarez, J.; Hevia, E.; Kennedy, A.R.; Klett, J.; Mulvey, R.E. Lewis base stabilized lithium TMP-aluminates: An unexpected fragmentation and capture reaction involving cyclic ether 1,4-dioxane. Chem. Commun. 2007, 23, 2402–2404. [Google Scholar] [CrossRef]
- Delferro, M.; Marks, T.J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Roesky, H.W. Assembling heterometals through oxygen: An efficient way to design homogeneous catalysts. Acc. Chem. Res. 2010, 43, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, R.E. s-Block metal inverse crowns: Synthetic and structural synergism in mixed alkali metal-magnesium (or zinc) amide chemistry. Chem. Commun. 2001, 12, 1049–1056. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Klett, J.; Mulvey, R.E.; Wright, D.S. Synergic sedation of sensitive anions: Alkali-mediated zincation of cyclic ethers and ethene. Science 2009, 326, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Marchand, A.; Mongin, F. Mixed AggregAte (MAA): A single concept for all dipolar organometallic aggregates. 1. Structural Data. Chem. Rev. 2013, 113, 7470–7562. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.E.H. Recent developments in the synthetic and structural chemistry of lithium zincates. New. J. Chem. 2004, 28, 435–443. [Google Scholar] [CrossRef]
- Conway, B.; Crosbie, E.; Kennedy, A.R.; Mulvey, R.E.; Robertson, S.D. Regioselective heterohalogenation of 4-halo-anisoles via a series of sequential ortho-aluminations and electrophilic halogenations. Chem. Commun. 2012, 48, 4674–4676. [Google Scholar] [CrossRef] [PubMed]
- Wittig, G.; Meyer, F.J.; Lange, G. Über das Verhalten von Diphenylmetallen als Komplexbildner. Justus Liebigs Ann. Chem. 1951, 571, 167–201. [Google Scholar] [CrossRef]
- Wittig, G. Komplexbildung und Reaktivität in der metallorganischen Chemie. Angew. Chem. 1958, 70, 65–71. [Google Scholar] [CrossRef]
- Mulvey, R.E.; Robertson, S.D. FascinATES: Mixed-metal ate compounds that function synergistically. Top. Organomet. Chem. 2014, 47, 129–158. [Google Scholar]
- Mulvey, R.E. Modern ate chemistry: Applications of synergic mixed alkali-metal-magnesium or -Zinc reagents in synthesis and structure building. Organometallics 2006, 25, 1060–1075. [Google Scholar] [CrossRef]
- Greer, J.A.; Blair, V.L.; Thompson, C.D.; Andrews, P.C. Simplifying metal-‘ate’ chemistry: Formation and comprehensive characterisation of a homo-metallic amido lithiate complex. Dalton Trans. 2016, 45, 10887–10890. [Google Scholar] [CrossRef] [PubMed]
- Mongin, F.; Harrison-Marchand, A. Mixed AggregAte (MAA): A single concept for all Dipolar organometallic aggregates. 2. Syntheses and Reactivities of Homo/HeteroMAAs. Chem. Rev. 2013, 113, 7563–7727. [Google Scholar] [CrossRef] [PubMed]
- Hatano, M.; Yamashita, K.; Mizuno, M.; Ito, O.; Ishihara, K. C-Selective and Diastereoselective Alkyl Addition to beta, gamma-Alkynyl-alpha-imino Esters with Zinc(II)ate Complexes. Angew. Chem. Int. Ed. 2015, 54, 2707–2711. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, R.E.; Blair, V.L.; Clegg, W.; Kennedy, A.R.; Klett, J.; Russo, L. Cleave and capture chemistry illustrated through bimetallic-induced fragmentation of tetrahydrofuran. Nat. Chem. 2010, 2, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, R.E.; Mongin, F.; Uchiyama, M.; Kondo, Y. Deprotonative Metalation Using Ate Compounds: Synergy, Synthesis, and Structure Building. Angew. Chem. Int. Ed. 2007, 46, 3802–3824. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, M.; Hevia, E. Polar organometallic strategies for regioselective C–H metallation of N-heterocyclic carbenes. Chem. Commun. 2018, 58, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Naka, H.; Uchiyama, M.; Matsumoto, Y.; Wheatley, A.E.H.; McPartlin, M.; Morey, J.V.; Kondo, Y. An Aluminum Ate Base: Its Design, Structure, Function, and Reaction Mechanism. J. Am. Chem. Soc. 2007, 129, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Naka, H.; Matsumoto, Y.; Ohwada, T. Regio-and chemoselective direct generation of functionalized aromatic aluminum compounds using aluminum ate base. J. Am. Chem. Soc. 2004, 126, 10526–10527. [Google Scholar] [CrossRef] [PubMed]
- Naka, H.; Morey, J.V.; Haywood, J.; Eisler, D.J.; McPartlin, M.; Garcia, F.; Kudo, H.; Kondo, Y.; Uchiyama, M.; Wheatley, A.E.H. Mixed Alkylamido Aluminate as a Kinetically Controlled Base. J. Am. Chem. Soc. 2008, 130, 16193–16200. [Google Scholar] [CrossRef] [PubMed]
- Krasovskiy, A.; Knochel, P. A LiCl-Mediated Br/Mg Exchange Reaction for the Preparation of Functionalized Aryl- and Heteroarylmagnesium Compounds from Organic Bromides. Angew. Chem. Int. Ed. 2004, 43, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Haag, B.; Mosrin, M.; Ila, H.; Malakhov, V.; Knochel, P. Regio- and Chemoselective Metalation of Arenes and Heteroarenes Using Hindered Metal Amide Bases. Angew. Chem. Int. Ed. 2011, 50, 9794–9824. [Google Scholar] [CrossRef] [PubMed]
- Hevia, E.; Chua, J.Z.; Garcia Alvarez, P.; Kennedy, A.R.; McCall, M.D. Exposing the hidden complexity of stoichiometric and catalytic metathesis reactions by elucidation of Mg-Zn hybrids. Proc. Natl. Acad. Sci. 2010, 107, 5294–5299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davin, L.; McLellan, R.; Kennedy, A.R.; Hevia, E. Ligand-induced reactivity of [small beta]-diketiminate magnesium complexes for regioselective functionalization of fluoroarenes via C-H or C-F bond activations. Chem. Commun. 2017, 53, 11650–11653. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Delgado, A.; Chen, E.Y. Single-Site Anionic Polymerization. Monomeric Ester Enolaluminate Propagator Synthesis, Molecular Structure, and Polymerization Mechanism. J. Am. Chem. Soc. 2005, 127, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.; Case, M.C.; Shusterman, A.J. Stereochemistry and Mechanism of the Ring-Opening Reaction of Cyclopropylenones with LiCu(Me)2. Organometallics 2012, 31, 7849–7854. [Google Scholar] [CrossRef]
- Harvey, M.J.; Proffitt, M.; Wei, P.; Atwood, D.A. Monomeric uni-ligated aluminates. Chem. Commun. 2001, 20, 2094–2095. [Google Scholar] [CrossRef]
- Singh, S.; Chai, J.; Pal, A.; Jancik, V.; Roesky, H.W.; Herbst-Irmer, R. Base free lithium-organoaluminate and the gallium congener: Potential precursors to heterometallic assemblies. Chem. Commun. 2007, 46, 4934–4936. [Google Scholar] [CrossRef]
- Pollard, V.A.; Orr, S.A.; McLellan, R.; Kennedy, A.R.; Hevia, E.; Mulvey, R.E. Lithium diamidodihydridoaluminates: Bimetallic cooperativity in catalytic hydroboration and metallation applications. Chem. Commun. 2018, 54, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Guo, Z.; Han, H.; Wei, X. N,N,O-Tridentate Mixed Lithium–Magnesium and Lithium–Aluminum Complexes: Synthesis, Characterization, and Catalytic Activities. Organometallics 2017, 36, 877–883. [Google Scholar] [CrossRef]
- Amstrong, D.R.; Crosbie, E.; Hevia, E.; Mulvey, R.E.; Ramsay, D.L.; Robertson, S.D. TMP (2,2,6,6-tetramethylpiperidide)-aluminate bases: Lithium-mediated alumination or lithiation-alkylaluminium-trapping reagents? Chem. Sci. 2014, 5, 3031–3045. [Google Scholar] [CrossRef]
- Mulvey, R.E.; Amstrong, D.R.; Conway, B.; Crosbie, E.; Kennedy, A.R.; Robertson, S.D. Structurally Powered Synergic 2,2,6,6-Tetramethylpiperidine Bimetallics: New Reflections through Lithium-Mediated Ortho Aluminations. Inorg. Chem. 2011, 50, 12241–12251. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.; García-Álvarez, P.; Kennedy, A.R.; Klett, J.; Mulvey, R.E.; Robertson, S.D. Structurally Engineered Deprotonation/Alumination of THF and THTP with Retention of Their Cycloanionic Structures. Angew. Chem. Int. Ed. 2010, 49, 9388–9391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohbogner, C.J.; Wunderlich, S.H.; Clososki, G.C.; Knochel, P. New Mixed Li/Mg and Li/Mg/Zn Amides for the Chemoselective Metallation of Arenes and Heteroarenes. Eur. J. Org. Chem. 2009, 11, 1781–1795. [Google Scholar] [CrossRef]
- Muñoz, M.T.; Urbaneja, C.; Temprado, M.; Mosquera, M.E.G.; Cuenca, T. Lewis acid fragmentation of a lithium aryloxide cage: Generation of new heterometallic aluminium-lithium species. Chem. Commun. 2011, 47, 11757–11759. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.T.; Cuenca, T.; Mosquera, M.E.G. Heterometallic aluminates: Alkali metals trapped by an aluminium aryloxide claw. Dalton Trans. 2014, 43, 14377–14385. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.T.; Barandika, G.; Bazán, B.; Cuenca, T.; Mosquera, M.E.G. Aluminum Alkali Metalate Derivatives: Factors Driving the Final Nuclearity in the Crystal Form. Eur. J. Inorg. Chem. 2017, 2017, 1994–2001. [Google Scholar]
- Fernández-Millán, M.; Temprado, M.; Cano, J.; Cuenca, T.; Mosquera, M.E.G. Synthesis of novel chiral heterometallic terpene oximates: Unusual generation of an aluminium enolate by a cooperative effect. Dalton Trans. 2016, 45, 10514–10518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jian, C.; Gao, Y.; Wang, L.; Tang, N.; Wu, J. Synthesis and Characterization of Multi-Alkali-Metal Tetraphenolates and Application in Ring-Opening Polymerization of Lactide. Inorg. Chem. 2012, 51, 13380–13389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiong, J.; Sun, Y.; Tang, N.; Wu, J. Highly Iso-Selective and Active Catalysts of Sodium and Potassium Monophenoxides Capped by a Crown Ether for the Ring-Opening Polymerization of rac-Lactide. Macromolecules 2014, 47, 7789–7796. [Google Scholar] [CrossRef]
- Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Stereoselective ring-opening polymerization of a racemic lactide by using achiral salen- and homosalen-aluminum complexes. Chem.-Eur. J. 2007, 13, 4433–4451. [Google Scholar] [CrossRef] [PubMed]
- Normand, M.; Dorcet, V.; Kirillov, E.; Carpentier, J.-F. {Phenoxy-imine} aluminum versus-indium complexes for the immortal ROP of lactide: Different stereocontrol, different mechanisms. Organometallics 2013, 32, 1694–1709. [Google Scholar] [CrossRef]
- Garcia-Valle, F.M.; Tabernero, V.; Cuenca, T.; Cano, J.; Mosquera, M.E.G. Schiff-base -ate derivatives with main group metals: Generation of a tripodal aluminate metalloligand. Dalton Trans. 2018, 47, 6499–6506. [Google Scholar] [CrossRef] [PubMed]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI Catalysts for Olefin Polymerization-A Comprehensive Treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef] [PubMed]
- Dagorne, S.; Janowska, I.; Welter, R.; Zakrzewski, J.; Jaouen, G. Synthesis and Structure of a Four-Coordinate Aluminum Alkyl Cation/HB(C6F5)3 Salt: Implication in a B(C6F5)3-Catalyzed Hydroalumination Reaction of Benzophenone or Benzaldehyde. Organometallics 2004, 23, 4706–4710. [Google Scholar] [CrossRef]
- Martínez, G.; Cuenca, T.; Mosquera, M.E.G. Effect of the Nitrogen Substituent on the Reactions of Alane towards Imino- and Aminophenols: Generation of a Dinuclear Aluminoxane. Eur. J. Inorg. Chem. 2012, 22, 3611–3617. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Estivill, R.; Gallegos, C.; Cuenca, T.; Mosquera, M.E.G.; Tabernero, V.; Cano, J. Metal and Ligand-Substituent Effects in the Immortal Polymerization of rac-Lactide with Li, Na, and K Phenoxo-imine Complexes. Organometallics 2015, 34, 477–487. [Google Scholar] [CrossRef]
- Iwasa, N.; Katao, S.; Liu, J.; Fujiki, M.; Furukawa, Y.; Nomura, K. Notable Effect of Fluoro Substituents in the Imino Group in Ring-Opening Polymerization of ε-Caprolactone by Al Complexes Containing Phenoxyimine Ligands. Organometallics 2009, 28, 2179–2187. [Google Scholar] [CrossRef]
- Saito, J.; Mitani, M.; Mohri, J.-I.; Yoshida, Y.; Matsui, S.; Ishii, S.-I.; Kojoh, S.-I.; Kashiwas, N.; Fujita, T. Living Polymerization of Ethylene with a Titanium Complex Containing Two Phenoxy-Imine Chelate Ligands. Angew. Chem. Int. Ed. 2001, 40, 2918–2920. [Google Scholar] [CrossRef]
- Kasumov, V.T.; Uçar, I.; Bulut, A. Synthesis, structural, spectroscopic and reactivity properties of a new N-2,3,4-trifluorophenyl-3,5-di-tert-butylsalicylaldimine ligand and its Cu(II) and Pd(II) complexes. J. Fluorine Chem. 2010, 131, 59–65. [Google Scholar] [CrossRef]
- Cambridge Structural Database (CSD version 5.39, May 2018). Available online: https://www.ccdc.cam.ac.uk/support-and-resources/ccdcresources/csd-2018-updates/ (accessed on 23 November 2018).
- Pang, W.; Zhao, J.-W.; Zhao, L.; Zhang, Z.-K.; Zhu, S.-Z. Synthesis, characterization and comparative study of a series of fluorinated Schiff bases containing different orientation CHN spacers. J. Mol. Struct. 2015, 1096, 21–28. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Tabernero, V.; Cuenca, T.; Mosquera, M.E.G.; Cano, J.; Milione, S. Biodegradable PHB from rac-β-Butyrolactone: Highly Controlled ROP Mediated by a Pentacoordinated Aluminum Complex. Organometallics 2018, 37, 837–840. [Google Scholar] [CrossRef]
- Maddock, L.C.H.; Nixon, T.; Kennedy, A.R.; Hevia, E.; Probert, M.R.; Clegg, W. Utilising Sodium-Mediated Ferration for Regioselective Functionalisation of Fluoroarenes via C-H and C-F Bond Activations. Angew. Chem. Int. Ed. 2018, 57, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Dietrich, H.M.; Schadle, C.; Maichle-Mossmer, C.; Tsurugi, H.; Tornroos, K.W.; Mashima, K.; Anwander, R. Synthesis of Rare-Earth-Metal Iminopyrrolyl Complexes from Alkyl Precursors: Ln→Al N-Ancillary Ligand Transfer. Organometallics 2013, 32, 1199–1208. [Google Scholar] [CrossRef]
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Crystallogr. Sect. A 1995, 51, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A 1990, 46, 194–201. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |






| [LiAlMe3La]·2C6D6 | [NaAlMe3La] | |
|---|---|---|
| Empirical formula | C32H34Al2Li2F4N2O2·2C6D6 | C32H34Al2Na2F4N2O2 |
| Formula weight | 790.59 | 654.55 |
| Colour, shape | Yellow/block | Yellow/block |
| Crystal size (mm) | 0.45 × 0.42 × 0.27 | 0.49 × 0.48 × 0.45 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21/c | P-1 |
| a (Å) | 11.699(3) | 8.5668(8) |
| b (Å) | 16.669(6) | 10.2749(8) |
| c (Å) | 10.7558(17) | 11.1625(8) |
| α (°) | 90 | 111.026(6) |
| β (°) | 90.35(2) | 91.093(5) |
| γ (°) | 90 | 112.001(6) |
| V (Å3) | 2097.5(9) | 836.87(13) |
| Z | 2 | 1 |
| ρcalcd. (mg m−3) | 1.233 | 1.299 |
| F000 | 816 | 340 |
| μ (mm−1) | 0.125 | 0.166 |
| θ Range (°) | 3.001 to 27.518 | 3.063 to 27.498 |
| Reflns. Collected | 16768 | 7166 |
| Indep. Reflns./R(int) | 4744/0.2050 | 3829/0.0734 |
| Data/restraints/param | 4744/147/203 | 3829/0/215 |
| R1/wR2 (I > 2σ(I)) a | 0.0982/0.2357 | 0.0498/0.1165 |
| R1/wR2 (all data)a | 0.1892/0.2927 | 0.1230/0.1505 |
| GOF | 0.873 | 0.918 |
| Max/min Δρ (e.Å−3) | 0.524 and −0.931 | 0.238 and −0.473 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Valle, F.M.; Tabernero, V.; Cuenca, T.; Cano, J.; Mosquera, M.E.G. Aluminates with Fluorinated Schiff Bases: Influence of the Alkali Metal–Fluorine Interactions in Structure Stabilization. Molecules 2018, 23, 3108. https://doi.org/10.3390/molecules23123108
García-Valle FM, Tabernero V, Cuenca T, Cano J, Mosquera MEG. Aluminates with Fluorinated Schiff Bases: Influence of the Alkali Metal–Fluorine Interactions in Structure Stabilization. Molecules. 2018; 23(12):3108. https://doi.org/10.3390/molecules23123108
Chicago/Turabian StyleGarcía-Valle, Francisco M., Vanessa Tabernero, Tomás Cuenca, Jesús Cano, and Marta E. G. Mosquera. 2018. "Aluminates with Fluorinated Schiff Bases: Influence of the Alkali Metal–Fluorine Interactions in Structure Stabilization" Molecules 23, no. 12: 3108. https://doi.org/10.3390/molecules23123108







