Anti-Amyloid Aggregation Activity of Black Sesame Pigment: Toward a Novel Alzheimer’s Disease Preventive Agent
Abstract
:1. Introduction
2. Results and Discussion
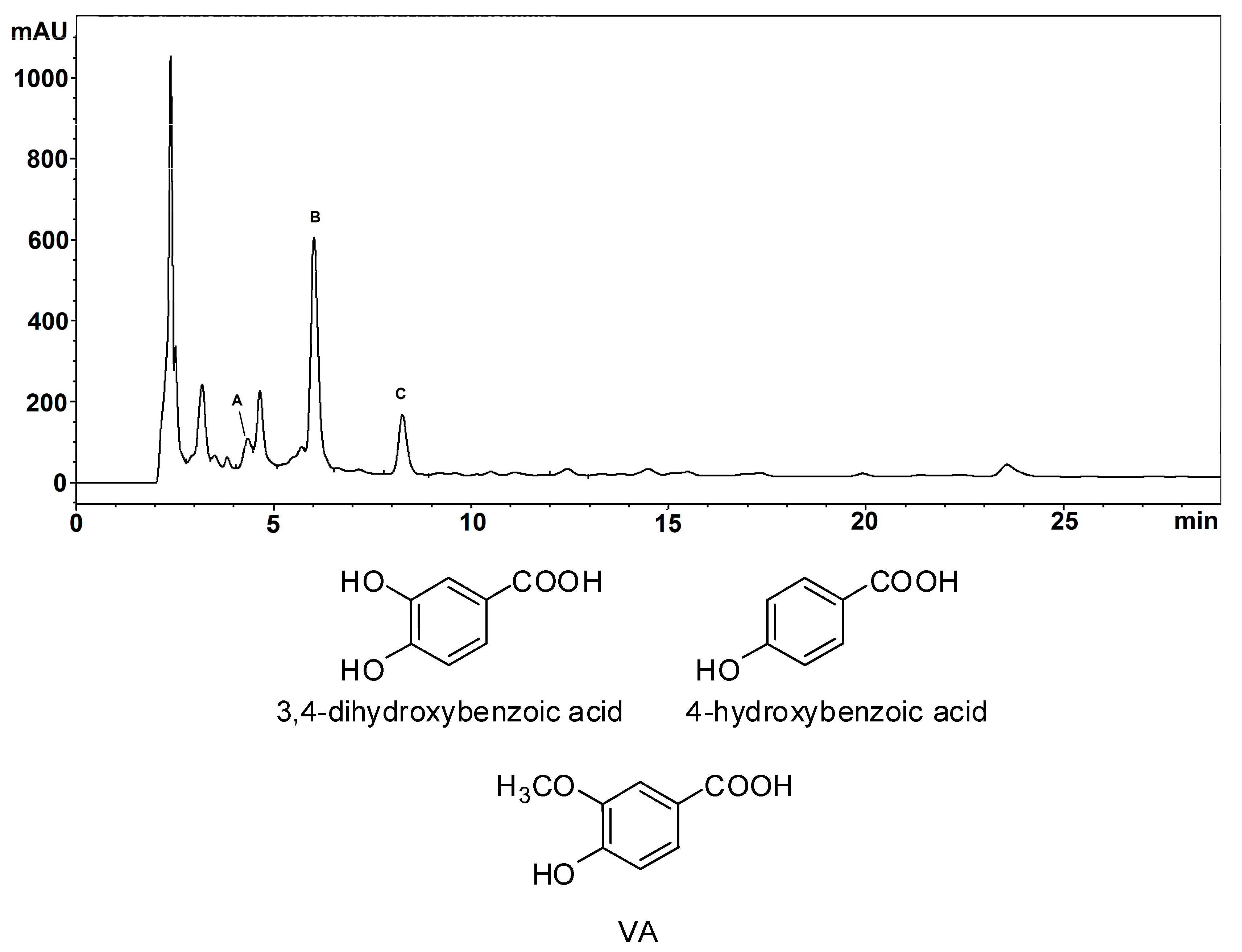
2.1. Structural Investigation of BSP Phenolic Components
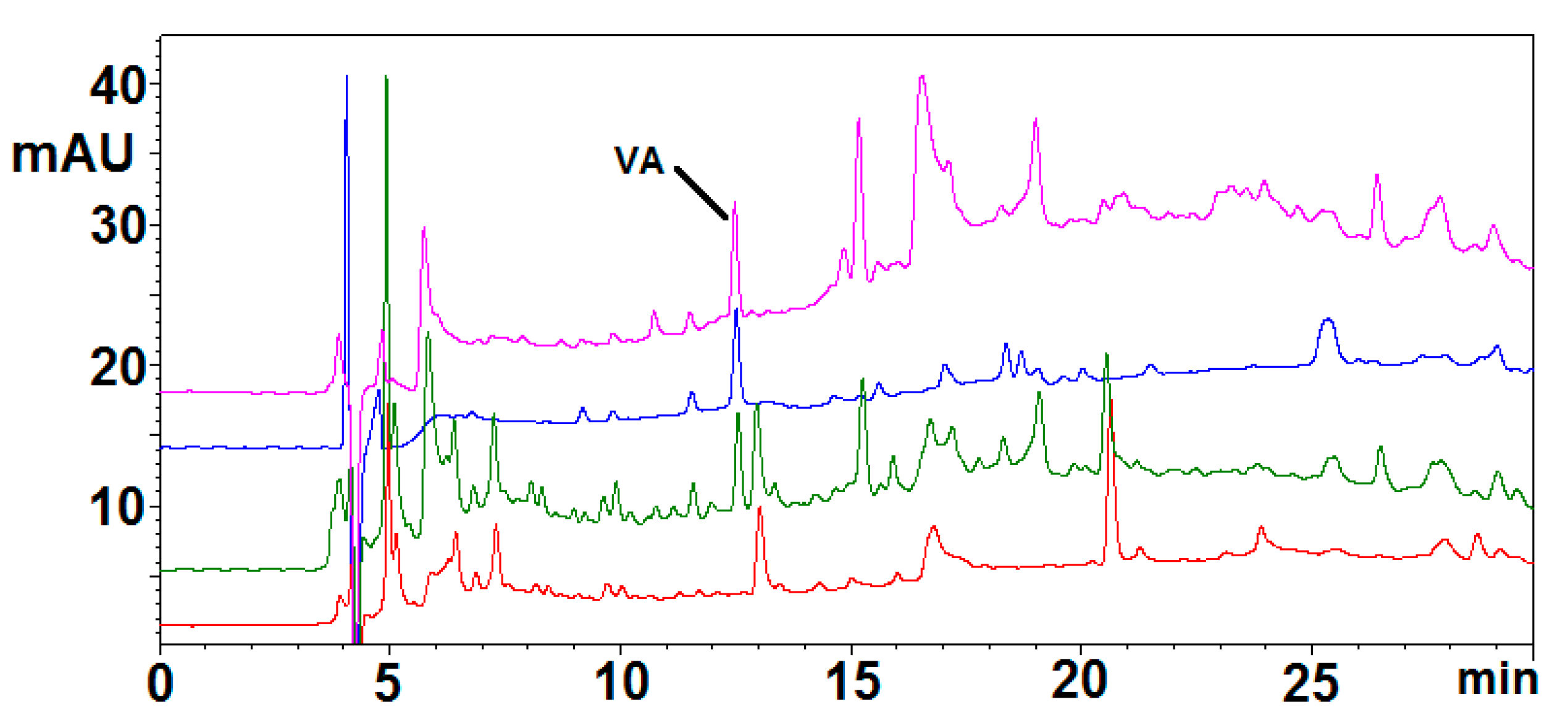
2.2. Simulated Gastrointestinal Digestion of BSP
2.3. Determination of the Activity of BSP toward AD Drug Targets
2.3.1. AChE and BChE Inhibition Assay
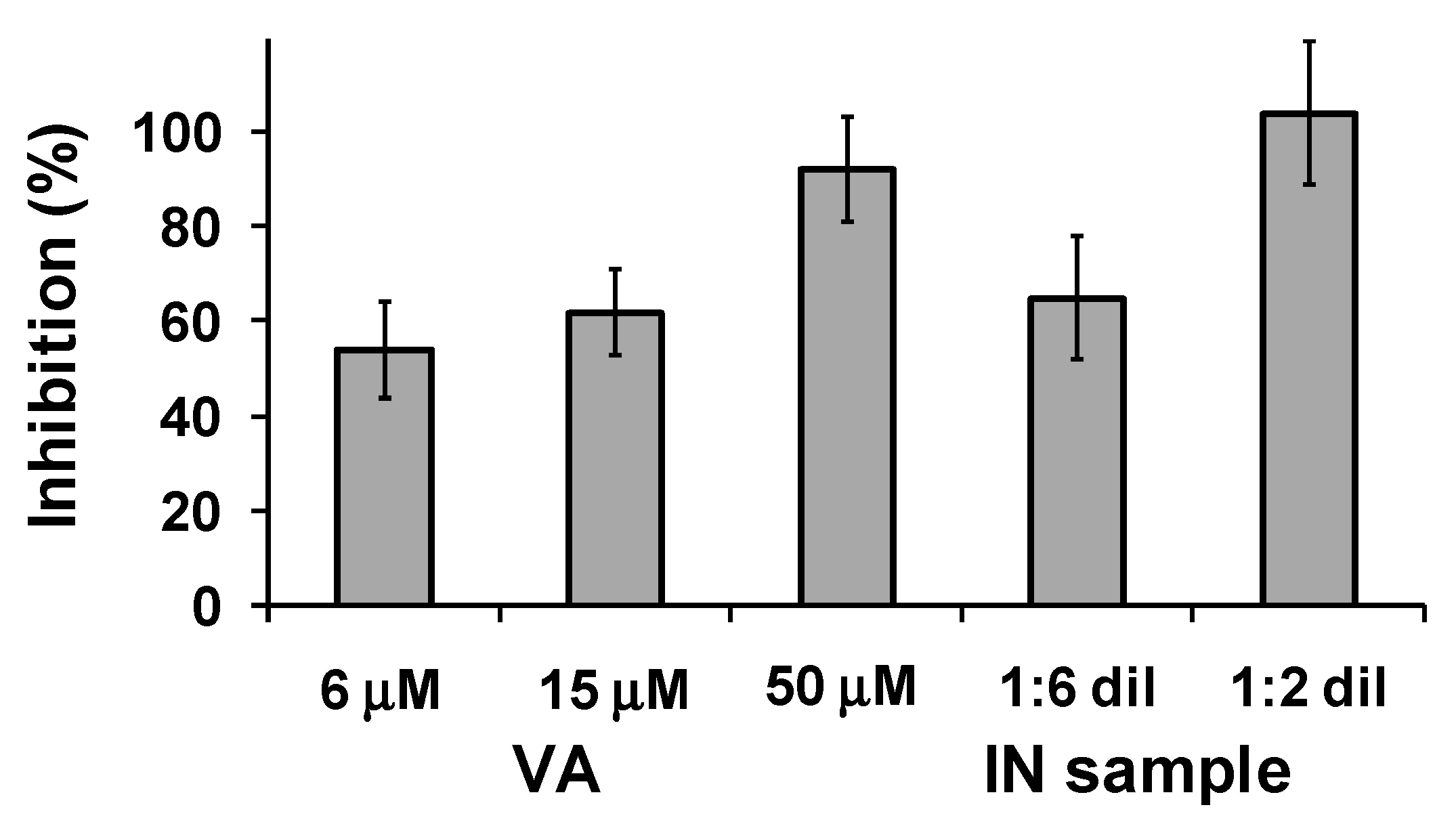
2.3.2. AChE-Induced Aβ1-40 Aggregation Inhibition Assay
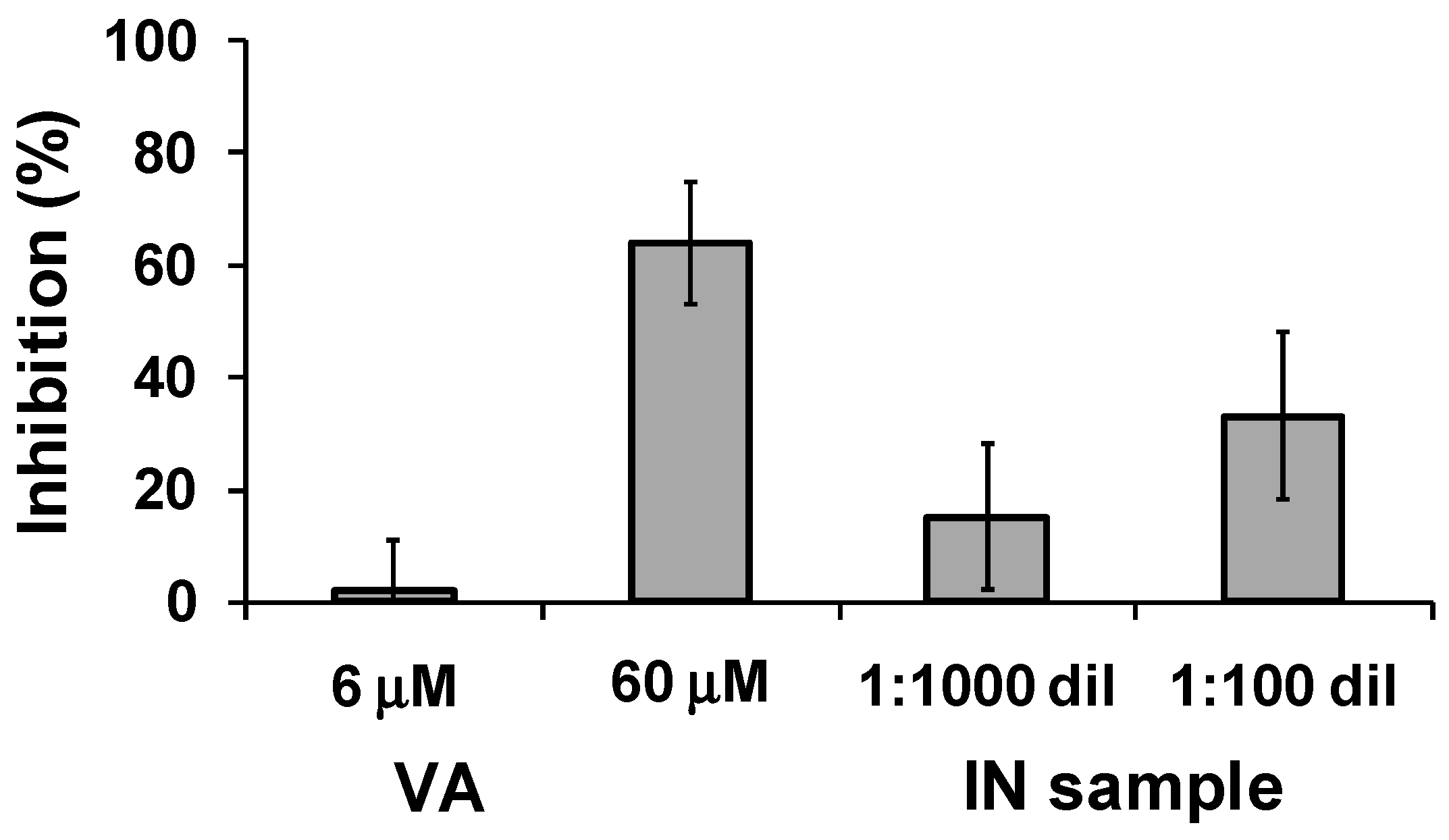
2.3.3. β-Amyloid Self-Aggregation Inhibition
2.3.4. BACE-1 Inhibition
3. Materials and Methods
3.1. Materials
3.2. General Experimental Methods
3.3. Alkali Fusion of BSP
3.4. Simulated Gastrointestinal Digestion of BSP
3.5. AChE and BChE Inhibition Assays
3.6. AChE-Induced Aβ1-40 Aggregation Inhibition Assay
3.7. Self-Induced Aβ1-42 Aggregation Inhibition Assay
3.8. BACE-1 Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Palanimuthu, D.; Poon, R.; Sahni, S.; Anjum, R.; Hibbs, D.; Lin, H.Y.; Bernhardt, P.V.; Kalinowski, D.S.; Richardson, D.R. A novel class of thiosemicarbazones show multi-functional activity for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Lei, P.; Bush, A.I. Metallostasis in Alzheimer’s disease. Free Radic. Biol. Med. 2013, 62, 76–89. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Oddo, S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol. Med. 2005, 11, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Viayna, E.; Sola, I.; Bartolini, M.; De Simone, A.; Tapia-Rojas, C.; Serrano, F.G.; Sabaté, R.; Juárez-Jiménez, J.; Pérez, B.; Luque, F.J.; et al. Synthesis and multitarget biological profiling of a novel family of rhein derivatives as disease-modifying anti-Alzheimer agents. J. Med. Chem. 2014, 57, 2549–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penke, B.; Bogár, F.; Fülöp, L. β-Amyloid and the pathomechanisms of Alzheimer’s disease: A comprehensive view. Molecules 2017, 22, 1692. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Kung, H.F. The β-amyloid hypothesis in Alzheimer’s disease: Seeing is believing. ACS Med. Chem. Lett. 2012, 3, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Bezerra da Silva, C.; Pott, A.; Elifio-Esposito, S.; Dalarmi, L.; Fialho do Nascimento, K.; Moura Burci, L.; de Oliveira, M.; de Fátima Gaspari Dias, J.; Warumby Zanin, S.M.; Gomes Miguel, O.; et al. Effect of donepezil, tacrine, galantamine and rivastigmine on acetylcholinesterase inhibition in Dugesia tigrina. Molecules 2016, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: Low-affinity, uncompetitive antagonism. Curr. Alzheimer Res. 2005, 2, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, X.; Li, L.; Zheng, J. Inhibition of amyloid-β aggregation in Alzheimer’s disease. Curr. Pharm. Des. 2014, 20, 1223–1243. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Piemontese, L. Heterocyclic compounds as key structures for the interaction with old and new targets in Alzheimer’s disease therapy. Neural Regen. Res. 2017, 12, 1256–1261. [Google Scholar] [PubMed]
- Bachurin, S.O.; Bovina, E.V.; Ustyugov, A.A. Drugs in clinical trials for alzheimer’s disease: The major trends. Med. Res. Rev. 2017, 37, 1186–1225. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garridom, J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Johnson, G.; Moore, S.W. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, A.-L.; Zhang, Y.-L.; Li, Z.; Ding, H.-W.; Huang, C.; Meng, X.-M.; Li, J. Design, synthesis and evaluation of hesperetin derivatives as potential multifunctional anti-Alzheimer agents. Molecules 2017, 22, 1067. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Pan, W.; Wang, K.; Ma, Q.; Yu, L.; Liu, W. Design, synthesis and biological evaluation of 3,4-dihydro-2(1H)-quinoline-O-alkylamine derivatives as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Jameel, E.; Meena, P.; Maqbool, M.; Kumar, J.; Ahmed, W.; Mumtazuddin, S.; Tiwari, M.; Hoda, N.; Jayaram, B. Rational design, synthesis and biological screening of triazine-triazolopyrimidine hybrids as multitarget anti-Alzheimer agents. Eur. J. Med. Chem. 2017, 136, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Qiang, X.; Li, Y.; Xu, R.; Cao, Z.; Song, Q.; Wang, T.; Zhang, X.; Liu, H.; Tan, Z.; et al. Design, synthesis and evaluation of scutellarein-O-acetamidoalkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 135, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Manral, A.; Jameel, E.; Kumar, J.; Saini, V.; Shandilya, A.; Tiwari, M.; Hoda, N.; Jayaram, B. Development of cyanopyridine-triazine hybrids as lead multitarget anti-Alzheimer agents. Bioorg. Med. Chem. 2016, 24, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Giacomo, S. Curcumin and resveratrol in the management of cognitive disorders: What is the clinical evidence? Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Napolitano, M.; Tedesco, I.; Moccia, S.; Milito, A.; Russo, G.L. Neuroprotective role of natural polyphenols. Curr. Top. Med. Chem. 2016, 16, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Devi, K.P. Dietary polyphenols for treatment of Alzheimer’s disease—Future research and development. Curr. Pharm. Biotechnol. 2014, 15, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Ngoungoure, V.L.; Schluesener, J.; Moundipa, P.F.; Schluesener, H. Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Mol. Nutr. Food Res. 2015, 59, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Stefani, M.; Rigacci, S. Beneficial properties of natural phenols: Highlight on protection against pathological conditions associated with amyloid aggregation. Biofactors 2014, 40, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Moncalvo, A.; Marinoni, L.; Dordoni, R.; Duserm Garrido, G.; Lavelli, V.; Spigno, G. Waste grape skins: Evaluation of safety aspects for the production of functional powders and extracts for the food sector. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L. Plant food supplements with antioxidant properties for the treatment of chronic and neurodegenerative diseases: Benefits or risks? J. Diet. Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Qiao, Q.; Sun, Y.; Liu, Q.; Ren, B.; Liu, X. Sesamol supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of nuclear factor kappaB. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Katayama, S.; Sugiyama, H.; Kushimoto, S.; Uchiyama, Y.; Hirano, M.; Nakamura, S. Effects of sesaminol feeding on brain Aβ accumulation in a senescence-accelerated mouse-prone 8. J. Agric. Food Chem. 2016, 64, 4908–4913. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Tiwari, V.; Kuhad, A.; Chopra, K. Modulation of nitrergic pathway by sesamol prevents cognitive deficits and associated biochemical alterations in intracerebroventricular streptozotocin administered rats. Eur. J. Pharmacol. 2011, 659, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Ahn, J.Y.; Kim, S.; Kim, M.K.; Ha, T.Y. Sesaminol glucosides protect β-amyloid peptide-induced cognitive deficits in mice. Biol. Pharm. Bull. 2009, 32, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lin, X.; Abbasi, A.M.; Zheng, B. Phytochemical contents and antioxidant and antiproliferative activities of selected black and white sesame seeds. Biomed. Res. Int. 2016, 2016, 8495630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seo, W.D.; Lee, S.K.; Lee, Y.B.; Park, C.H.; Ryu, H.W.; Lee, J.H. Comparative assessment of compositional components, antioxidant effects, and lignan extractions from Korean white and black sesame (Sesamum indicum L.) seeds for different crop years. J. Funct. Foods 2014, 7, 495–505. [Google Scholar] [CrossRef]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Namiki, M. The chemistry and physiological functions of sesame. Food Rev. Int. 1985, 11, 281–329. [Google Scholar] [CrossRef]
- Kuo, P.C.; Lin, M.C.; Chen, G.F.; Yiu, T.J.; Tzen, J.T.C. Identification of methanol-soluble compounds in sesame and evaluation of antioxidant potential of its lignans. J. Agric. Food Chem. 2011, 59, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Reshma, M.V.; Balachandran, C.; Arumughan, C.; Sunderasan, A.; Divya Sukumaran, S.T.; Saritha, S.S. Extraction, separation and characterisation of sesame oil lignan for nutraceutical applications. Food Chem. 2010, 120, 1041–1046. [Google Scholar] [CrossRef]
- Ryu, S.N.; Ho, C.T.; Osawa, T. High performance liquid chromatographic determination of antioxidant lignan glycosides in some varieties of sesame. J. Food Lipids 1998, 5, 17–28. [Google Scholar] [CrossRef]
- Dar, A.A.; Arumugam, N. Lignans of sesame: Purification methods, biological activities and biosynthesis—A review. Bioorg. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.-Z.; Chu, P.-Y.; Chandrasekaran, V.R.M.; Liu, M.-Y. Sesame lignan sesamol protects against aspirin-induced gastric mucosal damage in rats. J. Funct. Foods 2009, 1, 349–355. [Google Scholar] [CrossRef]
- Ma, J.Q.; Ding, J.; Zhang, L.; Liu, C.M. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem. Toxicol. 2014, 64, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ryu, S.N.; Bu, Y.; Kim, H.; Simon, J.E.; Kim, K.S. Antioxidant components as potential neuroprotective agents in sesame (Sesamum indicum L.). Food Rev. Int. 2010, 26, 103–121. [Google Scholar] [CrossRef]
- Saeed, M.; Khalid, H.; Sugimoto, Y.; Efferth, T. The lignan (-)-sesamin reveals cytotoxicity toward cancer cells: Pharmacogenomic determination of genes associated with sensitivity or resistance. Phytomedicine 2014, 21, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Eidenberger, T.; Napolitano, A.; d’Ischia, M. Black sesame pigment: DPPH assay-guided purification, antioxidant/antinitrosating properties and identification of a degradative structural marker. J. Agric. Food Chem. 2012, 60, 8895–8901. [Google Scholar] [CrossRef] [PubMed]
- Manini, P.; Panzella, L.; Eidenberger, T.; Giarra, A.; Cerruti, P.; Trifuoggi, M.; Napolitano, A. Efficient binding of heavy metals by black sesame pigment: Toward innovative dietary strategies to prevent bioaccumulation. J. Agric. Food Chem. 2016, 64, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Woimant, F.; Trocello, J.M. Disorders of heavy metals. Handb. Clin. Neurol. 2014, 120, 851–864. [Google Scholar] [PubMed]
- Caito, S.; Aschner, M. Neurotoxicity of metals. Handb. Clin. Neurol. 2015, 131, 169–189. [Google Scholar] [PubMed]
- Karas, M.; Jakubczyk, A.; Szymanowska, U.; Zlotek, U.; Zielinska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bohn, T.; McDougall, G.J.; Alegria, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martinez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Marze, S. Bioavailability of nutrients and micronutrients: Advances in modeling and in vitro approaches. Annu. Rev. Food Sci. Technol. 2017, 8, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, S.Y.; Chung, M.-S.; Hur, S.J. Development of novel in vitro human digestion systems for screening the bioavailability and digestibility of foods. J. Funct. Foods 2016, 22, 113–121. [Google Scholar] [CrossRef]
- Cilla, A.; González-Sarrías, A.; Tomás-Barberán, F.A.; Espín, J.C.; Barberá, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gökmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomas-Barberan, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Ito, H. Antioxidative properties of vanillic acid esters in multiple antioxidant assays. Biosci. Biotechnol. Biochem. 2012, 76, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Świsłocka, R.; Piekut, J.; Lewandowski, W. The relationship between molecular structure and biological activity of alkali metal salts of vanillic acid: Spectroscopic, theoretical and microbiological studies. Spectrochim. Acta A Mol. Biomol. Spetrosc. 2013, 100, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M.; Dhanasekar, K.; Rajakumar, S. Preventive effects of vanillic acid on lipids, bax, bcl-2 and myocardial infarct size on isoproterenol-induced myocardial infarcted rats: A biochemical and in vitro study. Cardiovasc. Toxicol. 2011, 11, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.C.; Um, J.Y.; Hong, S.H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef] [PubMed]
- Ul Amin, F.; Ali Shah, S.; Kim, M.O. Vanillic acid attenuates Aβ1–42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.C.; Kakalij, R.M.; Kshirsagar, R.P.; Kumar, B.H.; Komakula, S.S.; Diwan, P.V. Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharm. Biol. 2015, 53, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase inhibitors for the treatment of Alzheimer’s disease: Getting on and staying on. Curr. Ther. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef]
- Kwong, H.C.; Mah, S.H.; Chia, T.S.; Quah, C.K.; Lim, G.K.; Kumar, C.S.C. Cholinesterase inhibitory activities of adamantyl-based derivatives and their molecular docking studies. Molecules 2017, 22, 1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wehle, S.; Kuzmanovic, N.; Merget, B.; Holzgrabe, U.; König, B.; Sotriffer, C.A.; Decker, M. Acetylcholinesterase inhibitors with photoswitchable inhibition of β-amyloid aggregation. ACS Chem. Neurosci. 2014, 5, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Acheson, S.A.; Quinn, D.M. Anatomy of acetylcholinesterase catalysis: Reaction dynamics analogy for human erythrocyte and electric eel enzymes. Biochim. Biophys. Acta 1990, 1040, 199–205. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Takio, K.; Ogawara, M.; Selkoe, D.J. Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J. Biol. Chem. 1992, 267, 17082–17086. [Google Scholar] [PubMed]
- Schmidt, M.; Sachse, C.; Richter, W.; Xu, C.; Fändrich, M.; Grigorieff, N. Comparison of Alzheimer Abeta(1-40) and Abeta(1-42) amyloid fibrils reveals similar protofilament structures. Proc. Natl. Acad. Sci. USA 2009, 106, 19813–19818. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Sang, Z.; Yuan, W.; Li, Y.; Liu, Q.; Bai, P.; Shi, Y.; Ang, W.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 76, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Näslund, J.; Schierhorn, A.; Hellman, U.; Lannfelt, L.; Roses, A.D.; Tjernberg, L.O.; Silberring, J.; Gandy, S.E.; Winblad, B.; Greengard, P.; et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA 1994, 91, 8378–8382. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel tacrine-hydroxyphenylbenzimidazole hybrids as potential multitarget drug candidates for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 148, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Briante, R.; Febbraio, F.; Nucci, R. Antioxidant properties of low molecular weight phenols present in the mediterranean diet. J. Agric. Food Chem. 2003, 51, 6975–6981. [Google Scholar] [CrossRef] [PubMed]
- Rampa, A.; Tarozzi, A.; Mancini, F.; Pruccoli, L.; Di Martino, R.M.; Gobbi, S.; Bisi, A.; De Simone, A.; Palomba, F.; Zaccheroni, N.; et al. Naturally inspired molecules as multifunctional agents for Alzheimer’s disease treatment. Molecules 2016, 21, 643. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M.; Tang, J. Developing β-secretase inhibitors for treatment of Alzheimer’s disease. J. Neurochem. 2012, 120, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.J.; Kim, D.W.; Park, K.H. Inhibitory evaluation of sulfonamide chalcones on β-secretase and acylcholinesterase. Molecules 2013, 18, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Lee, E.J.; Kim, J.S.; Kang, S.S.; Lee, J.H.; Min, B.S.; Choi, J.S. Cholinesterase and BACE1 inhibitory diterpenoids from Aralia cordata. Arch. Pharm. Res. 2009, 32, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bachiller, M.I.; Pérez, C.; Monjas, L.; Rademann, J.; Rodríguez-Franco, M.I. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J. Med. Chem. 2012, 55, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The IN sample is available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzella, L.; Eidenberger, T.; Napolitano, A. Anti-Amyloid Aggregation Activity of Black Sesame Pigment: Toward a Novel Alzheimer’s Disease Preventive Agent. Molecules 2018, 23, 676. https://doi.org/10.3390/molecules23030676
Panzella L, Eidenberger T, Napolitano A. Anti-Amyloid Aggregation Activity of Black Sesame Pigment: Toward a Novel Alzheimer’s Disease Preventive Agent. Molecules. 2018; 23(3):676. https://doi.org/10.3390/molecules23030676
Chicago/Turabian StylePanzella, Lucia, Thomas Eidenberger, and Alessandra Napolitano. 2018. "Anti-Amyloid Aggregation Activity of Black Sesame Pigment: Toward a Novel Alzheimer’s Disease Preventive Agent" Molecules 23, no. 3: 676. https://doi.org/10.3390/molecules23030676
APA StylePanzella, L., Eidenberger, T., & Napolitano, A. (2018). Anti-Amyloid Aggregation Activity of Black Sesame Pigment: Toward a Novel Alzheimer’s Disease Preventive Agent. Molecules, 23(3), 676. https://doi.org/10.3390/molecules23030676






