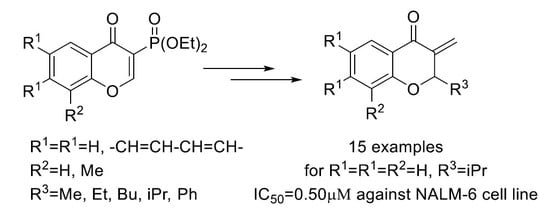
Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones
Abstract
:1. Introduction
2. Results and Discussion
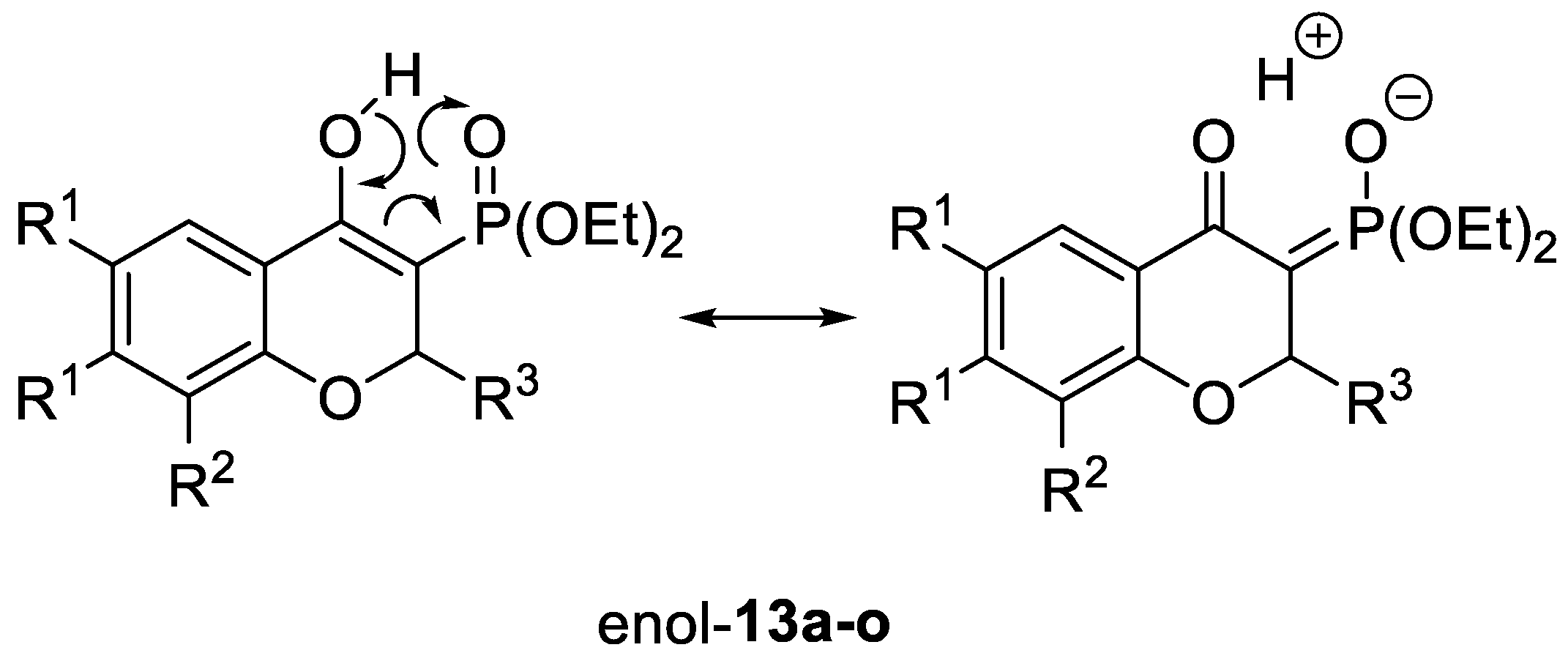
2.1. Chemistry
2.2. Biology
2.2.1. In Vitro Cytotoxicity of New Analogs Against Three Cancer Cell Lines
2.2.2. Apoptotic Cell Death Determination
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Synthesis of 2-Substituted 3-Metylidenechroman-4-ones 14a–o.
3.2. Biology
3.2.1. Cell Lines
3.2.2. In Vitro Cytotoxicity Assay
3.2.3. Annexin V and Propidium Iodide Assay
3.2.4. Caspase 3, Caspase 8, and Caspase 9 Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nibbs, A.E.; Scheidt, K.A. Asymmetric methods for the synthesis of flavanones, chromanones, and azaflavanones. Eur. J. Org. Chem. 2012, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhao, Y.; Zhou, X.; Wu, Y.; Wu, P.; Liu, T.; Yang, B.; Hu, Y.; Dong, X. Design, synthesis and biological evaluation of 3-benzylideneflavanone derivatives as cytotoxic agents. Med. Chem. Res. 2012, 21, 4150–4157. [Google Scholar] [CrossRef]
- Sidwell, W.T.L.; Tamm, C. The homo-isoflavones II. Isolation and structure of 4′-O-methyl-punctatin, autumnalin and 3, 9-dihydro-autumnalin. Tetrahedron Lett. 1970, 475–478. [Google Scholar] [CrossRef]
- Böhler, P.; Tamm, C. The homo-isoflavones, a new class of natural product. Isolation and structure of eucomin and eucomol. Tetrahedron Lett. 1967, 8, 3479–3483. [Google Scholar] [CrossRef]
- Desideri, N.; Bolasco, A.; Fioravanti, R.; Monaco, L.P.; Orallo, F.; Yañez, M.; Ortuso, F.; Alcaro, S. Homoisoflavonoids: Natural scaffolds with potent and selective monoamine oxidase-B inhibition properties. J. Med. Chem. 2011, 54, 2155–2164. [Google Scholar] [CrossRef]
- Desideri, N.; Monaco, L.P.; Fioravanti, R.; Biava, M.; Yañez, M.; Alcaro, S.; Ortuso, F. (E)-3-Heteroarylidenechroman-4-ones as potent and selective monoamine oxidase-B inhibitors. Eur. J. Med. Chem. 2016, 117, 292–300. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Gouranourimi, A.; Hekmat, S.; Asadipour, A.; Rahmani-Nezhad, S.; Moradi, A.; Nadri, H.; Moghadam, F.H.; Emami, S.; Foroumadi, A.; et al. Design, synthesis and anticholinesterase activity of novel benzylidenechroman-4-ones bearing cyclic amine side chain. Eur. J. Med. Chem. 2015, 97, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiang, X.; Luo, L.; Yang, X.; Xiao, G.; Zheng, Y.; Cao, Z.; Sang, Z.; Su, F.; Deng, Y. Multitarget drug design strategy against Alzheimer’s disease: Homoisoflavonoid mannich base derivatives serve as acetylcholinesterase and monoamine oxidase B dual inhibitors with multifuncional properties. Bioorg. Med. Chem. 2017, 25, 714–726. [Google Scholar] [CrossRef]
- Perjési, P.; Das, U.; De Clercq, E.; Balzarini, J.; Kawase, M.; Sakagami, H.; Stables, J.P.; Lorand, T.; Rozmer, Z.; Dimmock, J.R. Design, synthesis and antiproliferative activity of some 3-benzylidene-2, 3-dihydro-1-benzopyran-4-ones which display selective toxicity for malignant cells. Eur. J. Med. Chem. 2008, 43, 839–845. [Google Scholar] [CrossRef]
- Pordeli, P.; Nakhjiri, M.; Safavi, M.; Ardestani, S.K.; Foroumadi, A. Anticancer effects of synthetic hexahydrobenzo[g]chromen-4-one derivatives on human breast cancer cell lines. Breast Cancer 2017, 24, 299–311. [Google Scholar] [CrossRef]
- Pouget, C.; Fagnere, C.; Basly, J.-P.; Habrioux, G.; Chulia, A.-J.; New aromatase inhibitors. Synthesis and inhibitory activity of pyridinyl-Substituted flavanone derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 1059–1061. [Google Scholar] [CrossRef]
- Basavaiah, D.; Bakthadoss, M.; Pandiaraju, S. A new protocol for the syntheses of (E)-3-benzylidenechroman-4-ones: A simple synthesis of the methyl ether of bonducellin. Chem. Commun. 1998, 16, 1639–1640. [Google Scholar] [CrossRef]
- AI Nakib, T.; Bezjak, V.; Meegan, M.J.; Chandy, R. Synthesis and antifungal activity of some 3-benzylidenechroman-4-ones, 3-benzylidenethiochroman-4-ones and 2-benzylidene-1-tetralones. Eur. J. Med. Chem. 1990, 25, 455–462. [Google Scholar] [CrossRef]
- Takao, K.; Yamashita, M.; Yashiro, A.; Sugita, Y. Synthesis and Biological Evaluation of 3-Benzylidene-4-chromanone Derivatives as Free Radical Scavengers and α-Glucosidase Inhibitors. Chem. Pharm. Bull. 2016, 64, 1203–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddaiah, V.; Rao, C.V.; Venkateswarlu, S.; Krishnarajub, A.V.; Subbaraju, G.V. Synthesis, stereochemical assignments, and biological activities of homoisoflavonoids. Bioorg. Med. Chem. 2006, 14, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.M.; Cao Van, T.; Nguyen Tien, D. Homoisoflavonoid derivatives from the roots of Ophiopogon japonicus and their in vitro anti-inflammation activity. Bioorg. Med. Chem. Lett. 2010, 20, 2412–2416. [Google Scholar] [CrossRef]
- Ward, F.E.; Garling, D.L.; Buckler, R.T. Antimicrobial 3-Methylene flavanones. J. Med. Chem. 1981, 24, 1073–1077. [Google Scholar] [CrossRef]
- Wallén, E.A.A.; Dahlén, K.; Grøtli, M.; Luthman, K. Synthesis of 3-Aminomethyl-2-aryl-8-bromo-6-chlorochromones. Org.Lett. 2007, 9, 389–391. [Google Scholar]
- Okuro, K.; Alper, H. Palladium-Catalyzed Carbonylation ofo-Iodophenols with Allenes. J. Org. Chem. 1997, 62, 1566–1567. [Google Scholar] [CrossRef]
- Grigg, R.; Liu, A.; Shaw, D.; Suganthan, S.; Woodalla, D.E.; Yoganathan, G. Synthesis of quinol-4-ones and chroman-4-ones via a palladium-catalysed cascade carbonylation–allene insertion. Tetrahedron Lett. 2000, 41, 7125–7128. [Google Scholar] [CrossRef]
- Bugarin, A.; Jones, K.D.; Connell, B.T. Efficient, direct α-methylenation of carbonyls mediated by diisopropylammonium trifluoroacetate. Chem. Commun. 2010, 46, 1517–1717. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.V.; Wankhede, K.S.; Salunkhe, M.M.; Trivedi, G.K. Synthesis of Functionalized Benzopyrans via Intramolecular 1,3-Dipolar Cycloaddition of Nitrile Oxides. Synth. Commun. 2008, 38, 2392–2403. [Google Scholar] [CrossRef]
- Iwasaki, H.; Kume, T.; Yamamoto, Y.; Akiba, K. Reaction of 4-t-butyldimethylsiloxy-1-benzopyrylium salt with enol silyl ethers and active methylenes. Tetrahedron Lett. 1987, 28, 6355–6358. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Ishimaru, K.; Iwasaki, H.; Ohkata, K.; Akiba, K. Tandem Reactions In 4-Siloxy-1-benzopyrylium Salts: Introduction of Substituents and Cyclohexene and Cyclopentane Annulation in Chromones. J. Org. Chem. 1991, 56, 2058–2066. [Google Scholar] [CrossRef]
- Kitson, R.R.A.; Millemaggi, A.; Taylor, R.J.K. The Renaissance of α-Methylene-γ-butyrolactones: New Synthetic Approaches. Angew. Chem. Int. Ed. 2009, 48, 9426–9452. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, A.; Albrecht, Ł.; Janecki, T. Recent Advances in the Synthesis of α-Alkylidene-Substituted δ-Lactones, γ-Lactams and δ-Lactams. Eur. J. Org. Chem. 2011, 2011, 2747–2766. [Google Scholar] [CrossRef]
- Lagoutte, R.; Winssinger, N. Following the Lead from Nature with Covalent Inhibitors. CHIMIA 2017, 71, 703–711. [Google Scholar] [CrossRef]
- Lagoutte, R.; Pastor, M.; Berthet, M.; Winssinger, N. Rapid and scalable synthesis of chiral bromolactones as precursors to α-exo-methylene-γ--butyrolactone-containing sesquiterpene lactones. Tetrahedron 2018, 74, 6012–6021. [Google Scholar] [CrossRef]
- Janecki, T. Organophosphorus reagents as versatile tool in the synthesis of α-alkylidene-γ-butyrolactones and α-alkylidene-γ-butyrolactams. In Targets in Heterocyclic Systems; Italian Society of Chemistry: Rome, Italy, 2006; pp. 301–320. [Google Scholar]
- Modranka, J.; Albrecht, A.; Janecki, T. A Convenient Entry to 3-Methylidenechroman-2-ones and 2-Methylidenedihydrobenzochromen-3-ones. Synlett 2010, 19, 2867–2870. [Google Scholar]
- Pięta, M.; Kędzia, J.; Wojciechowski, J.; Janecki, T. Asymmetric synthesis of 1,4-disubstituted 3-methylidenedihydroquinolin-2(1H)-ones. Tetrahedron Asymm. 2017, 28, 567–576. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, Y.-J.; Zou, J.-P.; Zhang, W. Manganese(III) Acetate Mediated Free-Radical Phosphonylation of Flavones and Coumarins. Synthesis 2012, 44, 1043–1050. [Google Scholar] [CrossRef]
- Somu, R.V.; Boshoff, H.; Qiao, C.; Bennett, E.M.; Barry III, C.E.; Aldrich, C.C. Rationally Designed Nucleoside Antibiotics That Inhibit Siderophore Biosynthesis of Mycobacterium tuberculosis. J. Med. Chem. 2006, 49, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Janecki, T.; Wąsek, T. A novel route to substituted 3-methylidenechroman-2-ones and 3-methylchromen-2-ones. Tetrahedron 2004, 60, 1049–1050. [Google Scholar] [CrossRef]
- Koleva, A.I.; Petkova, N.I.; Nikolova, R.D. Ultrasound-Assisted Conjugate Addition of Organometallic Reagents to 3-Diethylphosphonocoumarin. Synlett 2016, 27, 2676–2680. [Google Scholar]
- Mahmudov, K.T.; Pombeiro, A.J.L. Resonance-Assisted Hydrogen Bonding as a Driving Force in Synthesis and a Synthon in the Design of Materials. Chem. Eur. J. 2016, 22, 16356–16398. [Google Scholar] [CrossRef] [PubMed]
- Koszuk, J.; Bartosik, T.; Wojciechowski, J.; Wolf, W.M.; Janecka, A.; Drogosz, J.; Długosz, A.; Krajewska, U.; Mirowski, M.; Janecki, T. Synthesis of 3-Methylidene-1-tosyl-2,3-dihydroquinolin-4(1H)-ones as Potent Cytotoxic Agents. Chem. Biodivers. 2018, 15, e1800242. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 10a–c,12a–c and 13a,c–d, f-h,k–l are available from the authors. |
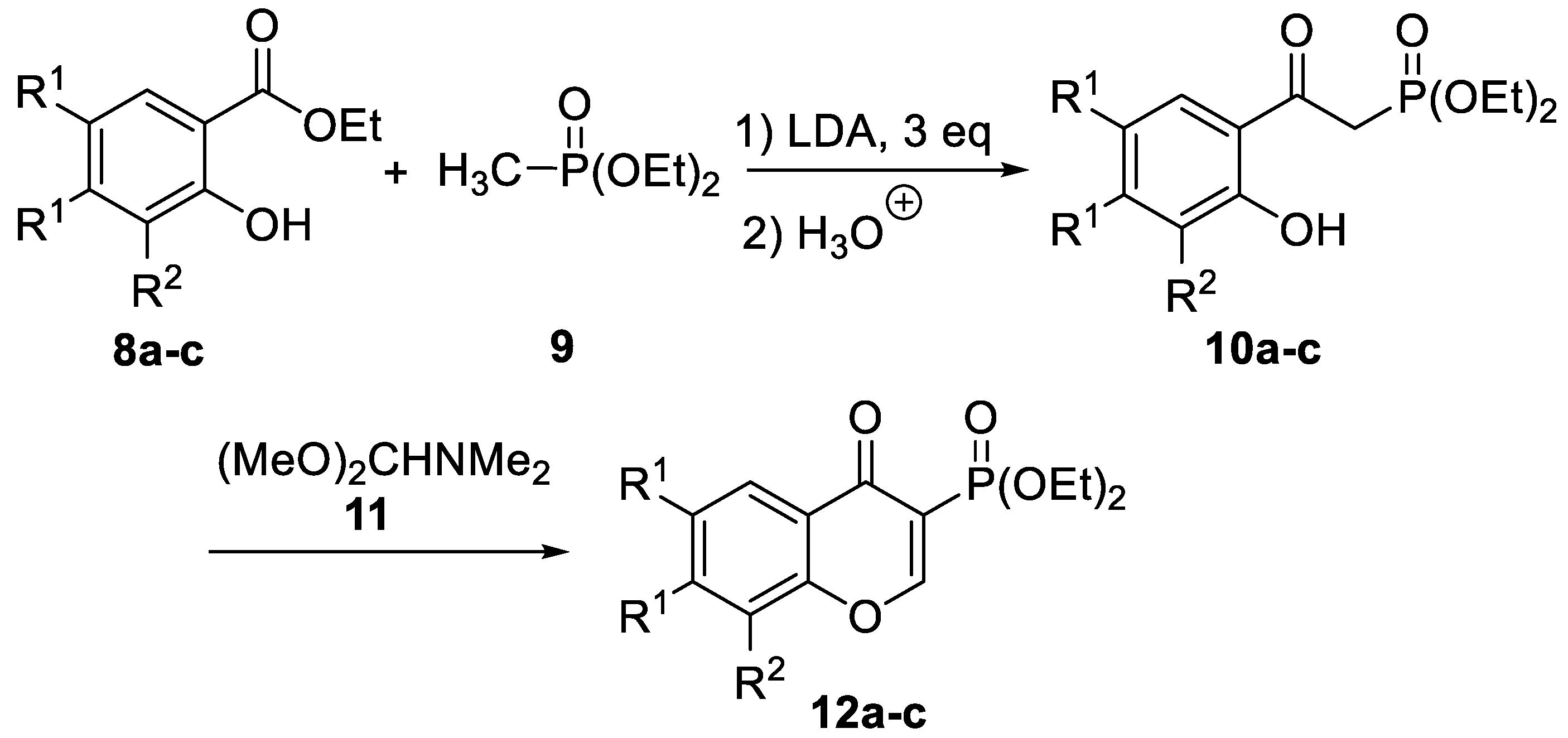


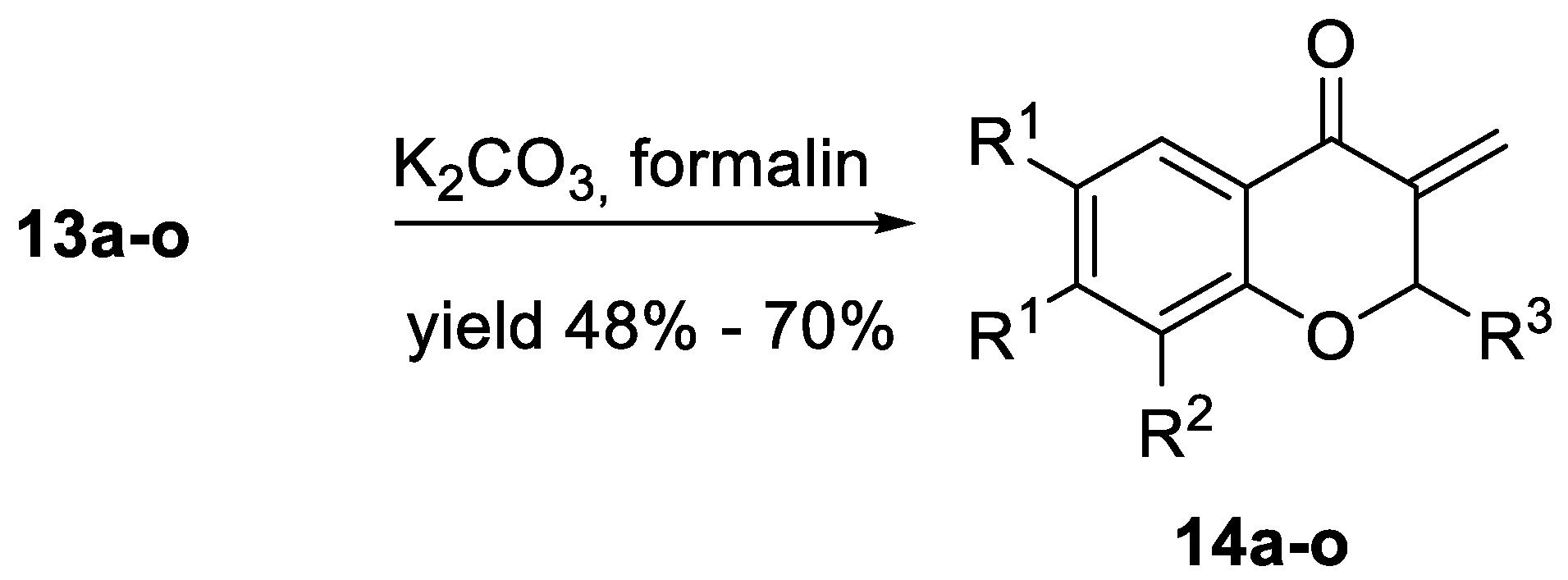


| Compound | R1,R1 | R2 | 10 Yield [%] 1 | 12 Yield [%] 1 |
|---|---|---|---|---|
| a | H,H | H | 80 | 92 |
| b | H,H | Me | 75 | 89 |
| c | CH=CH-CH=CH | H | 80 | 78 |
| Compound | R1,R1 | R2 | R3 | 13 | 14 Yield [%] 2 | |
|---|---|---|---|---|---|---|
| trans/cis/enol 1 | Yield [%] 2 | |||||
| a | H,H | H | Me | 67/30/3 | 84 | 68 |
| b | H,H | H | Et | 71/25/4 | 68 | 71 |
| c | H,H | H | n-Bu | 76/20/4 | 85 | 59 |
| d | H,H | H | iPr | 71/26/3 | 66 | 53 |
| e | H,H | H | Ph | 81/10/9 | 64 | 84 |
| f | H,H | Me | Me | 73/24/3 | 74 | 64 |
| g | H,H | Me | Et | 75/22/3 | 76 | 59 |
| h | H,H | Me | n-Bu | 72/25/3 | 83 | 52 |
| i | H,H | Me | iPr | 72/26/2 | 70 | 53 |
| j | H,H | Me | Ph | 83/11/6 | 84 | 59 |
| k | CH=CH-CH=CH | H | Me | 79/14/7 | 81 | 66 |
| l | CH=CH-CH=CH | H | Et | 89/4/7 | 64 | 67 |
| m | CH=CH-CH=CH | H | n-Bu | 73/16/11 | 53 | 60 |
| n | CH=CH-CH=CH | H | iPr | 81/15/4 | 66 | 48 |
| o | CH=CH-CH=CH | H | Ph | 65/6/29 | 76 | 70 |

| 14 | R1, R1 | R2 | R3 | IC50 [µM] 1 | ||
|---|---|---|---|---|---|---|
| HL-60 | NALM-6 | MCF-7 | ||||
| a | H, H | H | Me | 5.91 ± 0.32 | 2.13 ± 0.04 | 9.70 ± 0.80 |
| b | H, H | H | Et | 5.24 ± 0.52 | 0.60 ± 0.02 | 12.50 ± 0.71 |
| c | H, H | H | n-Bu | 8.79 ± 0.81 | 4.23 ± 0.46 | 16.00 ± 0.20 |
| d | H, H | H | iPr | 1.46 ± 0.16 | 0.50 ± 0.05 | 19.40 ± 0.80 |
| e | H, H | H | Ph | 23.86 ± 2.30 | 5,76 ± 0.23 | 17,10 ± 0.30 |
| f | H, H | Me | Me | 7.52 ± 0.81 | 3.28 ± 0.35 | 10.50 ± 0.34 |
| g | H, H | Me | Et | 20.87 ± 1.73 | 4.83 ± 0.45 | 11.60 ± 0.07 |
| h | H, H | Me | n-Bu | 32.16 ± 3.17 | 6.36 ± 0.36 | 13.50 ± 0.60 |
| i | H, H | Me | iPr | 3.45 ± 0.34 | 0.58 ± 0.05 | 8.48 ± 0.93 |
| j | H, H | Me | Ph | 30.51 ± 3.62 | 6.09 ± 0.61 | 30.00 ± 1.90 |
| k | CH=CH-CH=CH | H | Me | 6.96 ± 0.42 | 4.31 ± 0.36 | 13.50 ± 1.00 |
| l | CH=CH-CH=CH | H | Et | 47.00 ± 4.11 | 10.91 ± 2.8 | 34.60 ± 4.50 |
| m | CH=CH-CH=CH | H | n-Bu | 8.64 ± 0.53 | 6.00 ± 0.23 | 17.40 ± 2.50 |
| n | CH=CH-CH=CH | H | iPr | 10.47 ± 2.04 | 5.52 ± 0.25 | 15.70 ± 0.70 |
| o | CH=CH-CH=CH | H | Ph | 49.96 ± 3.89 | 6.59 ± 0.41 | 71.0 ± 7.50 |
| Carboplatin | 2.9 ± 0.1 | 0.7 ± 0.3 | 3.8 ± 0.45 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędzia, J.; Bartosik, T.; Drogosz, J.; Janecka, A.; Krajewska, U.; Janecki, T. Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones. Molecules 2019, 24, 1868. https://doi.org/10.3390/molecules24101868
Kędzia J, Bartosik T, Drogosz J, Janecka A, Krajewska U, Janecki T. Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones. Molecules. 2019; 24(10):1868. https://doi.org/10.3390/molecules24101868
Chicago/Turabian StyleKędzia, Jacek, Tomasz Bartosik, Joanna Drogosz, Anna Janecka, Urszula Krajewska, and Tomasz Janecki. 2019. "Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones" Molecules 24, no. 10: 1868. https://doi.org/10.3390/molecules24101868