Wetting Properties of Defective Graphene Oxide: A Molecular Simulation Study
Abstract
:1. Introduction
2. Models and Methodology
3. Results and Discussion
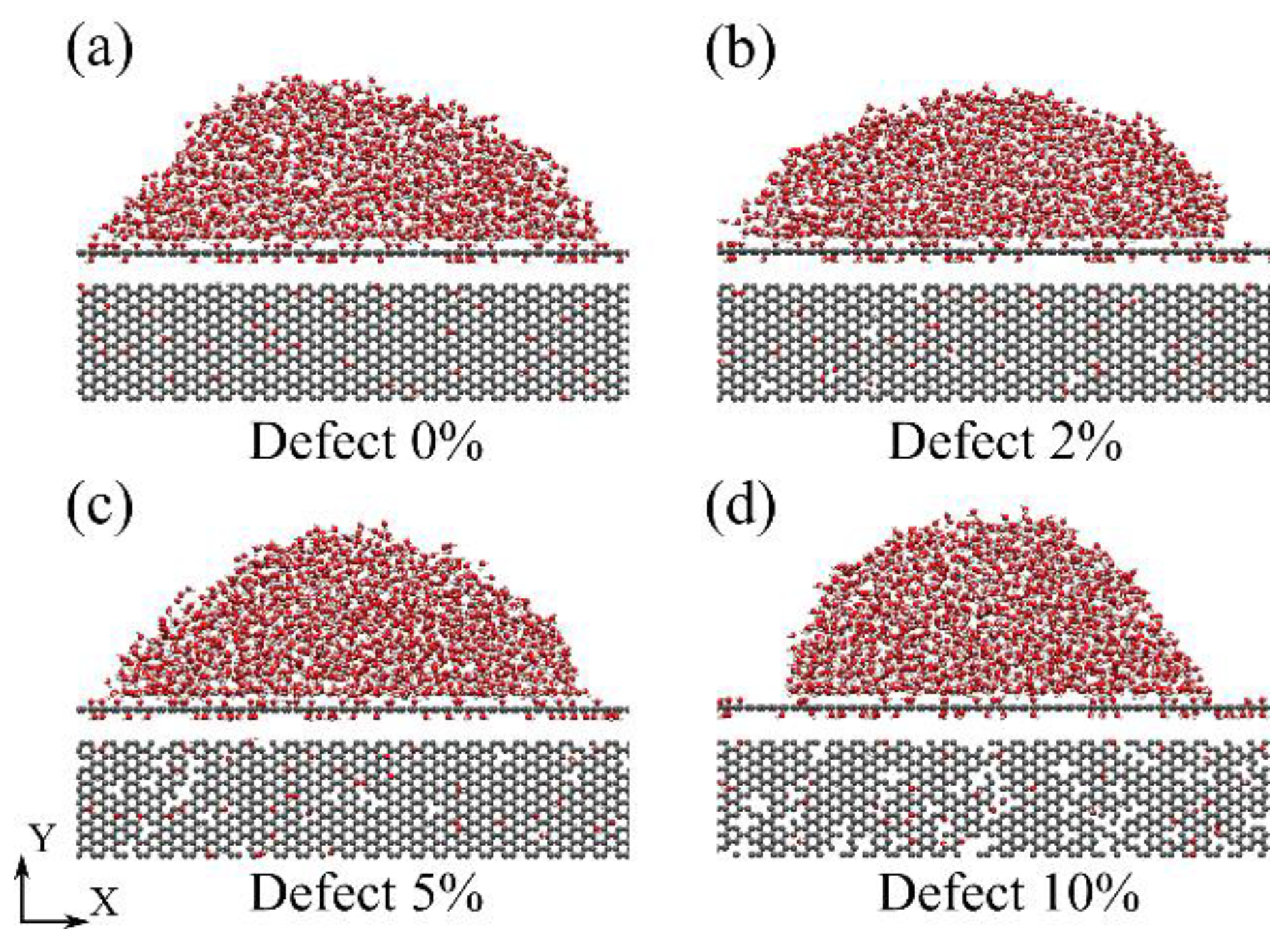
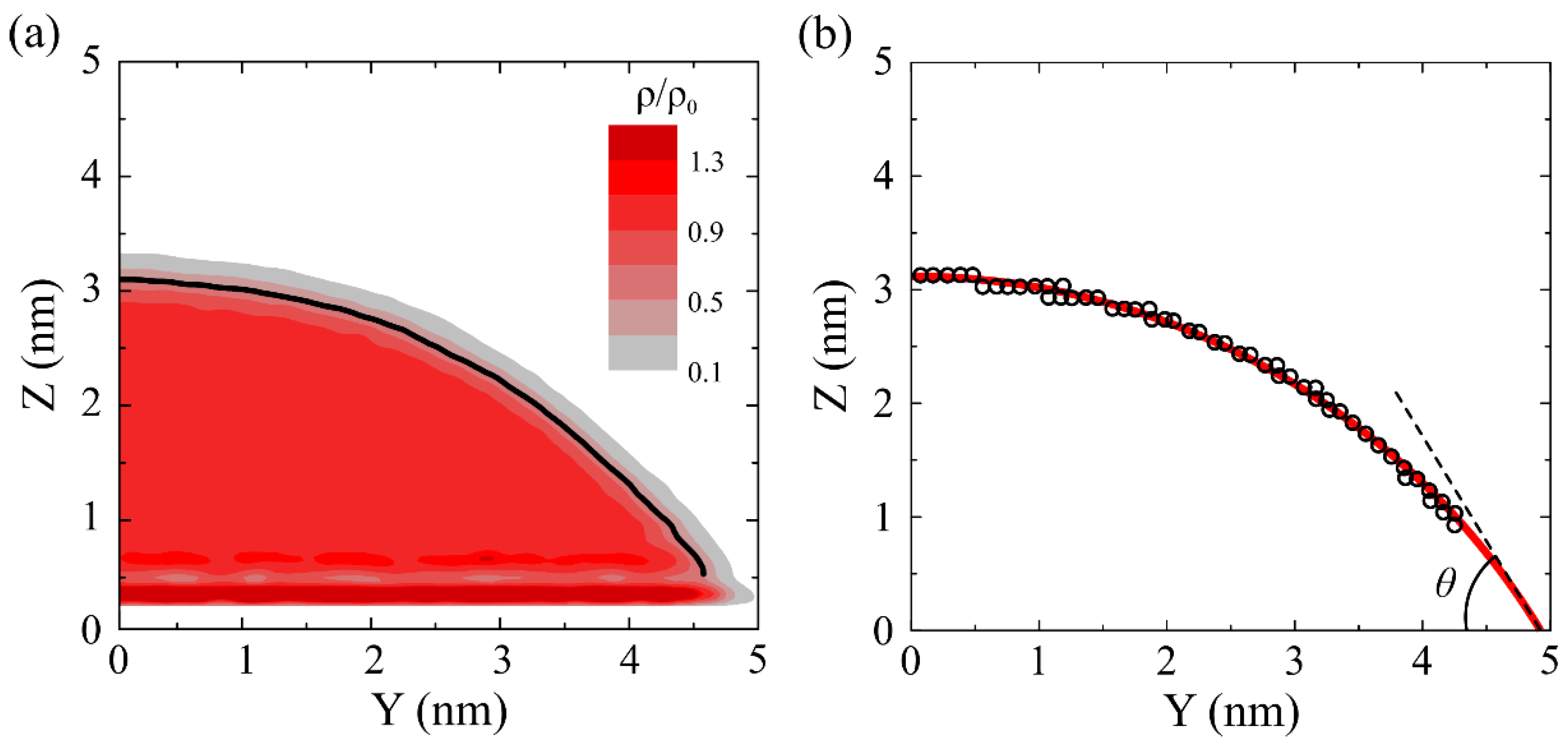
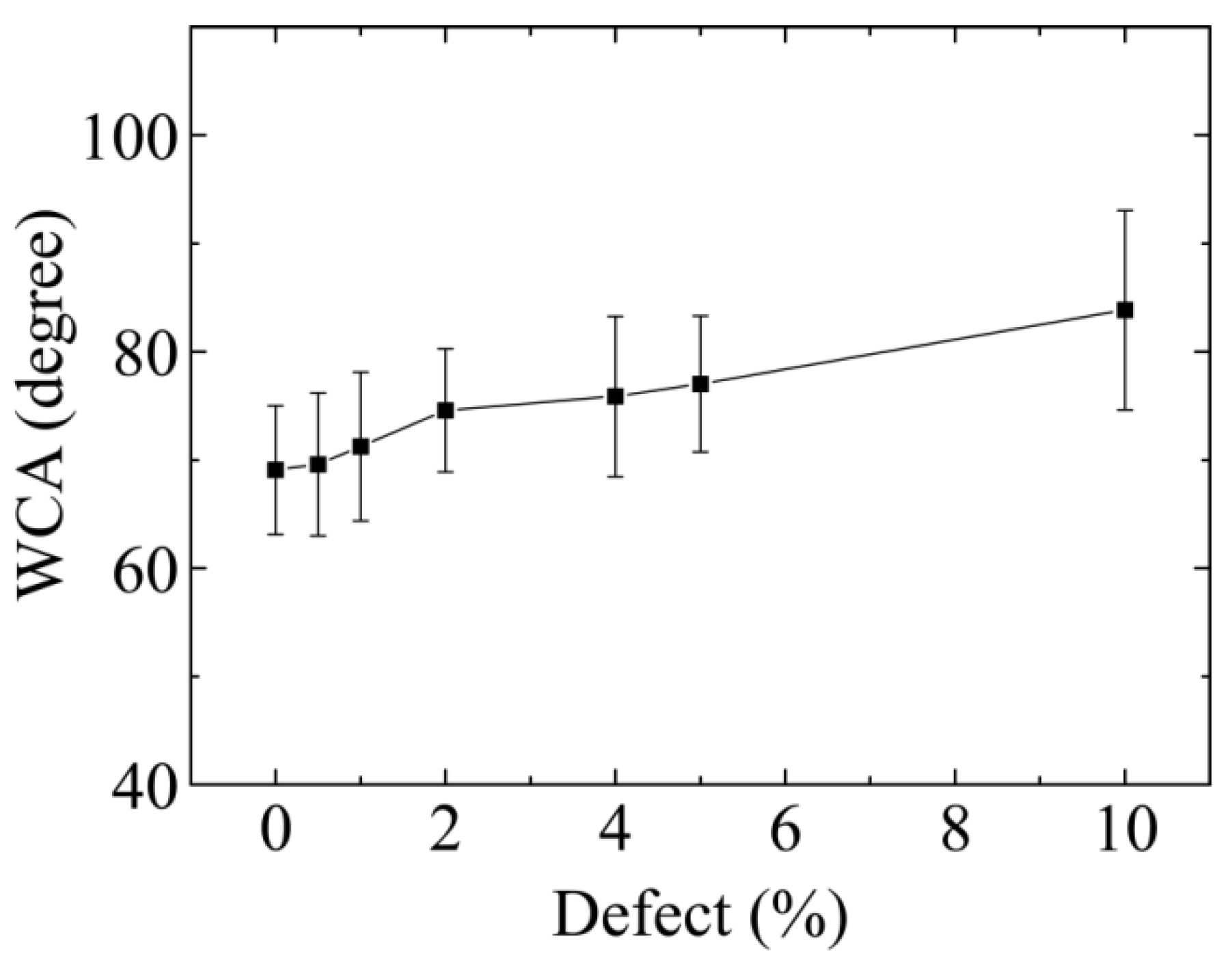
3.1. Wettability of Graphene Oxide
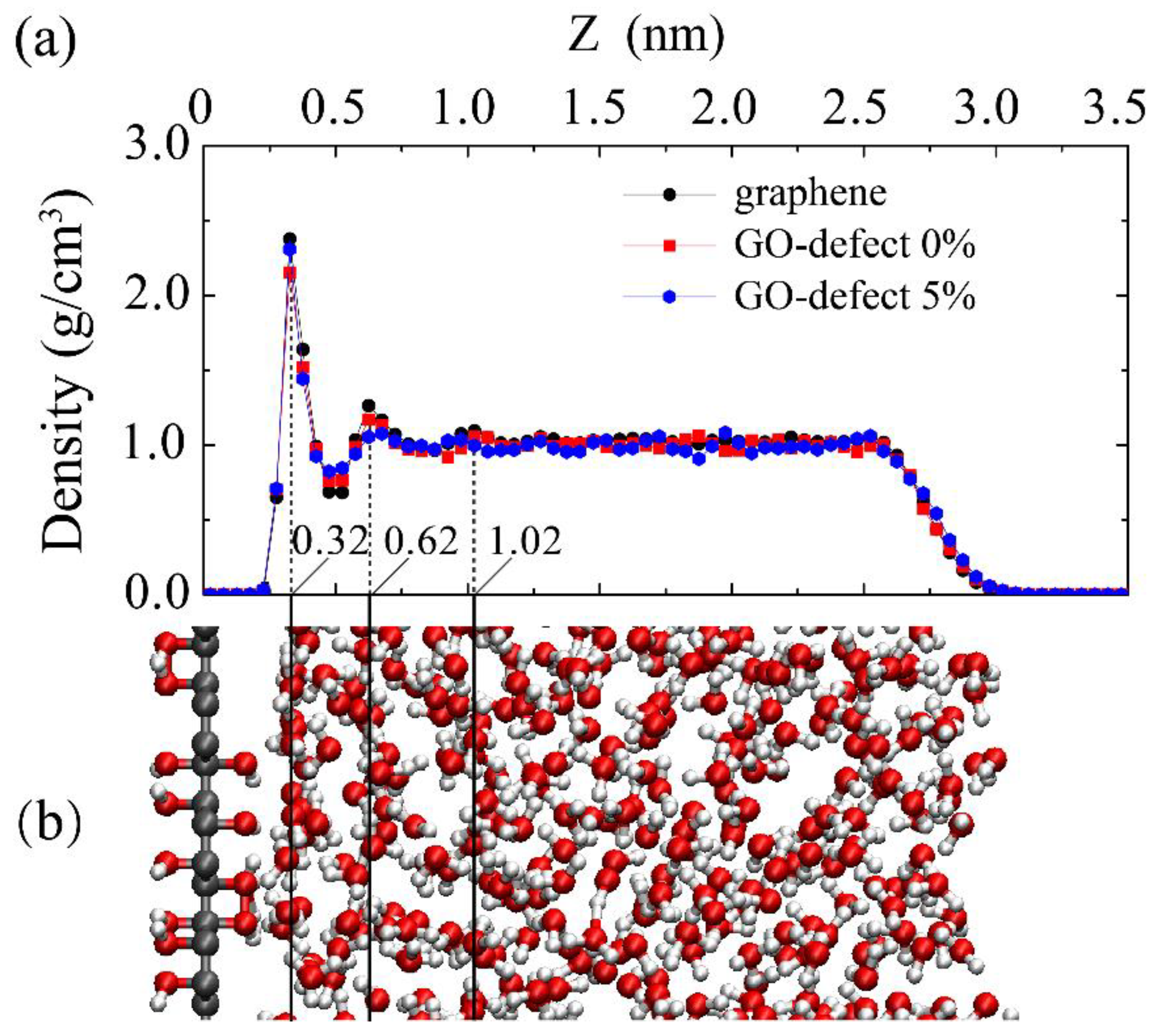
3.2. Properties of the Water–Graphene Oxide Interface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Hontoria-Lucas, C.; López-Peinado, A.J.; López-González, J.D.D.; Rojas-Cervantes, M.L.; Martín-Aranda, R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Huang, H.; Mao, Y.; Ying, Y.; Liu, Y.; Sun, L.; Peng, X. Salt concentration, pH and pressure controlled separation of small molecules through lamellar graphene oxide membranes. Chem. Commun. 2013, 49, 5963–5965. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.C.; Bennett, C.J.C.; Junkermeier, C.E.; Tsoi, S.D.; Bezares, F.J.; Stine, R.; Robinson, J.T.; Lock, E.H.; Boris, D.R.; Pate, B.D.; et al. Chemical gradients on graphene to drive droplet motion. ACS Nano 2013, 7, 4746–4755. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Lv, C.; Xu, Z. Wetting of graphene oxide: A molecular dynamics study. Langmuir 2014, 30, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, H.; Zhao, X.; Gao, C. Ultrastrong fibers assembled from giant graphene oxide sheets. Adv. Mater. 2013, 25, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Z.; Gao, C. Multifunctional, supramolecular, continuous artificial nacre fibres. Sci. Rep. 2012, 2, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, I.K.; Kim, J.I.; Lee, H.; Hur, K.; Kim, W.C.; Lee, H. 2D Graphene Oxide Nanosheets as an Adhesive Over-Coating Layer for Flexible Transparent Conductive Electrodes. Sci. Rep. 2013, 3, 1112. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective ion penetration of graphene oxide membranes. ACS Nano 2012, 7, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, Z.; Wei, N.; Shi, L.; Mao, Y.; Ying, Y.; Sun, L.; Xu, Z.; Peng, X. Ultrafast viscous water flow through nanostrand-channelled graphene oxide membranes. Nat. Commun. 2013, 4, 2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Zheng, F.; Zhu, M.; Song, Z.; Wang, K.; Zhong, M.; Wu, D.; Little, R.B.; Xu, Z.; Zhu, H. Selective trans-membrane transport of alkali and alkaline earth cations through graphene oxide membranes based on cation-π interactions. ACS Nano 2014, 8, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, F.; Jachak, A.; Kim, S.P.; Datta, D.; Liu, J.; Kulaots, I.; Vaslet, C.; Jang, H.D.; Huang, J. Aerosol synthesis of cargo-filled graphene nanosacks. Nano Lett. 2012, 12, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; He, X.; Zeng, G.; Pan, Y.; Li, G.; Liu, G.; Zhang, Y.; Chen, W.; Sun, Y. Strict molecular sieving over electrodeposited 2D-interspacing-narrowed graphene oxide membranes. Nat. Commun. 2017, 8, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The role of intercalated water in multilayered graphene oxide. ACS Nano 2010, 4, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; de Heer, W.; Bongiorno, A.; Riedo, E. Room-temperature metastability of multilayer graphene oxide films. Nat. Mater. 2012, 11, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Wang, Y.; Li, H.; Bian, X. Wetting and interfacial properties of water on the defective graphene. J. Phys. Chem. C 2013, 117, 14106–14112. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-Dependent Behavior of Graphene Oxide Aqueous Solutions: A Comparative Experimental and Molecular Dynamics Simulation Study. Langmuir 2011, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Liu, J.Z.; Ma, M.; Xu, Z.; Sheridan, J.; Zheng, Q. Strain engineering water transport in graphene nanochannels. Phys. Rev. E 2011, 84, 56329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Ma, M.; Jin, K.; Liu, J.Z.; Shen, L.; Zheng, Q.; Xu, Z. Nanoscale fluid-structure interaction: Flow resistance and energy transfer between water and carbon nanotubes. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2011, 84, 46314. [Google Scholar] [CrossRef] [PubMed]
- Hockney, R.W. Computer Simulation Using Particles; Taylor & Francis: New York, NY, USA, 1988. [Google Scholar]
- Wang, F.; Wu, H. Pinning and depinning mechanism of the contact line during evaporation of nano-droplets sessile on textured surfaces. Soft Matter 2013, 9, 5703–5709. [Google Scholar] [CrossRef]
- Werder, T.; Walther, J.H.; Jaffe, R.L.; Halicioglu, T.; Koumoutsakos, P. On the water-carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. J. Phys. Chem. B 2003, 107, 1345–1352. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Zhang, J.; Hao, X.; Zhang, C.; Wei, N.; Zhang, C. Wetting Properties of Defective Graphene Oxide: A Molecular Simulation Study. Molecules 2018, 23, 1439. https://doi.org/10.3390/molecules23061439
Xu K, Zhang J, Hao X, Zhang C, Wei N, Zhang C. Wetting Properties of Defective Graphene Oxide: A Molecular Simulation Study. Molecules. 2018; 23(6):1439. https://doi.org/10.3390/molecules23061439
Chicago/Turabian StyleXu, Ke, Jicheng Zhang, Xiaoli Hao, Chunbo Zhang, Ning Wei, and Chao Zhang. 2018. "Wetting Properties of Defective Graphene Oxide: A Molecular Simulation Study" Molecules 23, no. 6: 1439. https://doi.org/10.3390/molecules23061439
APA StyleXu, K., Zhang, J., Hao, X., Zhang, C., Wei, N., & Zhang, C. (2018). Wetting Properties of Defective Graphene Oxide: A Molecular Simulation Study. Molecules, 23(6), 1439. https://doi.org/10.3390/molecules23061439



