Photo-Oxidation of Bisphenol A in Aqueous Solutions at Near Neutral pH by a Fe(III)-Carboxylate Complex with Oxalacetic Acid as a Benign Molecule
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photolysis Experiments
2.3. Determination of the Complexation Ratio and the Equilibrium Constant
2.4. Analysis
3. Results and Discussion
3.1. Formation of Fe(III)-OA Complex
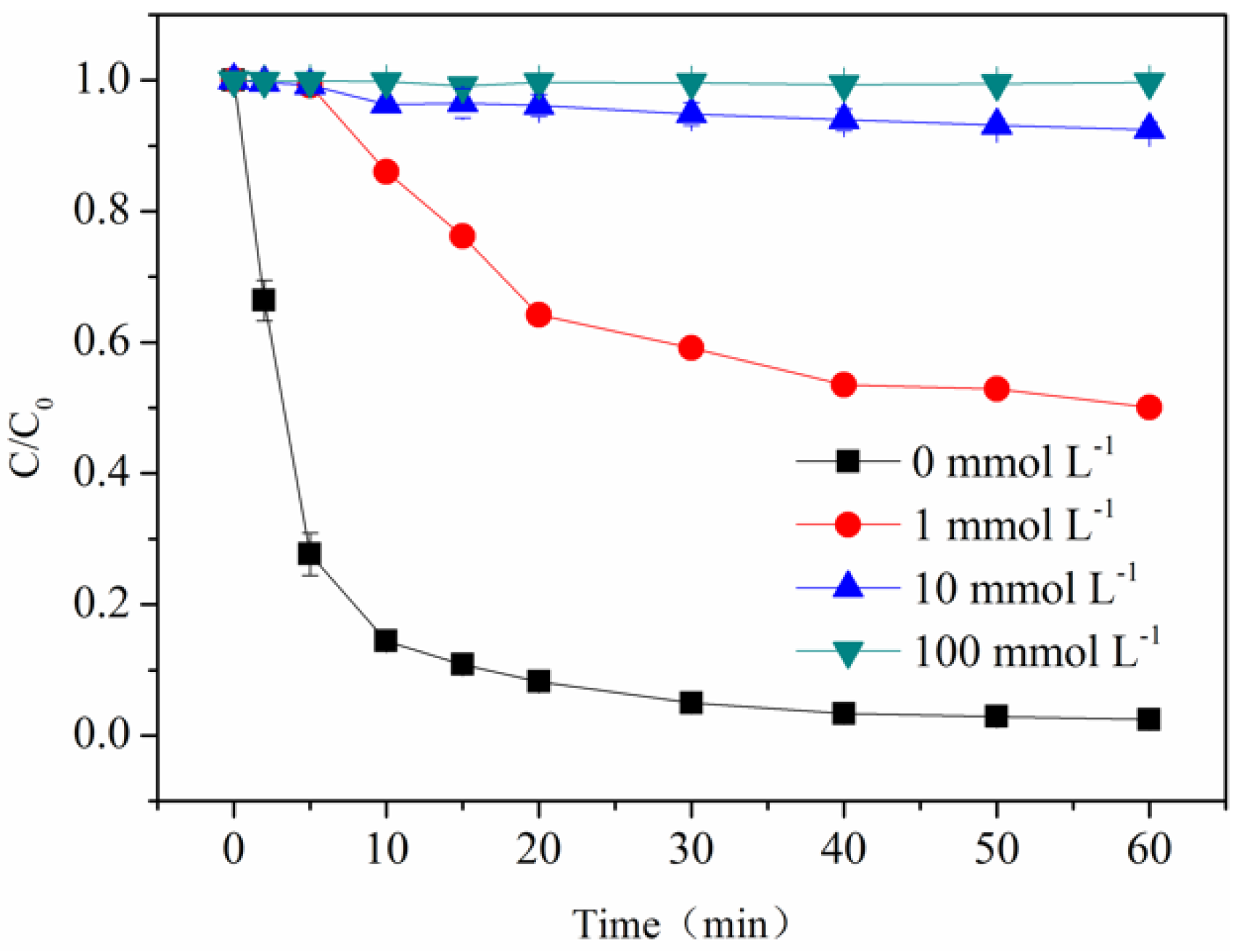
3.2. Photo-Degradation of BPA
3.3. Role of Hydroxyl Radicals
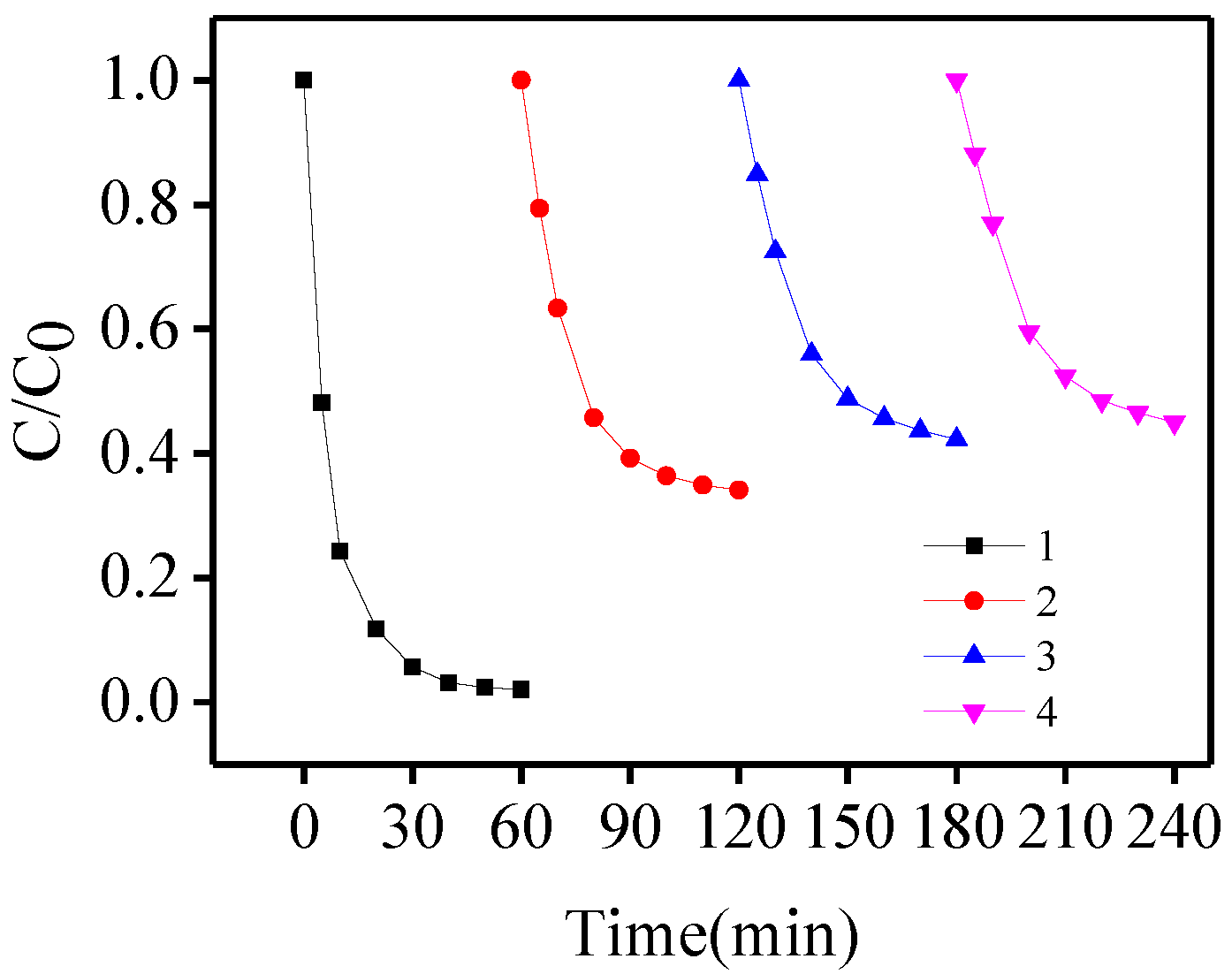
3.4. Reuse of Fe(III)/Fe(II) in the Solution
3.5. Photo-Degradation Products
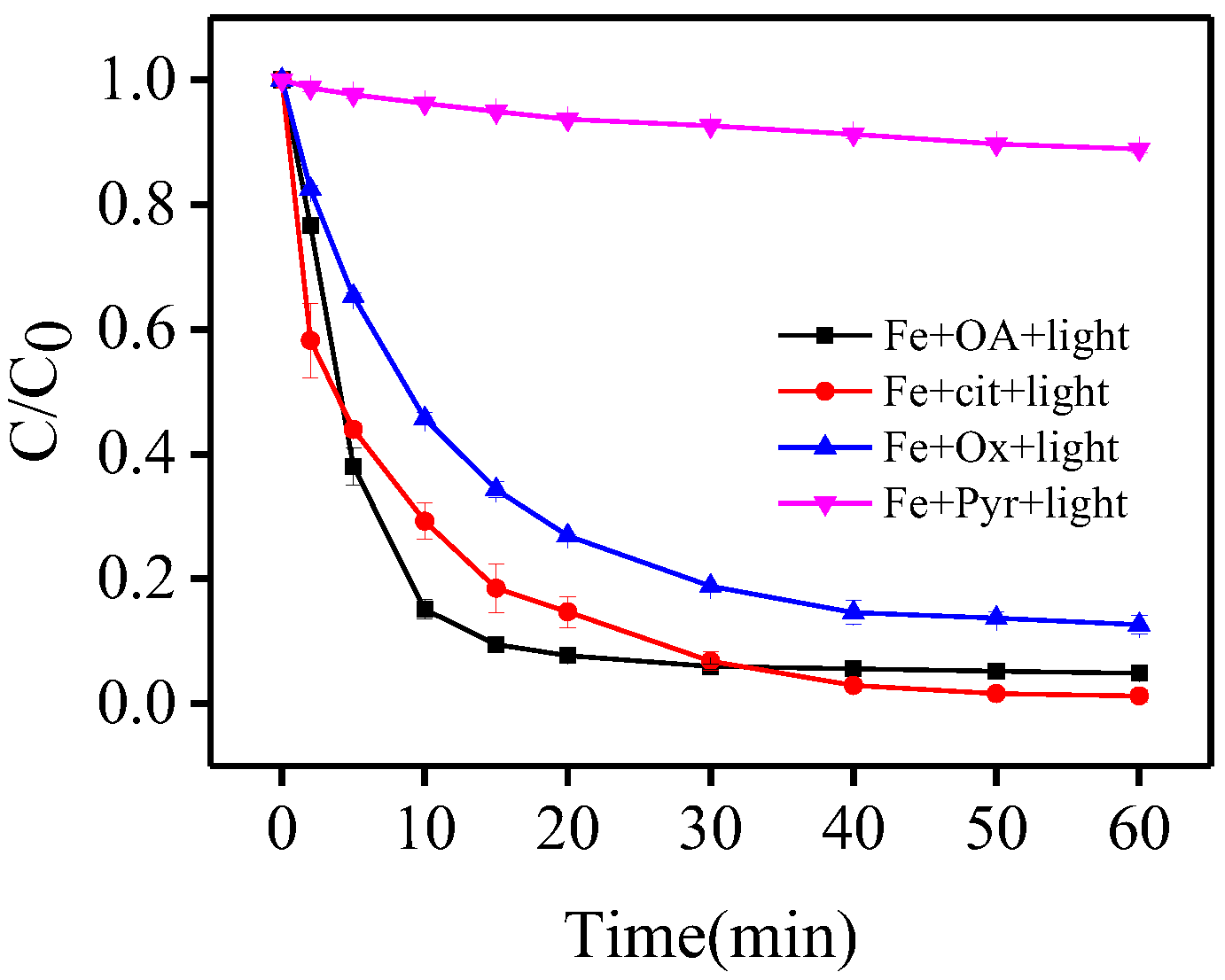
3.6. Comparison of Carboxylate Ligands
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zuo, Y.; Hoigne, J. Formation of hydrogen peroxide and depletion of oxalic acid in atmospheric water by photolysis of iron (III)–oxalato complexes. Environ. Sci. Technol. 1992, 26, 1014–1022. [Google Scholar] [CrossRef]
- Zuo, Y.; Hoigne, J. Photochemical decomposition of oxalic, glyoxalic and pyruvic acid catalysed by iron in atmospheric waters. Atmos. Environ. 1994, 28, 1231–1239. [Google Scholar] [CrossRef]
- Prousek, J.; Palacková, E.; Priesolová, S.; Marková, L.; Alevová, A. Fenton- and Fenton-like AOPs for wastewater treatment: From laboratory-to-plant-scale application. Sep. Sci. Technol. 2007, 42, 1505–1520. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.G.; Theng, B.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions-A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Silva, M.; Trovó, A.G.; Nogueira, R. Degradation of the herbicide tebuthiuron using solar photo–Fenton process and ferric citrate complex at circumneutral pH. J. Photochem. Photobiol. A 2007, 191, 187–192. [Google Scholar] [CrossRef]
- Carra, I.; Malato, S.; Jiménez, M.; Maldonado, M.I.; Sánchez Pérez, J.A. Microcontaminant removal by solar photo–Fenton at natural pH run with sequential and continuous iron additions. Chem. Eng. J. 2014, 235, 132–140. [Google Scholar] [CrossRef]
- Huang, Y.H.; Tsai, S.T.; Huang, Y.F.; Chen, C.Y. Degradation of commercial azo dye reactive Black B in photo/ferrioxalate system. J. Hazard. Mater. 2007, 140, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Abida, O.; Mailhot, G.; Litter, M.; Bolte, M. Impact of iron-complex (Fe(III)–NTA) on photoinduced degradation of 4–chlorophenol in aqueous solution. Photochem. Photobiol. Sci. 2006, 5, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mailhot, G.; Wu, F.; Deng, N.S. Photochemical efficiency of Fe(III)–EDDS complex: OH radical production and 17β–estradiol degradation. J. Photochem. Photobiol. A 2010, 212, 1–7. [Google Scholar] [CrossRef]
- Huang, W.Y.; Brigante, M.; Wu, F.; Mousty, C.; Hanna, K.; Mailhot, G. Assessment of the Fe(III)–EDDS complex in Fenton–like processes: From the radical formation to the degradation of bisphenol A. Environ. Sci. Technol. 2013, 47, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Molkenthin, M.; Olmez-Hanci, T.; Jekel, M.R.; Arslan-Alaton, I. Photo-Fenton-like treatment of BPA: Effect of UV light source and water matrix on toxicity and transformation products. Water Res. 2013, 47, 5052–5064. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zuo, Y.G. Bisphenol A and other alkylphenols in the environment-occurrence, fate, health effects and analytical techniques. Adv. Environ. Res. 2013, 2, 179–202. [Google Scholar] [CrossRef]
- Zuo, Y.G.; Zhu, Z. Simultaneous identification and quantification of 4-cumylphenol, 2,4-bis-(dimethylbenzyl)phenol and bisphenol A in prawn Macrobrachium rosenbergii. Chemosphere 2014, 107, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Guo, Y.Z.; Liu, Z.Z.; Feng, X.N.; Chen, Y.Q.; Tao, T. Catechin as a new improving agent for a photo–Fenton–like system at near-neutral pH for the removal of inderal. Photochem. Photobiol. Sci. 2015, 14, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Balmer, M.E.; Sulzberger, B. Atrazine degradation in irradiated iron/oxalate systems: Effects of pH and oxalate. Environ. Sci. Technol. 1999, 33, 2418–2424. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Feng, X.N.; Wang, Z.P.; Chen, Y.; Tao, T.; Wu, F.; Zuo, Y.G. Effect of Fe(III)/citrate concentrations and ratio on the photoproduction of hydroxyl radicals: Application on the degradation of diphenhydramine. Ind. Eng. Chem. Res. 2012, 51, 7007–7012. [Google Scholar] [CrossRef]
- Elshafei, G.; Yehia, F.Z.; Dimitry, O. Degradation of nitrobenzene at near neutral pH using Fe2+–glutamate complex as a homogeneous Fenton catalyst. Appl. Catal. B 2010, 99, 242–247. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, H.; Kaneco, S.; Suzuki, T.; Ohta, T.; Yobiko, Y. Photo–Fenton degradation of alachlor in the presence of citrate solution. J. Photochem. Photobiol. A 2006, 180, 38–45. [Google Scholar] [CrossRef]
- Lee, C.; Sedlak, D.L. A novel homogeneous Fenton–like system with Fe(III)–phosphotungstate for oxidation of organic compounds at neutral pH values. J. Mol. Catal. A Chem. 2009, 311, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Du, Y.Y.; Lan, Y.Q.; Mao, J.D. Photodegradation mechanism and kinetics of methyl orange catalyzed by Fe(III) and citric acid. J. Hazard. Mater. 2011, 186, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Zheng, S.R.; Xing, X.L.; Li, Y.F.; Yin, D.Q.; Ding, Y.S.; Pang, W.H. Fe(III)–oxalate complexes mediated photolysis of aqueous alkylphenol ethoxylates under simulated sunlight conditions. Chemosphere 2010, 78, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.N.; Wu, Y.; Feng, X.N.; Chen, Y.; Wang, Z.P.; Tao, T.; Wei, D.B. Photodegradation of hexabromocyclododecane (HBCD) by Fe(III) complexes/ H2O2, under simulated sunlight. Environ. Sci. Pollut. Res. 2014, 21, 6228–6233. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Feng, L.R.; Xu, N.; Chen, Z.G.; Wang, X.M. Magnetic nickel ferrite as a heterogeneous photo–Fenton catalyst for the degradation of rhodamine B in the presence of oxalic acid. Chem. Eng. J. 2012, 203, 432–439. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.Z.; Wang, Z.P.; Xue, M.M.; Zhu, X.C.; Tao, T. Photodegradation of propranolol by Fe(III)-citrate complexes: Kinetics, mechanism and effect of environmental media. J. Hazard. Mater. 2011, 194, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.N.; Chen, Y.; Fang, Y.; Wang, X.Y.; Wang, Z.P.; Tao, T.; Zuo, Y.G. Photodegradation of parabens by Fe(III)–citrate complexes at circumneutral pH: Matrix effect and reaction mechanism. Sci. Total Environ. 2014, 472, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Glebov, E.M.; Pozdnyakov, I.P.; Grivin, V.P.; Plyusnin, V.F.; Zhang, X.; Wu, F.; Deng, N.S. Intermediates in photochemistry of Fe (III) complexes with carboxylic acids in aqueous solutions. Photoch. Photobio. Sci. 2011, 10, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Vermilyea, A.W.; Voelker, B.M. Photo-Fenton reaction at near neutral pH. Environ. Sci. Technol. 2009, 43, 6927–6933. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, F.; Zhang, X.; Deng, N.S.; Bazhin, N.; Glebov, E.M. Fe(III)–pyruvate and Fe(III)–citrate induced photodegradation of glyphosate in aqueous solutions. J. Coord. Chem. 2007, 60, 2431–2439. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds mentioned in this paper are available from the authors. |


| m/z | Retention Time (min) | Structure |
|---|---|---|
| 228.6 | 11.2 | 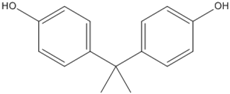 |
| 243.9 | 7.8 | 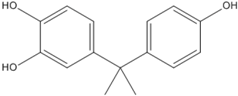 |
| 260.5 | 5.7 | 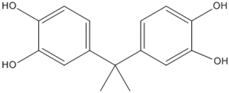 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhao, C.; Wang, T.; Yang, S.; Liu, Z. Photo-Oxidation of Bisphenol A in Aqueous Solutions at Near Neutral pH by a Fe(III)-Carboxylate Complex with Oxalacetic Acid as a Benign Molecule. Molecules 2018, 23, 1319. https://doi.org/10.3390/molecules23061319
Xu J, Zhao C, Wang T, Yang S, Liu Z. Photo-Oxidation of Bisphenol A in Aqueous Solutions at Near Neutral pH by a Fe(III)-Carboxylate Complex with Oxalacetic Acid as a Benign Molecule. Molecules. 2018; 23(6):1319. https://doi.org/10.3390/molecules23061319
Chicago/Turabian StyleXu, Jing, Chuxuan Zhao, Tianbei Wang, Shaojie Yang, and Zizheng Liu. 2018. "Photo-Oxidation of Bisphenol A in Aqueous Solutions at Near Neutral pH by a Fe(III)-Carboxylate Complex with Oxalacetic Acid as a Benign Molecule" Molecules 23, no. 6: 1319. https://doi.org/10.3390/molecules23061319






