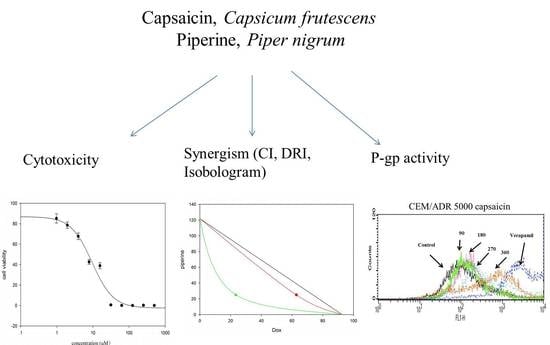
Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of Capsaicin and Piperine in Different Cancer Cell Lines
2.2. Capsaicin and Piperine Increased the Sensitivity of MDR Cancer Cells to Doxorubicin
2.3. Capsaicin and Piperine Inhibited the Activity of P-gp
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cytotoxicity Assays
4.4. Drug Combination Assays
4.5. Analysis of Combination Effects
4.6. Activity of ABC Transporters
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for anticancer drug development. Front. Plant Sci. 2016, 7, 1667. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.; Erba, P.; Mariani, G.; Gomes, C.M. Multidrug resistance in cancer: Its mechanism and its modulation. Drug News Perspect. 2007, 20, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 2012, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Geelen, M.J. The use of digitonin-permeabilized mammalian cells for measuring enzyme activities in the course of studies on lipid metabolism. Anal. Biochem. 2005, 347, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Yamashita, T.; Tsukiyama, T.; Kitamura, N.; Minami, N.; Yamada, M.; Imai, H. Reversible membrane permeabilization of mammalian cells treated with digitonin and its use for inducing nuclear reprogramming by Xenopus egg extracts. Cloning Stem Cells 2008, 10, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Sudji, I.R.; Subburaj, Y.; Frenkel, N.; García-Sáez, A.J.; Wink, M. Membrane disintegration caused by the steroid saponin digitonin is related to the presence of cholesterol. Molecules 2015, 20, 20146–20160. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine 2012, 19, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Digitonin synergistically enhances the cytotoxicity of plant secondary metabolites in cancer cells. Phytomedicine 2012, 19, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T. Overcoming multidrug resistance in human cancer cells by natural compounds. Toxins 2010, 2, 1207–1224. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. 2012, 33, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.; Jepson, M.A.; Tsuruo, T.; Simmons, N.L.; Hirst, B.H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. Kinetics of vinblastine secretion and interaction with modulators. J. Biol. Chem. 1993, 268, 14991–14997. [Google Scholar] [PubMed]
- Efferth, T.; Davey, M.; Olbrich, A.; Rücker, G.; Gebhart, E.; Davey, R. Activity of drugs from traditional Chinese medicine toward sensitive and MDR1-or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells Mol. Dis. 2002, 28, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cheng, X.; Wink, M. Natural lignans from Arctium lappa modulate P-glycoprotein efflux function in multidrug resistant cancer cells. Phytomedicine 2015, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Krstin, S.; Peixoto, H.S.; Wink, M. Combinations of alkaloids affecting different molecular targets with the saponin digitonin can synergistically enhance trypanocidal activity against Trypanosoma brucei brucei. Antimicrob. Agents Chemother. 2015, 59, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Keum, Y.S.; Seo, H.J.; Chun, K.S.; Lee, S.S.; Surh, Y.J. Capsaicin suppresses phorbol ester-induced activation of NF-kappaB/Rel and AP-1 transcription factors in mouse epidermis. Cancer Lett. 2001, 164, 119–126. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Doucette, C.D.; Hilchie, A.L.; Liwski, R.; Hoskin, D.W. Piperine, a dietary phytochemical, inhibits angiogenesis. J. Nutr. Biochem. 2013, 24, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; Pretoria: Briza, South Africa, 2017. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs and Poisons; Pretoria: Briza, South Africa, 2015. [Google Scholar]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Y.; Jia, Y.; Li, N.; Wink, M.; Ma, Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 2011, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Ternesten-Hasseus, E.; Johansson, E.L.; Millqvist, E. Cough reduction using capsaicin. Respir. Med. 2015, 109, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Laslett, L.L.; Jones, G. Capsaicin for osteoarthritis pain. Prog. Drug Res. 2014, 68, 277–291. [Google Scholar] [PubMed]
- Venier, N.A.; Yamamoto, T.; Sugar, L.; Fleshner, N.; Klotz, L.; Venkateswaran, V. Capsaicin reduces the metastatic burden in the transgenic adenocarcinoma of the mouse prostate model. Prostate 2015. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Motiwala, M.N.; Dumore, N.G.; Danao, K.R.; Ganjare, A.B. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J. Ethnopharmacol. 2015, 164, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.J.; Kolla, S.D.; Elshaari, F.; Elshaari, F.; Awamy, H.E.; Elfrady, M.; Singh, R.; Belkhier, A.; Srikumar, S.; Said, A.R.; et al. Effect of piperine on liver function of CF-1 albino mice. Infect. Disord. Drug Targets 2015, 15, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Capsaicin, piperine and digitonin are available from the authors. |

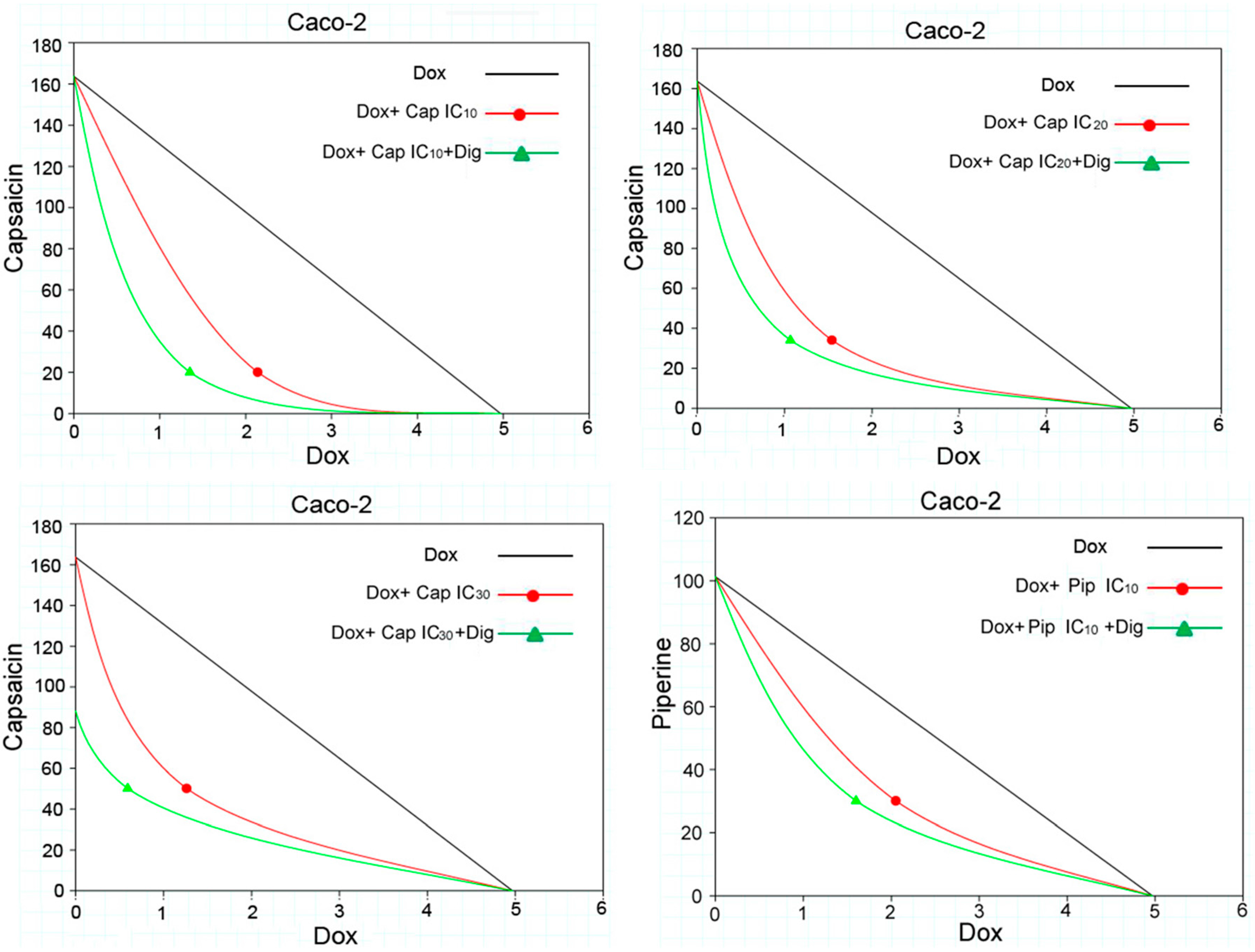
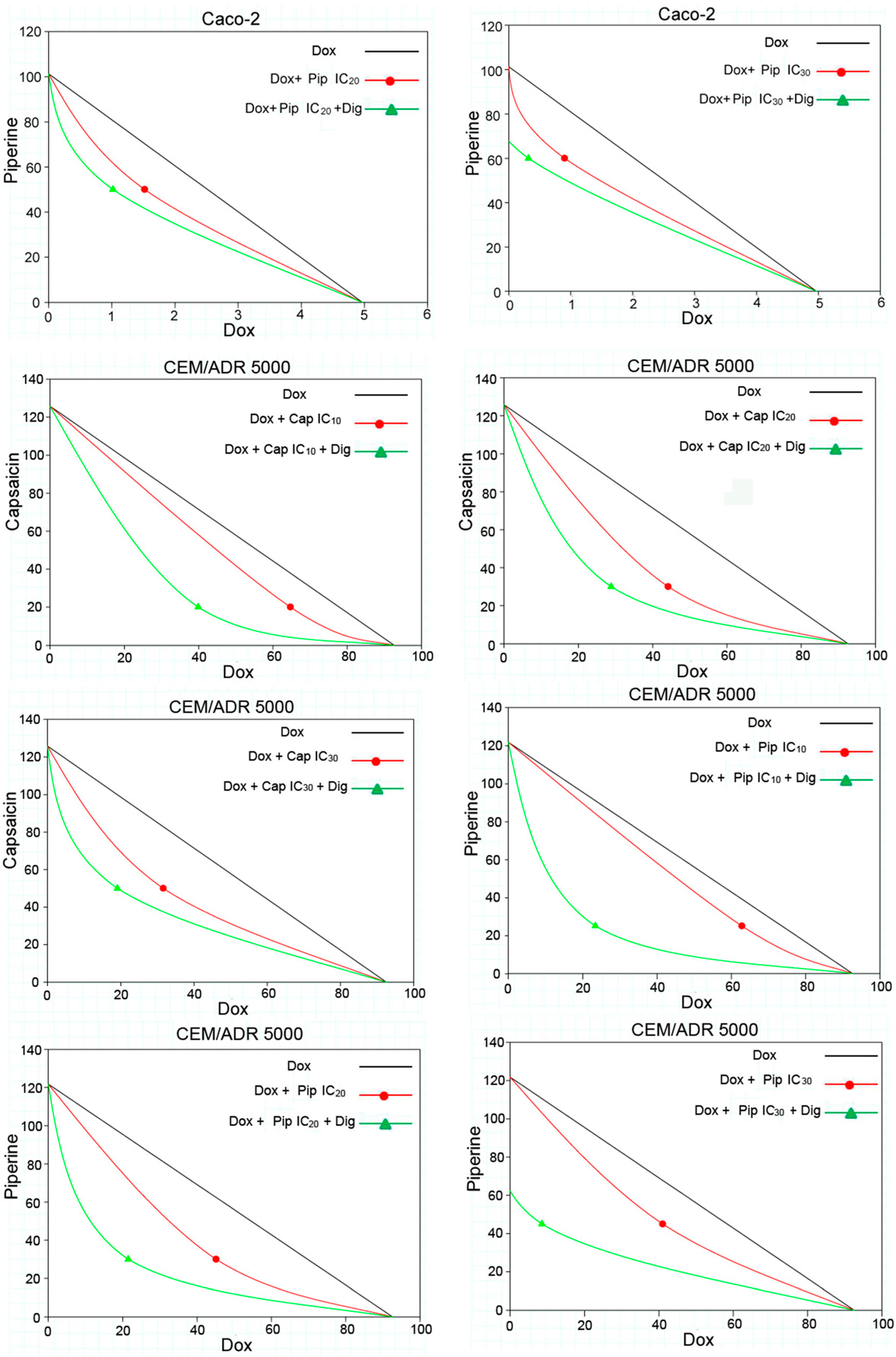

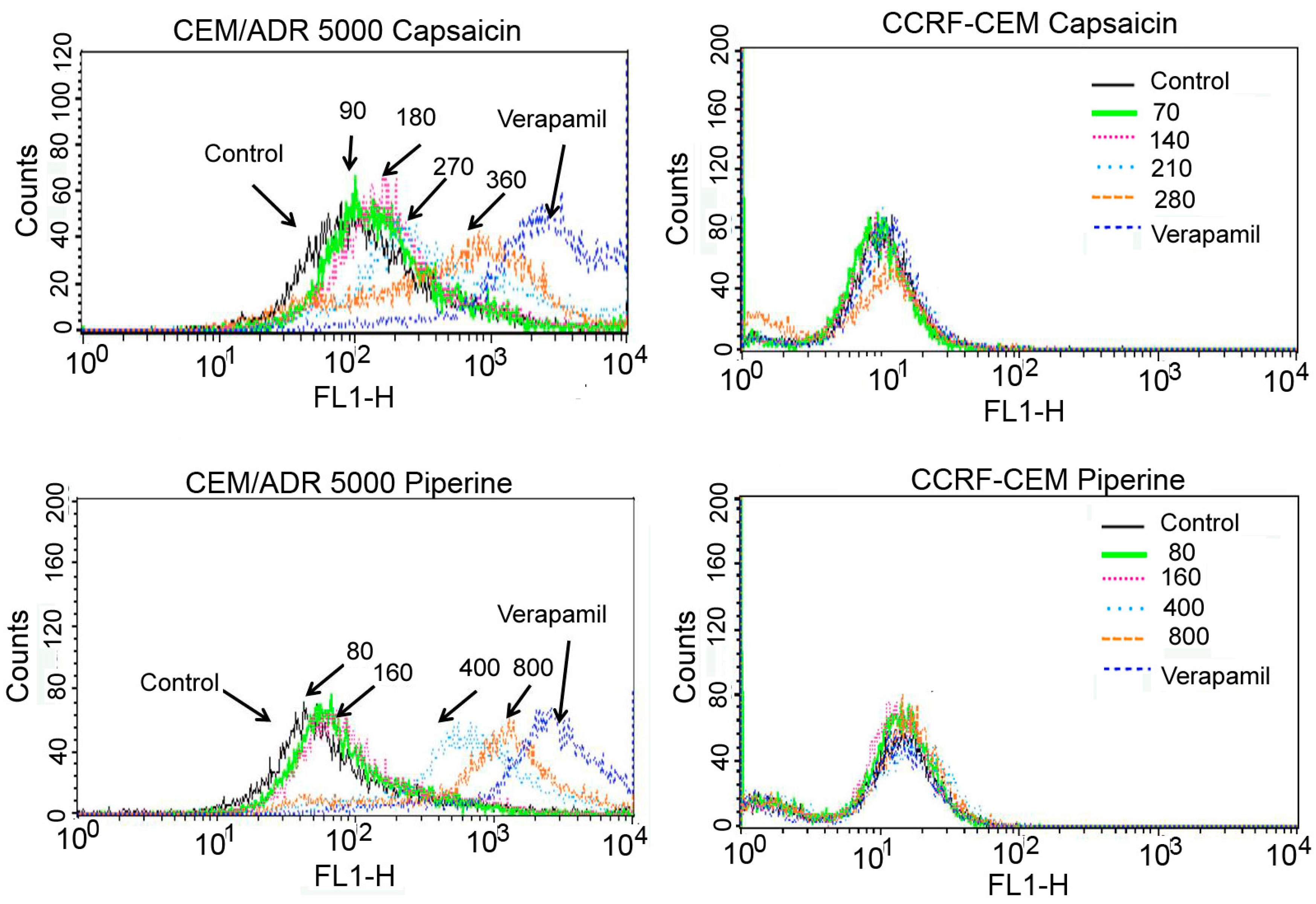
| Compounds | Caco-2 | HCT 116 | CEM/ADR 5000 | CCRF-CEM |
|---|---|---|---|---|
| Doxorubicin | 4.97 ± 0.36 | 0.92 ± 0.07 | 92.59 ± 7.90 | 0.37 ± 0.14 |
| Capsaicin | 163.70 ± 9.32 | 66.77 ± 10.78 | 125.85 ± 22.05 | 67.55 ± 6.29 |
| Piperine | 101.30 ± 6.97 | 74.30 ± 10.35 | 121.77 ± 17.35 | 102.18 ± 6.82 |
| Digitonin | 18.39 ± 1.44 | 9.04 ± 0.73 | 16.69 ± 2.06 | 10.34 ± 1.18 |
| Two-Drug Combinations | Three-Drug Combinations with Digitonin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 of Dox | IB | DRI | CI | Interpretation | IC50 of Dox | IB | DRI | CI | Interpretation | |
| Doxorubicin alone | 4.97 ± 0.36 | 1.00 | NR | NR | 4.97 ± 0.36 | 1.00 | NR | NR | ||
| Dox + Capsaicin | ||||||||||
| 20 μM (IC10) | 2.14 ± 0.37 | syn | 2.33 | 0.56 | + + + | 1.35 ± 0.56 | syn | 3.68 | 0.30 | + + + |
| 34 μM (IC20) | 1.54 ± 0.51 | syn | 3.22 | 0.42 | + + + | 1.07 ± 0.34 | syn | 4.66 | 0.35 | + + + |
| 50 μM (IC30) | 1.26 ± 0.04 | syn | 3.94 | 0.57 | + + + | 0.59 ± 0.12 | syn | 8.39 | 0.41 | + + + |
| Dox + Piperine | ||||||||||
| 30 μM (IC10) | 2.05 ± 0.39 | syn | 2.42 | 0.80 | + + | 1.60 ± 0.65 | syn | 3.11 | 0.77 | + + |
| 50 μM (IC20) | 1.52 ± 0.10 | syn | 3.27 | 0.81 | + + | 1.02 ± 0.13 | syn | 4.88 | 0.72 | + + |
| 65 μM (IC30) | 0.90 ± 0.14 | syn | 5.52 | 0.76 | + + | 0.32 ± 0.09 | syn | 15.65 | 0.63 | + + + |
| Two-Drug Combinations | Three-Drug Combinations with Digitonin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 of Dox | IB | DRI | CI | Interpretation | IC50 of Dox | IB | DRI | CI | Interpretation | |
| Doxorubicin alone | 92.59 ± 7.90 | 1.00 | NR | NR | 92.59 ± 7.90 | 1.00 | NR | NR | ||
| Dox + Capsaicin | ||||||||||
| 20 μM (IC10) | 64.73 ± 10.40 | syn | 1.43 | 0.86 | + | 39.87 ± 8.01 | syn | 2.32 | 0.59 | + + + |
| 30 μM (IC20) | 44.17 ± 7.78 | syn | 2.10 | 0.72 | + + | 28.84 ± 5.65 | syn | 3.21 | 0.55 | + + + |
| 50 μM (IC30) | 31.61 ± 9.56 | syn | 2.93 | 0.74 | + + | 19.10 ± 7.02 | syn | 4.85 | 0.60 | + + + |
| Dox + Piperine | ||||||||||
| 25 μM (IC10) | 62.85 ± 9.65 | syn | 1.47 | 0.88 | + | 23.39 ± 4.28 | syn | 3.96 | 0.46 | + + + |
| 30 μM (IC20) | 45.13 ± 7.92 | syn | 2.05 | 0.69 | + + + | 21.52 ± 1.02 | syn | 4.30 | 0.44 | + + + |
| 45 μM (IC30) | 41.02 ± 9.27 | syn | 2.26 | 0.65 | + + + | 8.54 ± 4.55 | syn | 10.84 | 0.30 | + + + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Krstin, S.; Wang, S.; Wink, M. Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules 2018, 23, 557. https://doi.org/10.3390/molecules23030557
Li H, Krstin S, Wang S, Wink M. Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules. 2018; 23(3):557. https://doi.org/10.3390/molecules23030557
Chicago/Turabian StyleLi, Hanmei, Sonja Krstin, Shihui Wang, and Michael Wink. 2018. "Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin" Molecules 23, no. 3: 557. https://doi.org/10.3390/molecules23030557
APA StyleLi, H., Krstin, S., Wang, S., & Wink, M. (2018). Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules, 23(3), 557. https://doi.org/10.3390/molecules23030557