An Efficient Chemoenzymatic Approach towards the Synthesis of Rugulactone
Abstract
:1. Introduction
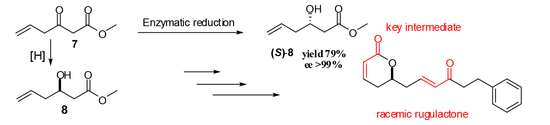
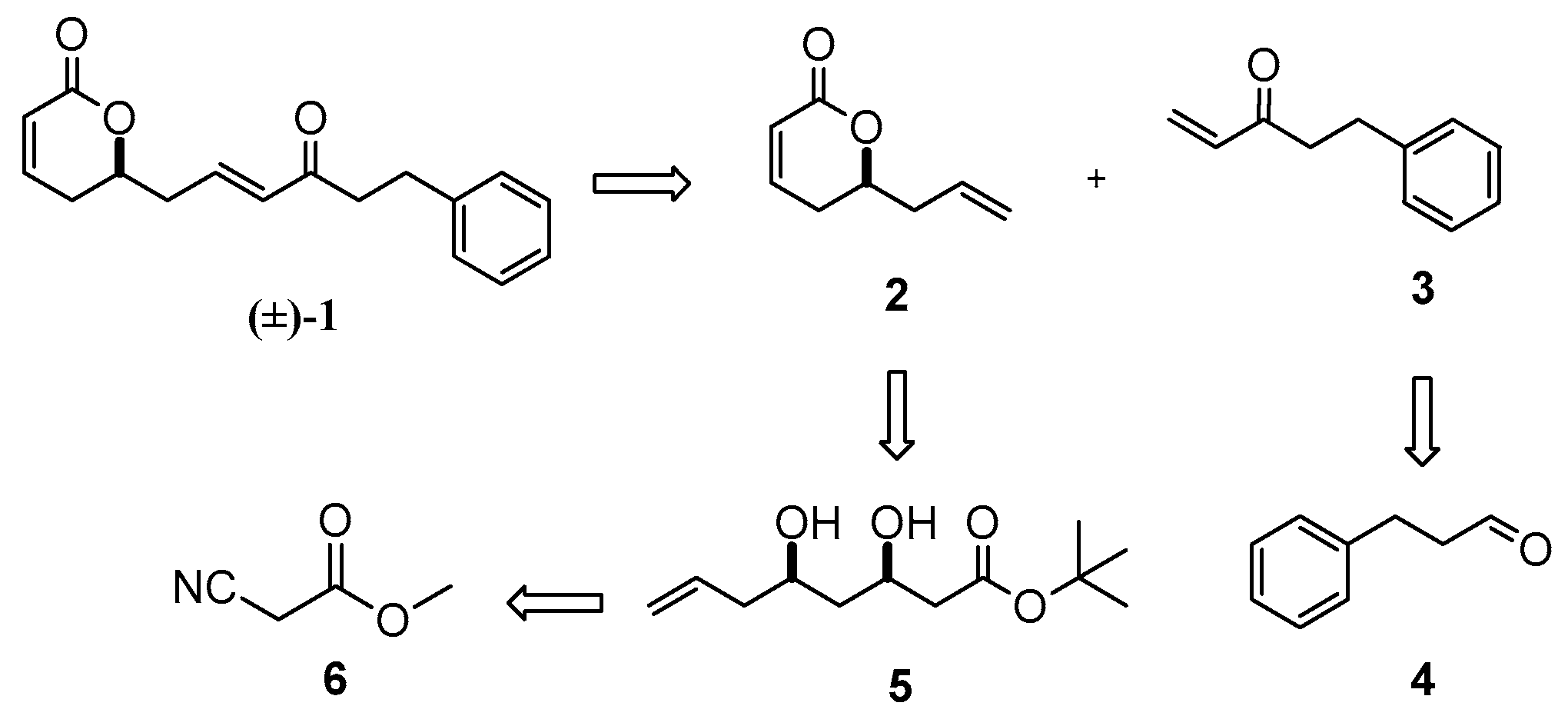
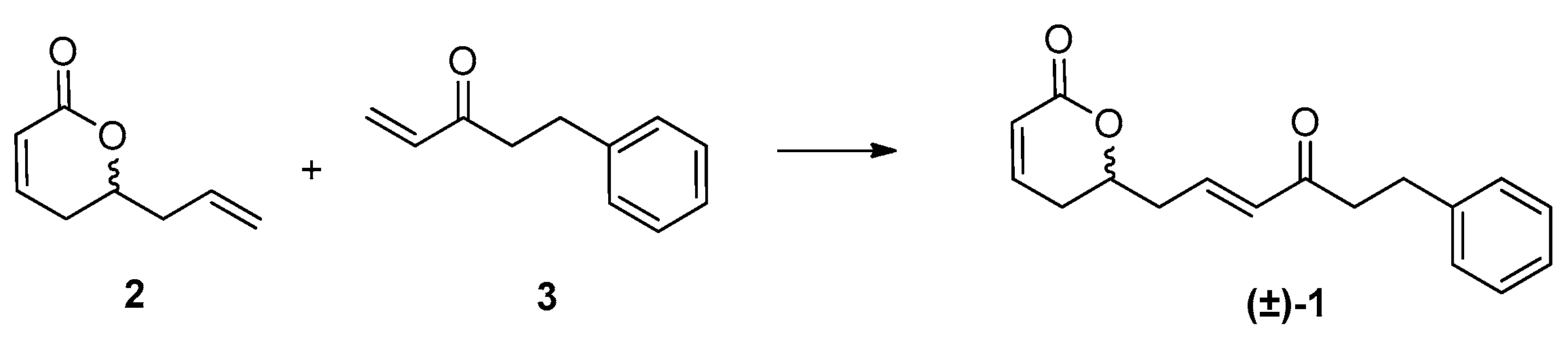
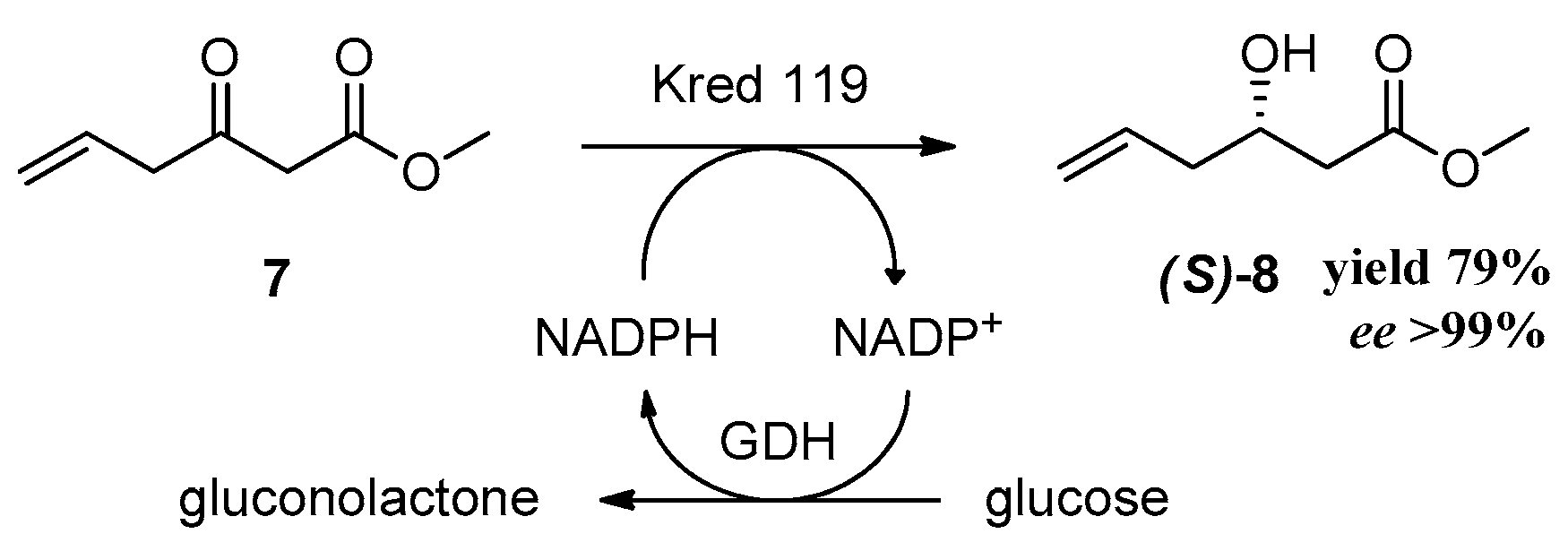
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthetic Procedures
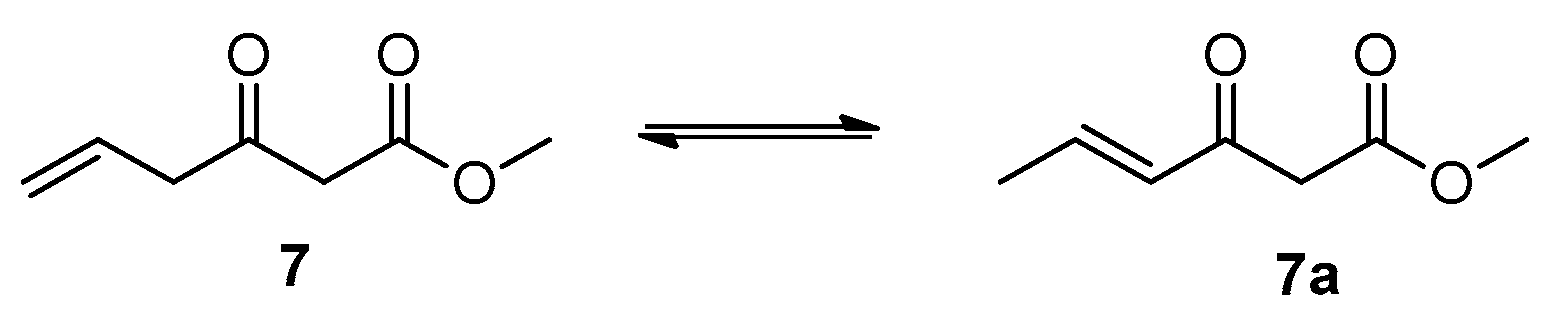
3.2.1. Methyl 3-Oxohex-5-enoate (7)
3.2.2. Methyl 3-Hydroxyhex-5-enoate (8)
3.2.3. tert-Butyl 3,5-dihydroxyoct-7-enoate (5)
3.2.4. Allyl-5,6-dihydro-2H-pyran-2-one (2)
3.2.5. 5-Phenylpent-1-en-3-ol (9)
3.2.6. 5-Phenylpent-1-en-3-one (3)
3.2.7. (E)-6-(4-Oxo-6-phenylhex-2-enyl)-5,6-dihydro-2H-pyran-2-one (1)
3.3. General Procedure for Enzymatic Reductions
(S)-Methyl 3-hydroxyhex-5-enoate, (S)-8
3.4. Preparation of MPA-Esters
3.4.1. General Method for the Synthesis of MPA Esters of Secondary Alcohols
(R)-MPA Ester of (S)-Methyl 3-hydroxyhex-5-enoate
(S)-MPA Ester of (S)-Methyl 3-hydroxyhex-5-enoate
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meragelman, T.L.; Scudiero, D.A.; Davis, R.E.; Staudt, L.M.; McCloud, T.G.; Cardellina, J.H., II; Shoemaker, R.H. Inhibitors of the NF-κB activation pathway from Cryptocarya rugulosa. J. Nat. Prod. 2009, 72, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Baltimore, D. Pillars article: Multiple nuclear factors interact with the immunoglobulin enhancer sequencies. J. Immunol. 1986, 177, 7485–7496. [Google Scholar]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and rel. proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Iyer, S.; Razani, B.; Cheng, G. NF-κB and Innate Immunity. In NF-κB in Health and Disease; Current Topics in Microbiology and Immunology; Karin, M., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; Volume 349, pp. 115–143. ISBN 978-3-642-16016-5. [Google Scholar]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, K.D.; Reddy, D.S.; Ramaiah, M.J.; Ghosh, S.; Pothula, V.; Lunavath, S.; Thomas, S.; Valli, S.N.C.V.L.P.; Pal Bahdra, M.; Yadav, S.J. Rugulactone derivatives act as inhibitors of NF-κB activation and modulates the transcription of NF-κB dependent genes in MDA-MB-231cells. Bioorg. Med. Chem. Lett. 2014, 24, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Karin, M. The IKK/NF-κB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004, 206, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Baltimore, D. Circuitry of nuclear factor κB signaling. Immunol. Rev. 2006, 210, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Leung, T.H.; Baltimore, D. Genetic analysis of NF-κB/Rel transcription factors defines functional specificities. EMBO J. 2003, 22, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Bacher, S.; Schmitz, M.L. The NF-κB pathway as a potential target for autoimmune disease therapy. Curr. Pharm. Des. 2004, 10, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.K.; Shekhar, V.; Prabhakar, P.; Chinna Babu, B.; Siddhardha, B.; Murthy, U.S.N.; Venkateswarlu, Y. Stereoselective synthesis and biological evaluation of (R)-rugulactone, (6R)-((4R)-hydroxy-6-phenyl-hex-2-enyl)-5,6-dihydro-pyran-2-one and its 4S epimer. Eur. J. Med. Chem. 2010, 45, 4657–4663. [Google Scholar] [CrossRef] [PubMed]
- Nodwell, M.B.; Menz, H.; Kirsh, S.F.; Sieber, S.A. Rugulactone and its analogues exert antibacterial effects through multiple mechanisms including inhibition of thiamine biosynthesis. ChemBioChem 2012, 13, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Cross, F.; Pelotier, B.; Piva, O. Regioselective tandem ring closing/cross metathesis of 1,5-hexadien-3-ol derivatives: Application to the total synthesis of rugulactone. Eur. J. Org. Chem. 2010, 2010, 5063–5070. [Google Scholar] [CrossRef]
- Mohapatra, D.K.; Pragna, P.D.; Reddy, D.S.; Yadav, S.J. First total syntheses and absolute configuration of rugulactone and 6(R)-(40-oxopent-20-enyl)-5,6-dihydro-2H-pyran-2-one. Tetrahedron Lett. 2009, 50, 5941–5944. [Google Scholar] [CrossRef]
- Reddy, D.K.; Shekhar, V.; Reddy, T.S.; Reddy, S.P.; Venkateswarlu, Y. Stereoselective first total synthesis of (R)-rugulactone. Tetrahedron Asymmetry 2009, 20, 2315–2319. [Google Scholar] [CrossRef]
- Reddipalli, G.; Venkataiah, M.; Fadnavis, W.N. Chemo-enzymatic synthesis of both enantiomers of rugulactone. Tetrahedron Asymmetry 2010, 21, 320–324. [Google Scholar] [CrossRef]
- Boese, D.; Fernadez, E.; Pietruszka, J. Stereoselective synthesis of both enantiomers of rugulactone. J. Org. Chem. 2011, 76, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Allais, F.; Aouhansou, M.; Majira, A.; Ducro, P.H. Asymmetric total synthesis of rugulactone, an a-pyrone from Cryptocarya rugulosa. Synthesis 2010, 16, 2787–2793. [Google Scholar] [CrossRef]
- Reddy, B.N.; Singh, R.P. A facile stereoselective total synthesis of (R)-rugulactone. ISRN Org. Chem. 2014, 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.; Saikia, P.P.; Saikia, B.; Chaturvedi, D.; Barua, N.C. An improved stereoselective total synthesis of (R)-rugulactone. Tetrahedron Lett. 2011, 52, 5133–5135. [Google Scholar] [CrossRef]
- Albarranan-Velo, J.; Gonzalez-Martınez, D.; Gotor-Fernandez, V. Stereoselective biocatalysis: A mature technology for the asymmetric synthesis of pharmaceutical building blocks. Biocatal. Biotransform. 2017, 36, 102–130. [Google Scholar] [CrossRef]
- Rudroff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- Gröger, H. Enzyme-catalyzed asymmetric synthesis. In Catalytic Asymmetric Synthesis, 3rd ed.; Ojima, I., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 269–342. ISBN 978-0-470-17577-4. [Google Scholar]
- Gröger, H. Cluster issue on chemoenzymatic synthesis. ChemCatChem 2011, 3, 239–240. [Google Scholar] [CrossRef]
- Anderson, G.W.; Halverstadt, I.F.; Miller, W.H.; Roblin, R.O., Jr. Studies in chemotherapy. X. Antithyroid compounds. synthesis of 5- and 6-substituted 2-thiouracils from oxoesters and thiourea. J. Am. Chem. Soc. 1945, 67, 2197–2200. [Google Scholar] [CrossRef] [PubMed]
- Hamana, H.; Sugasawa, T. Electrophilic reaction of allyltrimethylsilane with nitriles in the presence of boron trichloride. Chem. Lett. 1985, 14, 921–924. [Google Scholar] [CrossRef]
- Lee, A.S.-Y.; Lin, L.S. Synthesis of allyl ketone via Lewis acid promoted Barbier-type reaction. Tetrahedron Lett. 2000, 41, 8803–8806. [Google Scholar] [CrossRef]
- Fu, G.C.; Grubbs, R.H. Synthesis of cycloalkenes via alkylidene-mediated olefin metathesis and carbonyl olefination. J. Am. Chem. Soc. 1993, 115, 3800–3801. [Google Scholar] [CrossRef]
- Kallergi, M.; Kalaitzakis, D.; Smonou, I. Enzymatic total synthesis of banana volatile (S)-2-pentyl (R)-3-hydroxyhexanoate. Eur. J. Org. Chem. 2011, 2011, 3946–3950. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Smonou, I. Chemoenzymatic synthesis of stegobinone and stegobiol, components of the natural sex pheromone of the drugstore beetle (Stegobium paniceum L.). Eur. J. Org. Chem. 2012, 2012, 43–46. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Rozzell, J.D.; Kambourakis, S.; Smonou, I. Highly stereoselective reductions of α-alkyl-1,3-diketones and α-alkyl-β-keto esters catalyzed by isolated NADPH-dependent ketoreductases. Org. Lett. 2005, 7, 4799–4801. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, D.; Rozzell, J.D.; Kambourakis, S.; Smonou, I. Synthesis of valuable chiral intermediates by isolated ketoreductases: application in the synthesis of α-alkyl-β-hydroxy ketones and 1,3-diols. Adv. Synth. Catal. 2006, 348, 1958–1969. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Smonou, I. Highly diastereoselective synthesis of 2-substituted-1,3-diols catalyzed by ketoreductases. Tetrahedron 2010, 66, 9431–9439. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Smonou, I. A Two-step, one-pot enzymatic synthesis of 2-substituted 1,3-diols. J. Org. Chem. 2010, 75, 8658–8661. [Google Scholar] [CrossRef] [PubMed]
- Bariotaki, A.; Kalaitzakis, D.; Smonou, I. Enzymatic reductions for the regio- and stereoselective synthesis of hydroxy-keto esters and dihydroxy esters. Org. Lett. 2012, 14, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, D.; Rozzell, D.J.; Kambourakis, S.; Smonou, I. A Two-Step Chemoenzymatic Synthesis of the Natural Pheromone (+)-Sitophilure Utilizing isolated, NADPH-dependent ketoreductases. Eur. J. Org. Chem. 2006, 2309–2313. [Google Scholar] [CrossRef]
- Kalaitzakis, D.; Kambourakis, S.; Rozzell, D.J.; Smonou, I. Stereoselective chemoenzymatic synthesis of sitophilate: A natural pheromone. Tetrahedron Asymmetry 2007, 18, 2418–2426. [Google Scholar] [CrossRef]
- Fryszkowska, A.; Peterson, J.; Davies, N.L.; Dewar, C.; Evans, G.; Bycroft, M.; Triggs, N.; Fleming, T.; Gorantla, S.S.C.; Hoge, G.; et al. Development of a chemoenzymatic process for dehydroepiandrosterone acetate synthesis. Org. Proce. Res. Dev. 2016, 20, 1520–1528. [Google Scholar] [CrossRef]
- Seco, J.M.; Quinoa, E.; Riguera, R. The assignment of absolute configuration by NMR. Chem. Rev. 2004, 104, 17–117. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Substrate | Kred a | Conv. b (time) | Yield c | ee d | Product | |
|---|---|---|---|---|---|---|
| 7 | A1C | >99% | (12 h) | 62% | >99% | (S)-8 |
| A1D | >99% | (12 h) | 64% | 98% | (S)-8 | |
| 119 | >99% | (12 h) | 79% | >99% | (S)-8 | |
| B1F | >99% | (12 h) | 62% | 58.6% | (S)-8 | |
| 101 | >99% | (12 h) | 83% | 72% | (S)-8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyrikos-Ergas, T.; Giannopoulos, V.; Smonou, I. An Efficient Chemoenzymatic Approach towards the Synthesis of Rugulactone. Molecules 2018, 23, 640. https://doi.org/10.3390/molecules23030640
Tyrikos-Ergas T, Giannopoulos V, Smonou I. An Efficient Chemoenzymatic Approach towards the Synthesis of Rugulactone. Molecules. 2018; 23(3):640. https://doi.org/10.3390/molecules23030640
Chicago/Turabian StyleTyrikos-Ergas, Theodore, Vasileios Giannopoulos, and Ioulia Smonou. 2018. "An Efficient Chemoenzymatic Approach towards the Synthesis of Rugulactone" Molecules 23, no. 3: 640. https://doi.org/10.3390/molecules23030640