Aberrant Glycosylation Augments the Immuno-Stimulatory Activities of Soluble Calreticulin
Abstract
:1. Introduction
2. Results
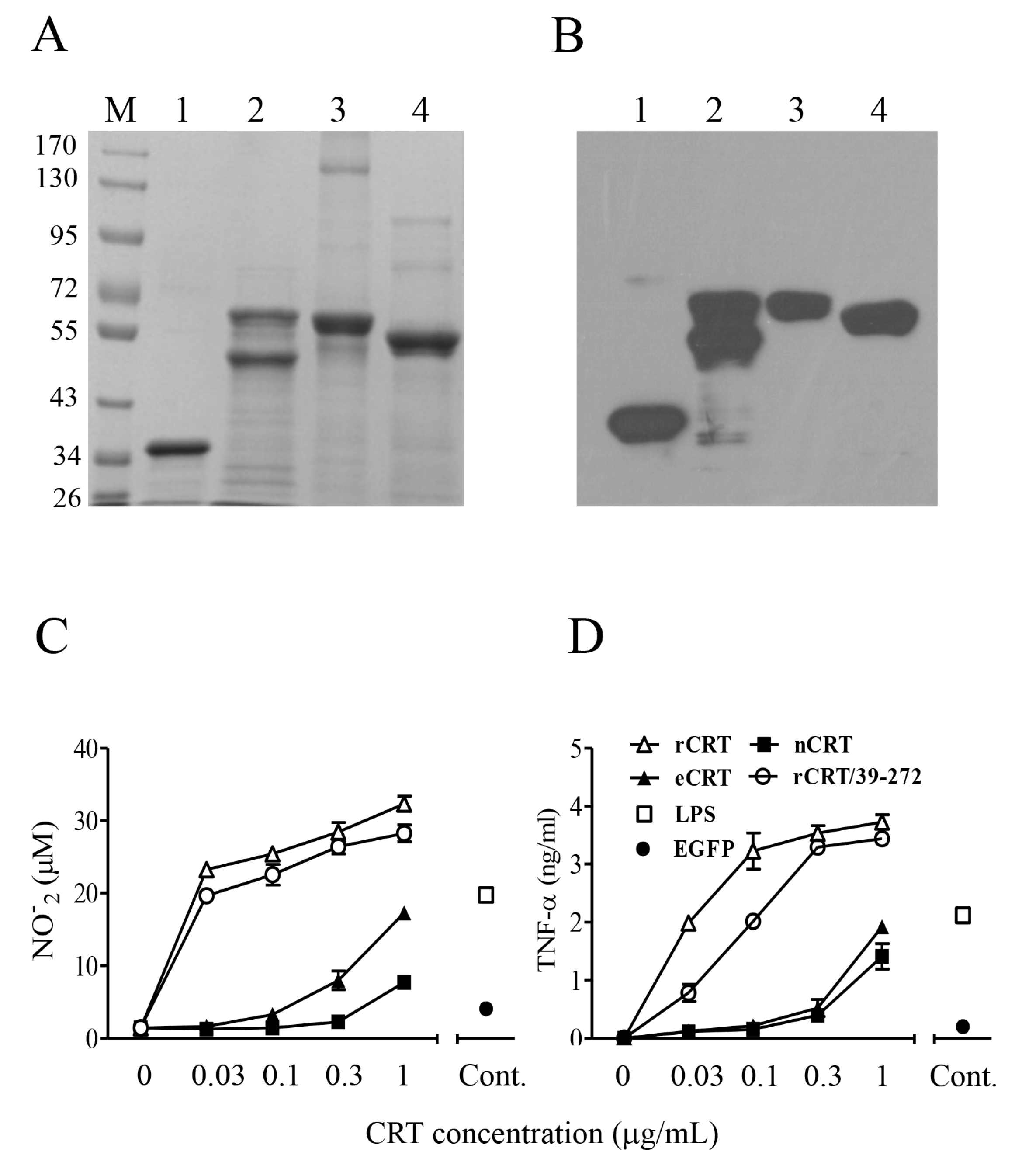
2.1. Expression, Purification and Functional Characterization of eCRT
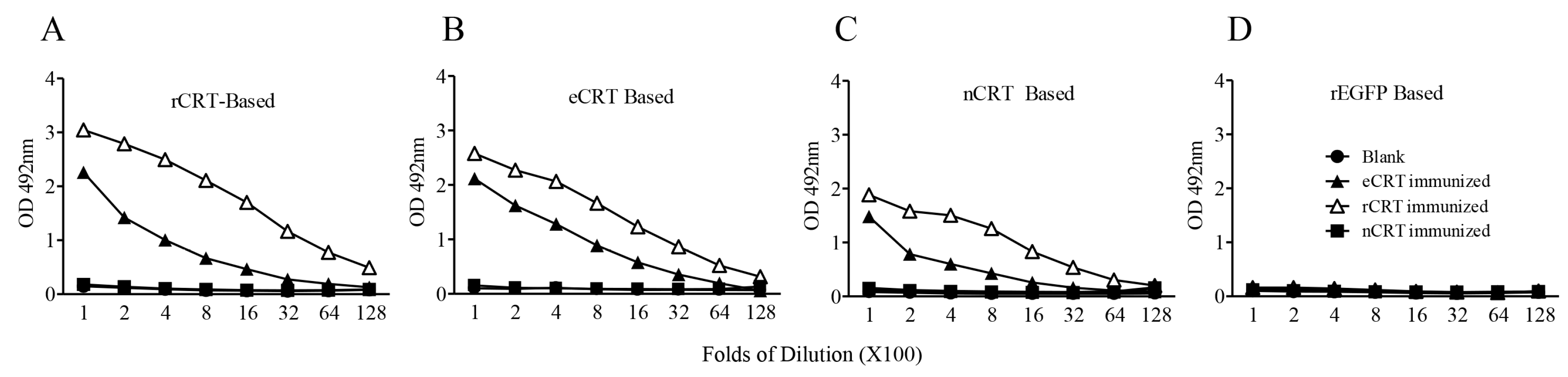
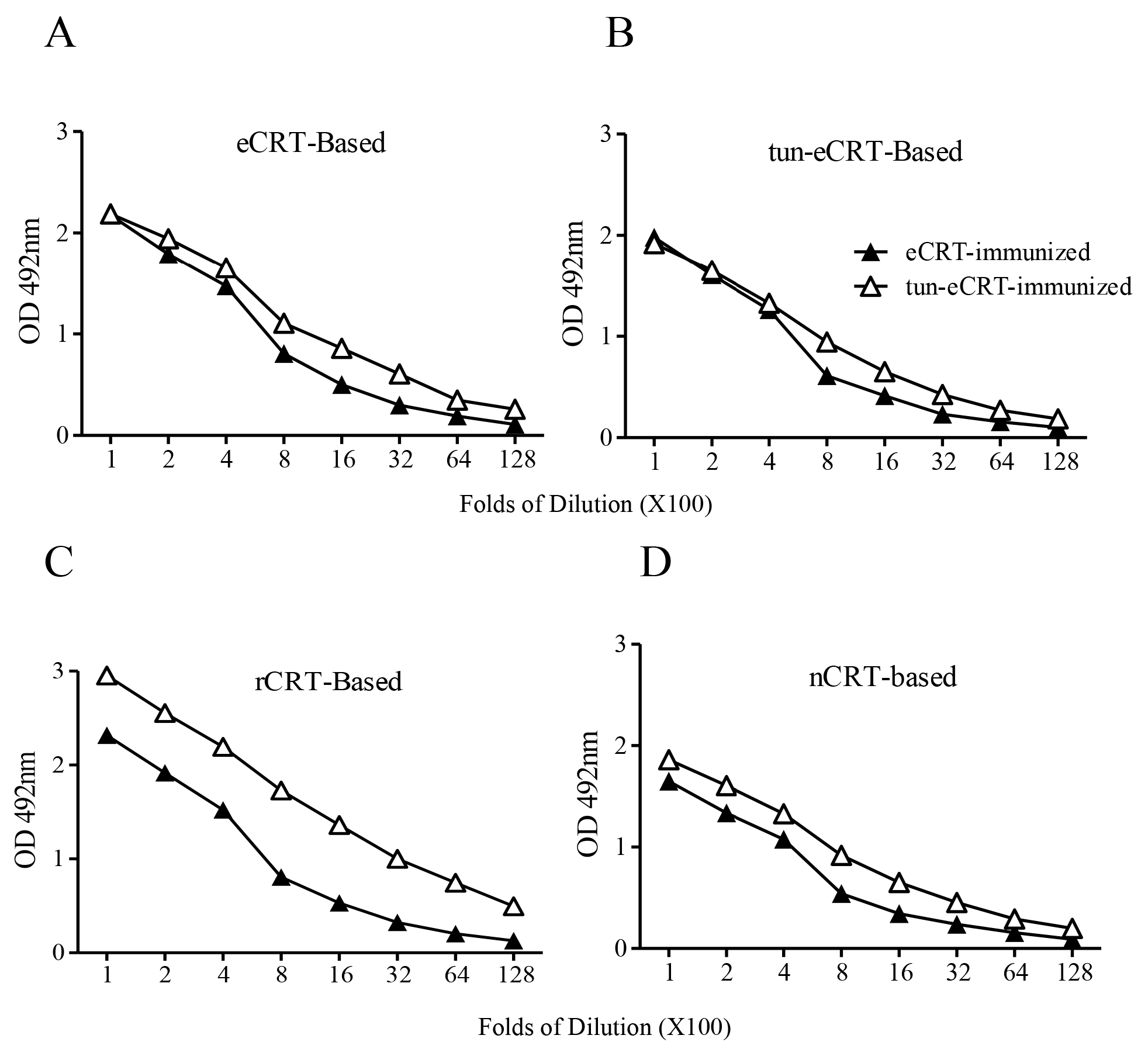
2.2. Immunogenicity of eCRT, rCRT and nCRT
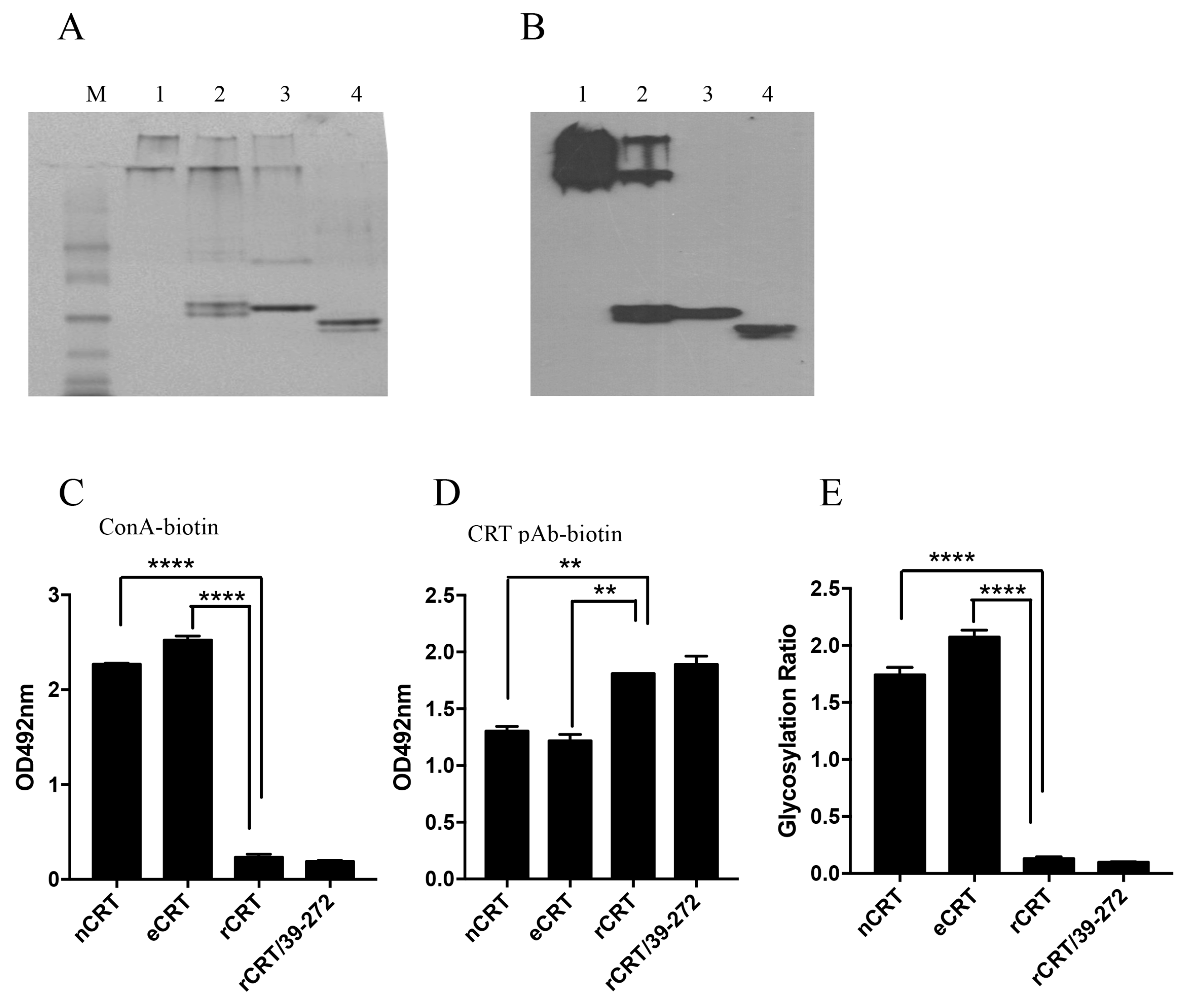
2.3. Biochemical Comparison of eCRT and nCRT
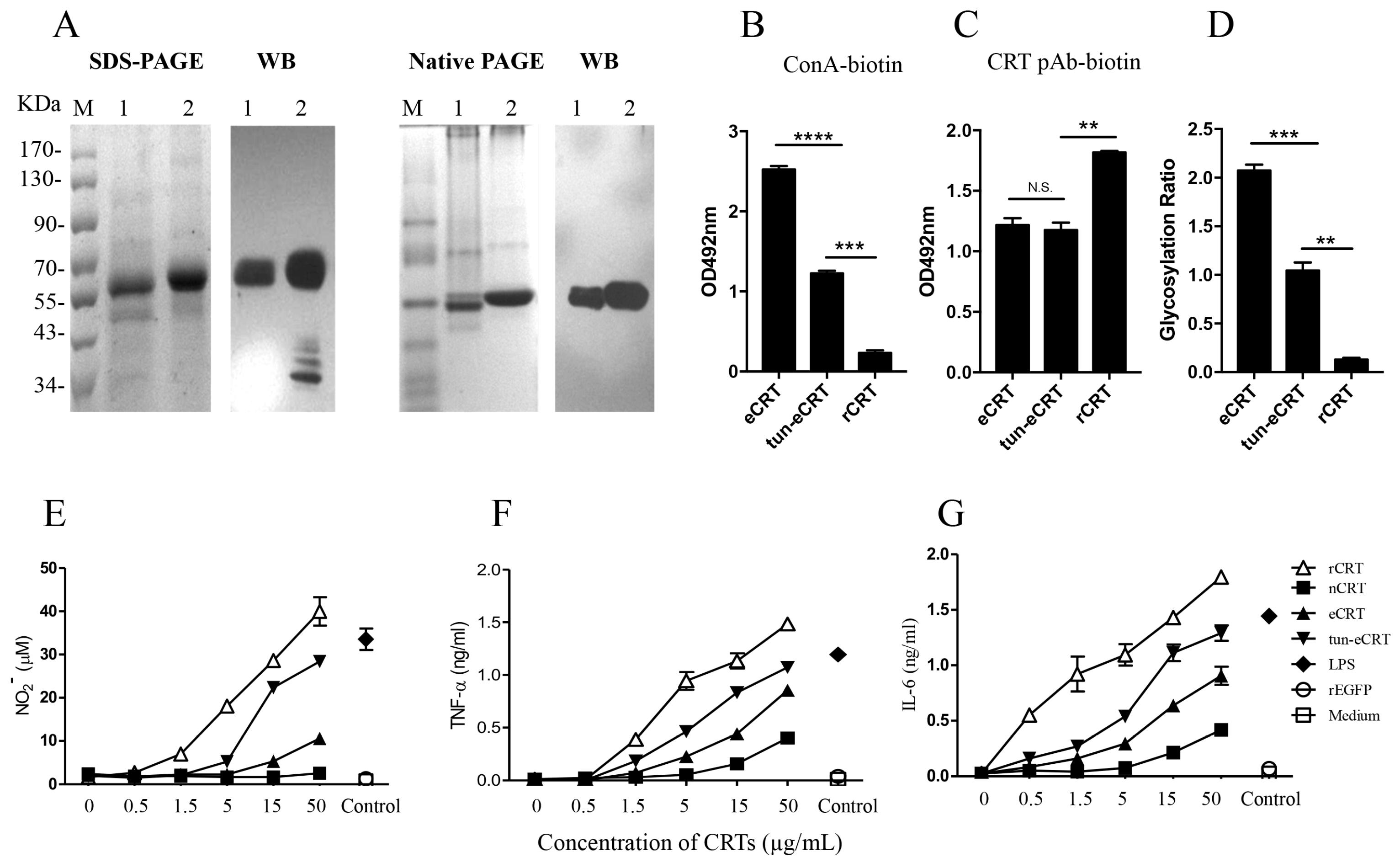
2.4. Dys-glycosylation of eCRT Correlates to Enhanced Immunological Activity
3. Discussion
4. Materials and Methods
4.1. Construction of Plasmid pIRES-eCRT
4.2. Expression and Purification of CRT
4.3. SDS-PAGE, Native PAGE and Western Blotting
4.4. Isolation and Culture of Mouse Peritoneal Macrophages
4.5. Immunization of Mice and Protein Based ELISA
4.6. Detection of N-glycosylation in CRTs
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zamanian, M.; Veerakumarasivam, A.; Abdullah, S.; Rosli, R. Calreticulin and cancer. Pathol. Oncol. Res. 2013, 19, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, D.; Forte, D.; Polverelli, N.; Perricone, M.; Romano, M.; Luatti, S.; Vianelli, N.; Cavo, M.; Palandri, F.; Catani, L. Circulating calreticulin is increased in myelofibrosis: Correlation with interleukin-6 plasma levels, bone marrow fibrosis, and splenomegaly. Mediat. Inflamm. 2016, 2016, 5860657. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Isono, T.; Matsuda, S.; Ushio, Y.; Satomura, S.; Terai, A.; Arai, Y.; Kawakita, M.; Okada, Y.; Yoshiki, T. Urinary calreticulin in the diagnosis of bladder urothelial carcinoma. Int. J. Urol. 2009, 16, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gong, J.; Chen, J.; Li, Q.; Song, C.; Zhang, J.; Li, Y.; Liu, Z.; Dong, Y.; Chen, L.; et al. Calreticulin as a potential diagnostic biomarker for lung cancer. Cancer Immunol. Immunother. 2012, 61, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Pike, S.E.; Yao, L.; Jones, K.D.; Cherney, B.; Appella, E.; Sakaguchi, K.; Nakhasi, H.; Teruya-Feldstein, J.; Wirth, P.; Gupta, G.; et al. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J. Exp. Med. 1998, 188, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Hong, C.; Wang, Y.; Liu, J.; Zhang, N.; Shen, C.; Wei, W.; Zheng, F. Calreticulin promotes angiogenesis via activating nitric oxide signalling pathway in rheumatoid arthritis. Clin. Exp. Immunol. 2014, 178, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Qiu, X.; Li, Y.; Huang, Q.; Zhong, Z.; Zhang, Y.; Liu, X.; Sun, L.; Lv, P.; Gao, X.M. Functional analysis of recombinant calreticulin fragment 39–272: Implications for immunobiological activities of calreticulin in health and disease. J. Immunol. 2010, 185, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Zhao, L.X.; Hong, C.; Duo, C.C.; Guo, B.N.; Zhang, L.J.; Gong, Z.; Xiong, S.D.; Gong, F.Y.; Gao, X.M. Self-oligomerization is essential for enhanced immunological activities of soluble recombinant calreticulin. PLoS ONE 2013, 8, e64951. [Google Scholar] [CrossRef] [PubMed]
- Dayer, J.M.; Choy, E. Therapeutic targets in rheumatoid arthritis: The interleukin-6 receptor. Rheumatology 2010, 49, 15–24. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, C.S.; Ryder, L.R.; Steino, A.; Hojrup, P.; Hansen, J.; Beyer, N.H.; Heegaard, N.H.; Houen, G. Dimerization and oligomerization of the chaperone calreticulin. Eur. J. Biochem. 2003, 270, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- He, M.C.; Wang, J.; Wu, J.; Gong, F.Y.; Hong, C.; Xia, Y.; Zhang, L.J.; Bao, W.R.; Gao, X.M. Immunological activity difference between native calreticulin monomers and oligomers. PLoS ONE 2014, 9, e105502. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: One protein, one gene, many functions. Biochem. J. 1999, 344, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Hirata, K. New function of calreticulin: Calreticulin-dependent mrna destabilization. Circ. Res. 2005, 97, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Jethmalani, S.M.; Henle, K.J.; Kaushal, G.P. Heat shock-induced prompt glycosylation. Identification of p-sg67 as calreticulin. J. Biol. Chem. 1994, 269, 23603–23609. [Google Scholar] [PubMed]
- Jeffery, E.; Peters, L.R.; Raghavan, M. The polypeptide binding conformation of calreticulin facilitates its cell-surface expression under conditions of endoplasmic reticulum stress. J. Biol. Chem. 2011, 286, 2402–2415. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.M.; Mancino, L.; Thammavongsa, V.; Cantley, R.L.; Raghavan, M. A polypeptide binding conformation of calreticulin is induced by heat shock, calcium depletion, or by deletion of the c-terminal acidic region. Mol. Cell 2004, 15, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Heifetz, A.; Keenan, R.W.; Elbein, A.D. Mechanism of action of tunicamycin on the udp-glcnac: Dolichyl-phosphate glc-nac-1-phosphate transferase. Biochemistry 1979, 18, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Huerta, E.; Mendoza-Hernandez, G.; Robles-Flores, M. Characterization of calreticulin as a protein interacting with protein kinase c. Biochem. J. 1999, 344, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.F.; Wassmann, K.; Berger, A.; Holz, S.; Wassmann, S.; Nickenig, G. Differential phosphorylation of calreticulin affects at1 receptor mrna stability in vsmc. Biochem. Biophys. Res. Commun. 2008, 370, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Carpio, M.A.; Decca, M.B.; Lopez Sambrooks, C.; Durand, E.S.; Montich, G.G.; Hallak, M.E. Calreticulin-dimerization induced by post-translational arginylation is critical for stress granules scaffolding. Int. J. Biochem. Cell Biol. 2013, 45, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Lopez Sambrooks, C.; Carpio, M.A.; Hallak, M.E. Arginylated calreticulin at plasma membrane increases susceptibility of cells to apoptosis. J. Biol. Chem. 2012, 287, 22043–22054. [Google Scholar] [CrossRef] [PubMed]
- Jethmalani, S.M.; Henle, K.J.; Gazitt, Y.; Walker, P.D.; Wang, S.Y. Intracellular distribution of heat-induced stress glycoproteins. J. Cell. Biochem. 1997, 66, 98–111. [Google Scholar] [CrossRef]
- Komatsu, S.; Yamada, E.; Furukawa, K. Cold stress changes the concanavalin a-positive glycosylation pattern of proteins expressed in the basal parts of rice leaf sheaths. Amino Acids 2009, 36, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cristina Castaneda-Patlan, M.; Razo-Paredes, R.; Carrisoza-Gaytan, R.; Gonzalez-Mariscal, L.; Robles-Flores, M. Protein kinase c is involved in the regulation of several calreticulin posttranslational modifications. Int. J. Biochem. Cell Biol. 2010, 42, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Van, P.N.; Peter, F.; Soling, H.D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J. Biol. Chem. 1989, 264, 17494–17501. [Google Scholar] [PubMed]
- Khanna, N.C.; Tokuda, M.; Waisman, D.M. Comparison of calregulins from vertebrate livers. Biochem. J. 1987, 242, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Seta, K.; Yamakawa, Y.; Okuyama, T.; Shinoda, T.; Isobe, T. Covalent structure of bovine brain calreticulin. Biochem. J. 1994, 298, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Denning, G.M.; Leidal, K.G.; Holst, V.A.; Iyer, S.S.; Pearson, D.W.; Clark, J.R.; Nauseef, W.M.; Clark, R.A. Calreticulin biosynthesis and processing in human myeloid cells: Demonstration of signal peptide cleavage and N-glycosylation. Blood 1997, 90, 372–381. [Google Scholar] [PubMed]
- Raghav, S.K.; Gupta, B.; Agrawal, C.; Saroha, A.; Das, R.H.; Chaturvedi, V.P.; Das, H.R. Altered expression and glycosylation of plasma proteins in rheumatoid arthritis. Glycoconj. J. 2006, 23, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Chui, D.; Sellakumar, G.; Green, R.; Sutton-Smith, M.; McQuistan, T.; Marek, K.; Morris, H.; Dell, A.; Marth, J. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc. Natl. Acad Sci. USA 2001, 98, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.G.; Palotai, R.; Antal, P.; Tokatly, I.; Tothfalusi, L.; Lund, O.; Nagy, G.; Falus, A.; Buzas, E.I. Critical role of glycosylation in determining the length and structure of t cell epitopes. Immunome Res. 2009, 5, 4. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, F.-Y.; Gong, Z.; Duo, C.-C.; Wang, J.; Hong, C.; Gao, X.-M. Aberrant Glycosylation Augments the Immuno-Stimulatory Activities of Soluble Calreticulin. Molecules 2018, 23, 523. https://doi.org/10.3390/molecules23030523
Gong F-Y, Gong Z, Duo C-C, Wang J, Hong C, Gao X-M. Aberrant Glycosylation Augments the Immuno-Stimulatory Activities of Soluble Calreticulin. Molecules. 2018; 23(3):523. https://doi.org/10.3390/molecules23030523
Chicago/Turabian StyleGong, Fang-Yuan, Zheng Gong, Cui-Cui Duo, Jun Wang, Chao Hong, and Xiao-Ming Gao. 2018. "Aberrant Glycosylation Augments the Immuno-Stimulatory Activities of Soluble Calreticulin" Molecules 23, no. 3: 523. https://doi.org/10.3390/molecules23030523
APA StyleGong, F. -Y., Gong, Z., Duo, C. -C., Wang, J., Hong, C., & Gao, X. -M. (2018). Aberrant Glycosylation Augments the Immuno-Stimulatory Activities of Soluble Calreticulin. Molecules, 23(3), 523. https://doi.org/10.3390/molecules23030523



