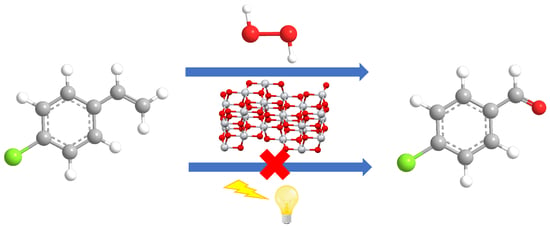
Titania-Catalyzed H2O2 Thermal Oxidation of Styrenes to Aldehydes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oxidation of 4-Chlorostyrene with H2O2 over a TiO2 Catalyst
2.2. Scope and Limitations
2.3. Detection of Active Species for the Oxidation and Catalyst Recyclability
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bäckvall, J.-E. Modern Oxidation Methods, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Diaper, D.G. Ozonolysis of 1-substituted cycloolefins. Can. J. Chem. 1955, 33, 1720–1723. [Google Scholar] [CrossRef]
- Pappo, R.; Allen, D.S.; Lemieux, R.U.; Johnson, W.S. Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds. J. Org. Chem. 1956, 21, 478–479. [Google Scholar] [CrossRef]
- Spannring, P.; Bruijnincx, P.C.A.; Weckhuysen, B.M.; Gebbink, R.J.M. Transition metal-catalyzed oxidative double bond cleavage of simple and bio-derived alkenes and unsaturated fatty acids. Catal. Sci. Technol. 2014, 4, 2182–2209. [Google Scholar] [CrossRef]
- Urgoitia, G.; SanMartin, R.; Herrero, M.T.; Domínguez, E. Aerobic Cleavage of Alkenes and Alkynes into Carbonyl and Carboxyl Compounds. ACS Catal. 2017, 7, 3050–3060. [Google Scholar] [CrossRef]
- Reddy, J.S.; Khire, U.R.; Ratnasamy, P.; Mitra, R.B. Cleavage of the Carbon-Carbon Bond over Zeolites using Hydrogen Peroxide. J. Chem. Soc. Chem. Commun. 1992, 17, 1234–1235. [Google Scholar] [CrossRef]
- Tong, J.; Li, W.; Bo, L.; Wang, H.; Hu, Y.; Zhang, Z.; Mahboob, A. Selective oxidation of styrene catalyzed by cerium-doped cobalt ferrite nanocrystals with greatly enhanced catalytic performance. J. Catal. 2016, 344, 474–481. [Google Scholar] [CrossRef]
- Adam, F.; Iqbal, A. The liquid phase oxidation of styrene with tungsten modified silica as a catalyst. Chem. Eng. J. 2011, 171, 1379–1386. [Google Scholar] [CrossRef]
- Ghosh, S.; Acharyya, S.S.; Bal, R. One-pot preparation of nanocrystalline Ag–WO3 catalyst for the selective oxidation of styrene. RSC Adv. 2015, 5, 37610–37616. [Google Scholar] [CrossRef]
- Sun, W.; Hu, J. Oxidation of styrene to benzaldehyde with hydrogen peroxide in the presence of catalysts obtained by the immobilization of H3PW12O40 on SBA-15 mesoporous material. Reac. Kinet. Mech. Cat. 2016, 119, 305–318. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Transition-Metal-Substituted Phosphomolybdates: Catalytic and Kinetic Study for Liquid-Phase Oxidation of Styrene. Ind. Eng. Chem. Res. 2013, 52, 11913–11919. [Google Scholar] [CrossRef]
- Sharma, P.; Patel, A. Chemical Supported 12-molybdophosphoricacid: Characterization and non-solvent liquid phase oxidation of styrene. J. Mol. Catal. A Chem. 2009, 299, 37–43. [Google Scholar] [CrossRef]
- Zhang, L.; Hua, Z.; Dong, X.; Li, L.; Chen, H.; Shi, J. Preparation of highly ordered Fe-SBA-15 by physical-vapor-infiltration and their application to liquid phase selective oxidation of styrene. J. Mol. Catal. A Chem. 2007, 268, 155–162. [Google Scholar] [CrossRef]
- Hulea, V.; Dumitriu, E. Styrene oxidation with H2O2 over Ti-containing molecular sieves with MFI, BEA and MCM-41 topologies. Appl. Catal. A 2004, 277, 99–106. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Shishido, T.; Takehira, K. Characterizations of Iron-Containing MCM-41 and Its Catalytic Properties in Epoxidation of Styrene with Hydrogen Peroxide. J. Catal. 2002, 209, 186–196. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Hao, S.; Guan, J.; Ding, H.; Shang, F. Heterogenization of functionalized Cu (II) and VO (IV) Schiff base complexes by direct immobilization onto amino-modified SBA-15: Styrene oxidation catalysts with enhanced reactivity. Appl. Catal. A 2010, 381, 274–281. [Google Scholar] [CrossRef]
- Li, B.; Zhu, Y.; Jin, X. Synthesis of cobalt-containing mesoporous catalysts using the ultrasonic-assisted “pH-adjusting” method: Importance of cobalt species in styrene oxidation. J. Solid State Chem. 2015, 221, 230–239. [Google Scholar] [CrossRef]
- Wang, H.; Qian, W.; Chen, J.; Wu, Y.; Xu, X.; Wang, J.; Kong, Y. Spherical V-MCM-48: The synthesis, characterization and catalytic performance in styrene oxidation. RSC Adv. 2014, 4, 50832–50839. [Google Scholar] [CrossRef]
- Chen, D.; Li, N.; Sun, P.; Kong, Y. Catalytic Performance of Ti-MCM-41 for Styrene Oxidation. Chin. J. Catal. 2009, 30, 643–648. [Google Scholar]
- Zhang, Y.; Shen, J.; Zhu, J.; Sun, Y. Study on preparation process of benzaldehyde by catalytic oxidation of styrene on catalyst V-SBA-15. Petrochem. Tech. Appl. 2012, 30, 124–128. [Google Scholar]
- Gao, D.; Gao, Q. Selective oxidation of styrene to benzaldehyde over VSB-5 and isomorphously substituted cobalt VSB-5. Catal. Commun. 2007, 8, 681–685. [Google Scholar] [CrossRef]
- Maurya, M.R.; Chandrakar, A.K.; Chand, S. Oxidation of phenol, styrene and methyl phenyl sulfide with H2O2 catalysed by dioxovanadium (V) and copper (II) complexes of 2-aminomethylbenzimidazole-based ligand encapsulated in zeolite-Y. J. Mol. Catal. A Chem. 2007, 263, 227–237. [Google Scholar] [CrossRef]
- Tanglumlert, W.; Imae, T.; White, T.J.; Wongkasemjit, S. Styrene oxidation with H2O2 over Fe- and Ti-SBA-1 mesoporous silica. Catal. Commun. 2009, 10, 1070–1073. [Google Scholar] [CrossRef]
- Campelo, J.M.; Conesa, D.; Gracia, J.; Jurado, J.; Luque, R.; Maria, J.; Angel, A. Microwave facile preparation of highly active and dispersed SBA-12 supported metal nanoparticles. Green Chem. 2008, 10, 853–858. [Google Scholar] [CrossRef]
- Zou, H.; Hu, C.; Chen, K.; Xiao, G.; Peng, X. Cobalt vanadium oxide supported on reduced graphene oxide for the oxidation of styrene derivatives to aldehydes with hydrogen peroxide as oxidant. Synlett 2018, 29, 2181–2184. [Google Scholar]
- Zou, H.; Xiao, G.; Chen, K.; Peng, X. Noble metal-free V2O5/g-C3N4 composites for selective oxidation of olefins using hydrogen peroxide as an oxidant. Dalton Trans. 2018, 47, 13565–13572. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, M.; Ghahramaninezhad, M.; Rezaeifard, A. Catalytic activity and selectivity of reusable α- MoO3 nanobelts toward oxidation of olefins and sulfides using economical peroxides. RSC Adv. 2014, 4, 1601–1608. [Google Scholar] [CrossRef]
- Shi, F.; Tse, M.K.; Pohl, M.; Brückner, A.; Zhang, S.; Beller, M. Tuning Catalytic Activity between Homogeneous and Heterogeneous Catalysis: Improved Activity and Selectivity of Free Nano-Fe2O3 in Selective Oxidations. Angew. Chem. Int. Ed. 2007, 46, 8866–8868. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Kin, M.; Pohl, M.; Radnik, J.; Brückner, A.; Zhang, S.; Beller, M. Nano-iron oxide-catalyzed selective oxidations of alcohols and olefins with hydrogen peroxide. J. Mol. Catal. A Chem. 2008, 292, 28–35. [Google Scholar] [CrossRef]
- Xie, L.; Wang, H.; Lu, B.; Zhao, J. Highly selective oxidation of styrene to benzaldehyde over Fe3O4 using H2O2 aqueous solution as oxidant. Reac. Kinet. Mech. Cat. 2018, 125, 743–756. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water Purification by Semiconductor Photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Lachheb, H.; Guillard, C.; Lassoued, H.; Haddaji, M.; Rajah, M.; Houas, A. Photochemical oxidation of styrene in acetonitrile solution in presence of H2O2, TiO2/H2O2 and ZnO/H2O2. J. Photochem. Photobiol. A 2017, 346, 462–469. [Google Scholar] [CrossRef]
- Wiedmer, D.; Sagstuen, E.; Welch, K.; Haugen, H.J.; Tiainen, H. Oxidative power of aqueous non-irradiated TiO2-H2O2 suspensions: Methylene blue degradation and the role of reactive oxygen species. Appl. Catal. B 2016, 198, 9–15. [Google Scholar] [CrossRef]
- Sánchez, L.D.; Taxt-lamolle, S.F.M.; Hole, E.O.; Krivokapić, A.; Sagstuen, E.; Haugen, H.J. TiO2 suspension exposed to H2O2 in ambient light or darkness: Degradation of methylene blue and EPR evidence for radical oxygen species. Appl. Catal. B 2013, 143, 662–667. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, D.P.; Wu, R.; Gazi, S.; Soo, H.S.; Sritharan, T.; Chen, Z. New insights into the photocatalytic activity of 3-D core–shell P25@silica nanocomposites: Impact of mesoporous coating. Dalton Trans. 2017, 46, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, S.; Li, L.; Gao, W.; Cong, R.; Yang, T. Octahedral-based redox molecular sieve M-PKU-1: Isomorphous metal-substitution, catalytic oxidation of sec-alcohol and related catalytic mechanism. J. Catal. 2017, 352, 130–141. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]



| Entry | TiO2 (mg) | H2O2 (mmol) | Conversion (%) a | Yield (%) a |
|---|---|---|---|---|
| 1 | 100 | 1 | 47 | 31 |
| 2 | 100 | 3 | 86 | 60 |
| 3 | 100 | 5 | 92 | 65 |
| 4 | 100 | 7 | 90 | 56 |
| 5 | 0 | 5 | 8 | 1 |
| 6 b | 100 | 5 | 98 | 63 |
| 7 c | 100 | 5 | 85 | 45 d |

| Entry | Substrate | Product | Conversion (%) a | Yield (%) a |
|---|---|---|---|---|
| 1 |  |  | 92 | 65 |
| 2 |  |  | 82 | 54 |
| 3 |  |  | 92 | 57 |
| 4 |  |  | 63 | 23 |
| 5 |  |  | 91 | 65 |
| 6 |  |  | 79 | 58 |
| 7 |  |  | 92 | 72 |
| 8 |  |  | 35 | 15 |
| 9 |  |  | 22 | 7 |
| 10 |  |  | 5 | 3 |
| 11 |  |  | 76 | 43 |
| 12 |  |  | 7 | 0 |
| 13 |  |  | 84 | 30 b |
| 14 |  |  | 65 | 23 b |

| Entry | Additive | Atmosphere | Irradiation | Solvent | Conversion (%) a | Yield (%) a |
|---|---|---|---|---|---|---|
| 1 | - | Air | Room light | MeCN | 82 | 31 |
| 2 | - | Argon | Room light | MeCN | 82 | 51 |
| 3 | - | Air | Darkness | MeCN | 83 | 52 |
| 4 b | - | Air | hv (365 nm) c | MeCN | 1 | 0 |
| 5 | - | Air | hv (365 nm) c | MeCN | 91 | 51 |
| 6 | BHT (1 equiv.) | Air | Room light | MeCN | 11 | 1 |
| 7 | - | Air | Room light | t-BuOH | 85 | 55 |
| 8 | BQ (0.5 equiv.) | Air | Room light | MeCN | 2 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Kon, Y.; Nakashima, T.; Hong, D.; Konno, H.; Ino, D.; Sato, K. Titania-Catalyzed H2O2 Thermal Oxidation of Styrenes to Aldehydes. Molecules 2019, 24, 2520. https://doi.org/10.3390/molecules24142520
Ito S, Kon Y, Nakashima T, Hong D, Konno H, Ino D, Sato K. Titania-Catalyzed H2O2 Thermal Oxidation of Styrenes to Aldehydes. Molecules. 2019; 24(14):2520. https://doi.org/10.3390/molecules24142520
Chicago/Turabian StyleIto, Satoru, Yoshihiro Kon, Takuya Nakashima, Dachao Hong, Hideo Konno, Daisuke Ino, and Kazuhiko Sato. 2019. "Titania-Catalyzed H2O2 Thermal Oxidation of Styrenes to Aldehydes" Molecules 24, no. 14: 2520. https://doi.org/10.3390/molecules24142520