Change of Petals′ Color and Chemical Components in Oenothera Flowers during Senescence
Abstract
:1. Introduction
2. Results
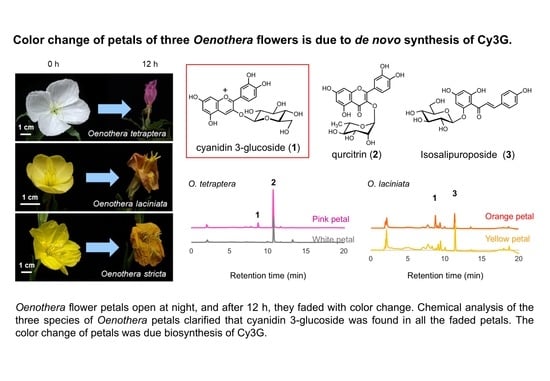
2.1. Analysis of Petal Components of O. tetraptera
2.2. Analysis of the Components of the Petals of O. laciniata and O. stricta
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. HPLC and Structural Analysis of the Flavonoids in the Petals
4.3. Quantitative Analysis of the Flavonoids by HPLC
4.4. UV/Vis and Color Parameter Measurements
4.5. Petal pH Measurements
4.6. Isolation and Characterization of Isosalipurposide from Oenothera Laciniata
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dement, W.A.; Raven, P.H. Pigments responsible for ultraviolet patterns in flowers of Oenothera (Onagraceae). Nature 1974, 252, 705–706. [Google Scholar] [CrossRef]
- Weiss, M.R. Floral color change: A widespread functional convergence. Am. J. Bot. 1995, 82, 167–185. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Schaefer, H.M. Floral colour change as a potential signal to pollinators. Curr. Opin. Plant Biol. 2016, 32, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kondo, T.; Okazaki, Y.; Katou, K. Cause of blue petal colour. Nature 1995, 373, 291. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, N.; Momonoi, K.; Kawachi, M.; Katou, K.; Okazaki, Y.; Uozumi, N.; Maeshima, M.; Kondo, T. Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly Blue. Proc. Jpn. Acad. Ser. B 2009, 85, 187–197. [Google Scholar] [CrossRef]
- Li, M.; Cao, Y.T.; Ye, S.R.; Irshad, M.; Pan, T.F.; Qiu, D.L. Isolation of CHS gene from Brunfelsia acuminata flowers and its regulation in anthocyanin biosysthesis. Molecules 2017, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Forkmann, G.; Dangelmayr, B. Genetic control of chalcone isomerase activity in flowers of Dianthus caryophyllus. Biochem. Genet. 1980, 18, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Heller, W.; Forkmann, G. Biosynthesis of flavonoids. In The Flavonoids: Advances in Research since 1986; Harborne, J.B., Ed.; Chapman & Hall: London, UK, 1994; pp. 499–536. ISBN 0412480700. [Google Scholar]
- Springob, K.; Nakajima, J.; Yamazaki, M.; Saito, K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 2003, 20, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Heller, W.; Britsch, L.; Forkmann, G.; Grisebach, H. Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br. Planta 1985, 163, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sato, Y.; Okuno, R.; Kameda, K.; Isobe, M.; Kondo, T. Structural analysis and measurement of anthocyanins from colored seed coats of Vigna, Phaseolus, and Glycine legumes. Biosci. Biotechnol. Biochem. 1996, 60, 589–593. [Google Scholar] [CrossRef]
- Harborne, J.B. Comparative biochemistry of flavonoids-I. Distribution of chalcone and aurone pigments in plants. Phytochemistry 1966, 5, 111–115. [Google Scholar] [CrossRef]
- Huang, S.; Fujioka, T.; Yoshida, M.; Ishimaru, K. A new chalcone glycoside from Sapium sebiferum. J. Nat. Med. 2007, 61, 339–341. [Google Scholar] [CrossRef]
- Howard, G.Z.; Mabry, T.J.; Raven, P.H. Distribution of flavonoids in twenty-one species of Oenothera. Phytochemistry 1972, 11, 289–291. [Google Scholar] [CrossRef]
- Dement, W.A.; Raven, P.H. Distribution of the chalcone, isosalipurposide, in the Onagraceae. Phytochemistry 1973, 12, 807–808. [Google Scholar] [CrossRef]
- Averett, J.E.; Huang, S.; Wagner, W.L. Flavonoid survey of Oenothera (Onagraceae): Sects. Gauropsis, Hartmannia, Kneiffia, Paradoxus, and Xylopleurum. Am. J. Bot. 1988, 75, 476–483. [Google Scholar] [CrossRef]
- Zapesochnaya, G.G.; Kurkin, V.A.; Braslavskii, V.B.; Filatova, N.V. Phenolic compounds of Salix acutifolia bark. Chem. Nat. Compd. 2002, 38, 314–318. [Google Scholar] [CrossRef]
- Ghribia, L.; Ghouilaa, H.; Omrib, A.; Besbesb, M.; Janneta, H.B. Antioxidant and anti-acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac. J. Trop. Biomed. 2014, 4, S417–S423. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Schwemmle, B. Flavonoids from Oenothera-seedlings: Identification and extranuclear control of their biosynthesis. J. Plant Physiol. 1993, 142, 135–143. [Google Scholar] [CrossRef]
- Davies, K.M.; Bloor, S.J.; Spiller, G.B.; Deroles, S.C. Production of yellow colour in flowers: Redirection of flavonoid biosynthesis in Petunia. Plant J. 1998, 13, 259–266. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 3 are available from the authors. |






| Sample | CIELAB Color Coordinate 1 | PH | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| 0 h | 99.52 | −1.5 | 3.33 | 5.73 |
| 4 h | 93.13 | 8.5 | −1.11 | 5.86 |
| 7 h | 87.74 | 17.33 | −3.94 | 5.66 |
| 12 h | 82.39 | 19.62 | −5.71 | 5.52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teppabut, Y.; Oyama, K.-i.; Kondo, T.; Yoshida, K. Change of Petals′ Color and Chemical Components in Oenothera Flowers during Senescence. Molecules 2018, 23, 1698. https://doi.org/10.3390/molecules23071698
Teppabut Y, Oyama K-i, Kondo T, Yoshida K. Change of Petals′ Color and Chemical Components in Oenothera Flowers during Senescence. Molecules. 2018; 23(7):1698. https://doi.org/10.3390/molecules23071698
Chicago/Turabian StyleTeppabut, Yada, Kin-ichi Oyama, Tadao Kondo, and Kumi Yoshida. 2018. "Change of Petals′ Color and Chemical Components in Oenothera Flowers during Senescence" Molecules 23, no. 7: 1698. https://doi.org/10.3390/molecules23071698
APA StyleTeppabut, Y., Oyama, K.-i., Kondo, T., & Yoshida, K. (2018). Change of Petals′ Color and Chemical Components in Oenothera Flowers during Senescence. Molecules, 23(7), 1698. https://doi.org/10.3390/molecules23071698