Gold-Catalyzed Addition of β-Ketoesters to Alkenes: Influence of Electronic and Steric Effects in the Reaction Outcome
Abstract
:1. Introduction
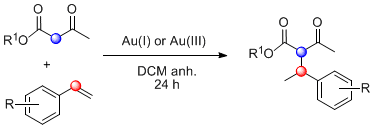
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Instrumental and Physical Data
3.3. Synthetic Procedures
3.4. Analytical Data of Individual Compounds





4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arcadi, A. Alternative Synthetic Methods through New Developments in Catalysis by Gold. Chem. Rev. 2008, 108, 3266–3325. [Google Scholar] [CrossRef] [PubMed]
- Furstner, A.; Davies, P.W. Catalytic Carbophilic Activation: Catalysis by Platinum and Gold pi-Acids. Angew. Chem. Int. Ed. 2007, 46, 3410–3449. [Google Scholar] [CrossRef] [PubMed]
- Furstner, A. Gold and platinum catalysis-a convenient tool for generating molecular complexity. Chem. Soc. Rev. 2009, 38, 3208–3221. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.; Rudolph, M. Gold catalysis in total synthesis. Chem. Soc. Rev. 2008, 37, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.; Hashmi, A.S. Gold catalysis in total synthesis-an update. Chem. Soc. Rev. 2012, 41, 2448–2462. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.C. Recent advances in syntheses of heterocycles and carbocycles via homogeneous gold catalysis. Part 1: Heteroatom addition and hydroarylation reactions of alkynes, allenes, and alkenes. Tetrahedron 2008, 64, 3885–3903. [Google Scholar] [CrossRef]
- Shen, H.C. Recent advances in syntheses of carbocycles and heterocycles via homogeneous gold catalysis. Part 2: Cyclizations and cycloadditions. Tetrahedron 2008, 64, 7847–7870. [Google Scholar] [CrossRef]
- Gorin, D.J.; Sherry, B.D.; Toste, F.D. Ligand Effects in Homogeneous Au Catalysis. Chem. Rev. 2008, 108, 3351–3378. [Google Scholar] [CrossRef] [PubMed]
- Pflasterer, D.; Hashmi, A.S. Gold catalysis in total synthesis - recent achievements. Chem. Soc. Rev. 2016, 45, 1331–1367. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.A.; Sajid, M.A.; Khan, Z.A.; Canseco-Gonzalez, D. Gold catalysis in organic transformations: A review. Synth. Commun. 2017, 47, 735–755. [Google Scholar] [CrossRef]
- Echavarren, A.M.; Hashmi, A.S.; Toste, F.D. Gold Catalysis—Steadily Increasing in Importance. Adv. Synth. Catal. 2016, 358, 1347. [Google Scholar] [CrossRef]
- Dyker, G. An Eldorado for Homogeneous Catalysis? Angew. Chem. Int. Ed. 2000, 39, 4237–4239. [Google Scholar] [CrossRef]
- Hashmi, A.S. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007, 107, 3180–3211. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S. Homogeneous Gold Catalysis Beyond Assumptions and Proposals—Characterized Intermediates. Angew. Chem. Int. Ed. 2010, 49, 5232–5241. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S. Dual Gold Catalysis. Acc. Chem. Res. 2014, 47, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, Y.; Liu, H. Recent advances in the gold-catalyzed additions to C-C multiple bonds. Beilstein J. Organ. Chem. 2011, 7, 897–936. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Brouwer, C.; He, C. Gold-Catalyzed Organic Transformations. Chem. Rev. 2008, 108, 3239–3265. [Google Scholar] [CrossRef] [PubMed]
- Skouta, R.; Li, C.J. Gold-catalyzed reactions of C-H bonds. Tetrahedron 2008, 64, 4917–4938. [Google Scholar] [CrossRef]
- Gorin, D.J.; Toste, F.D. Relativistic effects in homogeneous gold catalysis. Nature 2007, 446, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Leyva-Perez, A.; Sabater, M.J. Gold-Catalyzed Carbon-Heteroatom Bond-Forming Reactions. Chem. Rev. 2011, 111, 1657–1712. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S. Homogeneous gold catalysts and alkynes: A successful liaison. Gold Bull. 2003, 36, 3–9. [Google Scholar] [CrossRef]
- Jimenez-Nunez, E.; Echavarren, A.M. Molecular diversity through gold catalysis with alkynes. Chem. Commun. 2007, 43, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Nuñez, E.; Echavarren, A.M. Gold-Catalyzed Cycloisomerizations of Enynes: A Mechanistic Perspective. Chem. Rev. 2008, 108, 3326–3350. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Smith, J.J.; Staben, S.T.; Toste, F.D. Gold(I)-Catalyzed Conia-Ene Reaction of beta-Ketoesters with Alkynes. J. Am. Chem. Soc. 2004, 126, 4526–4527. [Google Scholar] [CrossRef] [PubMed]
- Muzart, J. Gold-catalysed reactions of alcohols: Isomerisation, inter- and intramolecular reactions leading to C-C and C-heteroatom bonds. Tetrahedron 2008, 64, 5815–5849. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef] [PubMed]
- Chiarucci, M.; Bandini, M. New developments in gold-catalyzed manipulation of inactivated alkenes. Beilstein J. Organ. Chem. 2013, 9, 2586–2614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, W.; Petryna, E.M.; Rogers, B.R.; Day, C.S.; Jones, A.C. Insights into Alkene Activation by Gold: Nucleophile Activation with Base as a Trigger for Generation of Lewis Acidic Gold. ACS Catal. 2016, 6, 7357–7362. [Google Scholar] [CrossRef]
- Bay, S.; Englert, A.; Nalivela, K.S.; Hashmi, A.S.; Larsen, M.H. Gold catalysis: Experimental mechanistic insights into the anellation of phenols with 1,3-dienes. J. Organomet. Chem. 2015, 795, 58–62. [Google Scholar] [CrossRef]
- Hashmi, A.S.; Ramamurthi, T.D.; Rominger, F. Synthesis, structure and reactivity of organogold compounds of relevance to homogeneous gold catalysis. J. Organomet. Chem. 2009, 694, 592–597. [Google Scholar] [CrossRef]
- Klatt, G.; Xu, R.; Pernpointner, M.; Molinari, L.; Quang-Hung, T.; Rominger, F.; Hashmi, A.S.; Koppel, H. Are beta-H-Eliminations or Alkene Insertions Feasible Elementary Steps in Catalytic Cycles Involving Gold(I) Alkyl Species or Gold(I) Hydrides? Chem. A Eur. J. 2013, 19, 3954–3961. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Monari, M.; Romaniello, A.; Tragni, M. Gold-Catalyzed Direct Activation of Allylic Alcohols in the Stereoselective Synthesis of Functionalized 2-Vinyl-Morpholines. Chem. A Eur. J. 2010, 16, 14272–14277. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Widenhoefer, R.A. Gold(I)-Catalyzed Intramolecular Hydroamination of Alkenyl Carbamates. Angew. Chem. Int. Ed. 2006, 45, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Hamasaki, A.; Nakamura, A.; Tokunaga, M. Enhancement of Reaction Efficiency by Functionalized Alcohols on Gold(I)-Catalyzed Intermolecular Hydroalkoxylation of Unactivated Olefins. Org. Lett. 2009, 11, 5510–5513. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; Muñiz, K. Oxidative Interception of the Hydroamination Pathway: A Gold-Catalyzed Diamination of Alkenes. Chem. A Eur. J. 2009, 15, 10563–10569. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.G.; He, C. Gold(I)-Catalyzed Intermolecular Addition of Phenols and Carboxylic Acids to Olefins. J. Am. Chem. Soc. 2005, 127, 6966–6967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cui, L.; Wang, Y.; Zhang, L. Homogeneous Gold-Catalyzed Oxidative Carboheterofunctionalization of Alkenes. J. Am. Chem. Soc. 2010, 132, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, C.G.; He, C. Gold(I)-Catalyzed Intra- and Intermolecular Hydroamination of Unactivated Olefins. J. Am. Chem. Soc. 2006, 128, 1798–1799. [Google Scholar] [CrossRef] [PubMed]
- Denes, F.; Perez-Luna, A.; Chemla, F. Addition of Metal Enolate Derivatives to Unactivated Carbon-Carbon Multiple Bonds. Chem. Rev. 2010, 110, 2366–2447. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Presset, M.; Guerinot, A.; Bour, C.; Bezzenine-Lafollee, S.; Gandon, V. Cationic gold(I)-catalyzed enantioselective hydroalkylation of unactivated alkenes: Influence of the chloride scavenger on the stereoselectivity. Org. Chem. Front. 2014, 1, 608–613. [Google Scholar] [CrossRef]
- Porcel, S.; López-Carrillo, V.; García-Yebra, C.; Echavarren, A.M. Gold-Catalyzed Allyl-Allyl Coupling. Angew. Chem. Int. Ed. 2008, 47, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.P.; Liu, X.Y.; Che, C.M. Efficient Gold(I)-Catalyzed Direct Intramolecular Hydroalkylation of Unactivated Alkenes with alpha-Ketones. Angew. Chem. Int. Ed. 2011, 50, 4937–4941. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.V.; Yao, X.Q.; Bohle, D.S.; Li, C.J. Gold- and Silver-Catalyzed Highly Regioselective Addition of Active Methylenes to Dienes, Triene, and Cyclic Enol Ethers. Org. Lett. 2005, 7, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Vayer, M.; Fang, W.; Guillot, R.; Bezzenine-Lafollee, S.; Bour, C.; Gandon, V. Acid-catalysed intramolecular addition of beta-ketoesters to 1,3-dienes. Org. Biomol. Chem. 2017, 15, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, C.J. Highly Efficient Addition of Activated Methylene Compounds to Alkenes Catalyzed by Gold and Silver. J. Am. Chem. Soc. 2004, 126, 6884–6885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Che, C.M. Highly Efficient Au(I)-Catalyzed Intramolecular Addition of beta-Ketoamide to Unactivated Alkenes. J. Am. Chem. Soc. 2007, 129, 5828–5829. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Koppolu, S.R. Scope of AuCl3 in the activation of per-O-acetylglycals. Tetrahedron 2009, 65, 8139–8142. [Google Scholar] [CrossRef]
- Fang, W.; Presset, M.; Guerinot, A.; Bour, C.; Bezzenine-Lafollee, S.; Gandon, V. Silver-Free Two-Component Approach in Gold Catalysis: Activation of [LAuCl] Complexes with Derivatives of Copper, Zinc, Indium, Bismuth, and Other Lewis Acids. Chem. Eur. J. 2014, 20, 5439–5446. [Google Scholar] [CrossRef] [PubMed]
- Guerinot, A.; Fang, W.; Sircoglou, M.; Bour, C.; Bezzenine-Lafollee, S.; Gandon, V. Copper Salts as Additives in Gold(I)-Catalyzed Reactions. Angew. Chem. Int. Ed. 2013, 52, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.V.; Li, C.J. An Annulation toward Fused Bicyclolactones. J. Am. Chem. Soc. 2005, 127, 17184–17185. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, A.R.; Youn, S.W. AuI-Catalyzed Intramolecular Cyclization of 2-Alkenylphenyl Carbonyl Compounds: Exploring the Oxophilic Lewis Acidity of AuI Species. Eur. J. Org. Chem. 2011, 2011, 3904–3910. [Google Scholar] [CrossRef]
- Zhang, X.; Corma, A. Effective Au(III)-CuCl2-catalyzed addition of alcohols to alkenes. Chem. Commun. 2007, 43, 3080–3082. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, D.C.; Shekhar, S.; Takemiya, A.; Utsunomiya, M.; Hartwig, J.F. Hydroamination and Hydroalkoxylation Catalyzed by Triflic Acid. Parallels to Reactions Initiated with Metal Triflates. Org. Lett. 2006, 8, 4179–4182. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.; Schafer, S.; Goker, V.; Eisenbach, C.D.; Dirnberger, K.; Zhao-Karger, Z.; Crewdson, P. Gold Catalysis: AuCl-induced Polymerization of Styrene and n-Butylvinylether. Aust. J. Chem. 2014, 67, 500–506. [Google Scholar] [CrossRef]
- Urbano, J.; Hormigo, A.J.; de Fremont, P.; Nolan, S.P.; Diaz-Requejo, M.M.; Perez, P.J. Gold-promoted styrene polymerization. Chem. Commun. 2008, 759–761. [Google Scholar] [CrossRef]
- Hashmi, A.S.; Frost, T.M.; Bats, J.W. Highly Selective Gold-Catalyzed Arene Synthesis. J. Am. Chem. Soc. 2000, 122, 11553–11554. [Google Scholar] [CrossRef]
- Hashmi, A.S.; Schwarz, L.; Choi, J.H.; Frost, T.M. A New Gold-Catalyzed C-C Bond Formation. Angew. Chem. Int. Ed. 2000, 39, 2285–2288. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2011; ISBN 978-3-527-32473-6. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| 1 | R1 | Catalyst System | T | Yield 1 | |
|---|---|---|---|---|---|
| 1 | 1a | Et | AuCl3 (5 mol %) AgSbF6 (15 mol %) | rt | 75% |
| 2 | 1a | Et | AuCl3 (5 mol %) AgOTf (15 mol %) | rt | 70% |
| 3 | 1a | Et | AuCl (5 mol %) AgSbF6 (5 mol %) | rt | 40% |
| 4 | 1a | Et | AuCl3 (5 mol %) AgSbF6 (15 mol %) CuCl2 (10 mol %) | 0 °C | 60% |
| 5 | 1a | Et | TfOH (2 mol %) | 0 °C | 20% |
| 6 | 1a | Et | AgOTf (15 mol %) | rt | NR 2 |
| 7 | 1b | tBu | AuCl3 (5 mol %) AgSbF6 (15 mol %) | rt | NR 2 |

| Alkene | Product 1 | Recovered Starting Material 2 | Polymeric by-Products 3 | |
|---|---|---|---|---|
| 1 | 2a, R2 = p-Me | 3a, 75% | 1a | Yes |
| 2 | 2b, R2 = p-Et | 3b, 50% | 1a | Yes |
| 3 | 2c, R2 = p-tBu | 3c, 20% | 1a | Yes |
| 4 | 2d, R2 = p-CH2Cl | 3d, 10% (35%) 4 | 1a | Yes |
| 5 | 2e, R2 = p-OAc | 3e, traces | 1a | Yes |
| 6 | 2f, R2 = p-OMe | 3f, 7% (25%) 4 | 1a | Yes |
| 7 | 2g, R2 = p-OtBu | 3g, traces | 1a | No |
| 8 | 2h, R2 = p-CO2Me | NP | 1a + 2h | No |
| 9 | 2i, R2 = p-Br | NP | 1a + 2i | No |
| 10 | 2j, R2 = o-Br | NP | 1a + 2j | No |
| 11 | 2k, R2 = o-Me | NP | 1a + 2k | Yes |
| 12 | 2l | NP | 1a + 2l | No |
| 13 | 2m | NP | 1a 5 | No |
| 14 | 2n | NP | 1a + 2n | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La-Venia, A.; Mischne, M.P.; Mata, E.G. Gold-Catalyzed Addition of β-Ketoesters to Alkenes: Influence of Electronic and Steric Effects in the Reaction Outcome. Molecules 2018, 23, 629. https://doi.org/10.3390/molecules23030629
La-Venia A, Mischne MP, Mata EG. Gold-Catalyzed Addition of β-Ketoesters to Alkenes: Influence of Electronic and Steric Effects in the Reaction Outcome. Molecules. 2018; 23(3):629. https://doi.org/10.3390/molecules23030629
Chicago/Turabian StyleLa-Venia, Agustina, Mirta P. Mischne, and Ernesto G. Mata. 2018. "Gold-Catalyzed Addition of β-Ketoesters to Alkenes: Influence of Electronic and Steric Effects in the Reaction Outcome" Molecules 23, no. 3: 629. https://doi.org/10.3390/molecules23030629