Synthesis and Biological Evaluation of Disubstituted Pyrimidines as Selective 5-HT2C Agonists
Abstract
:1. Introduction
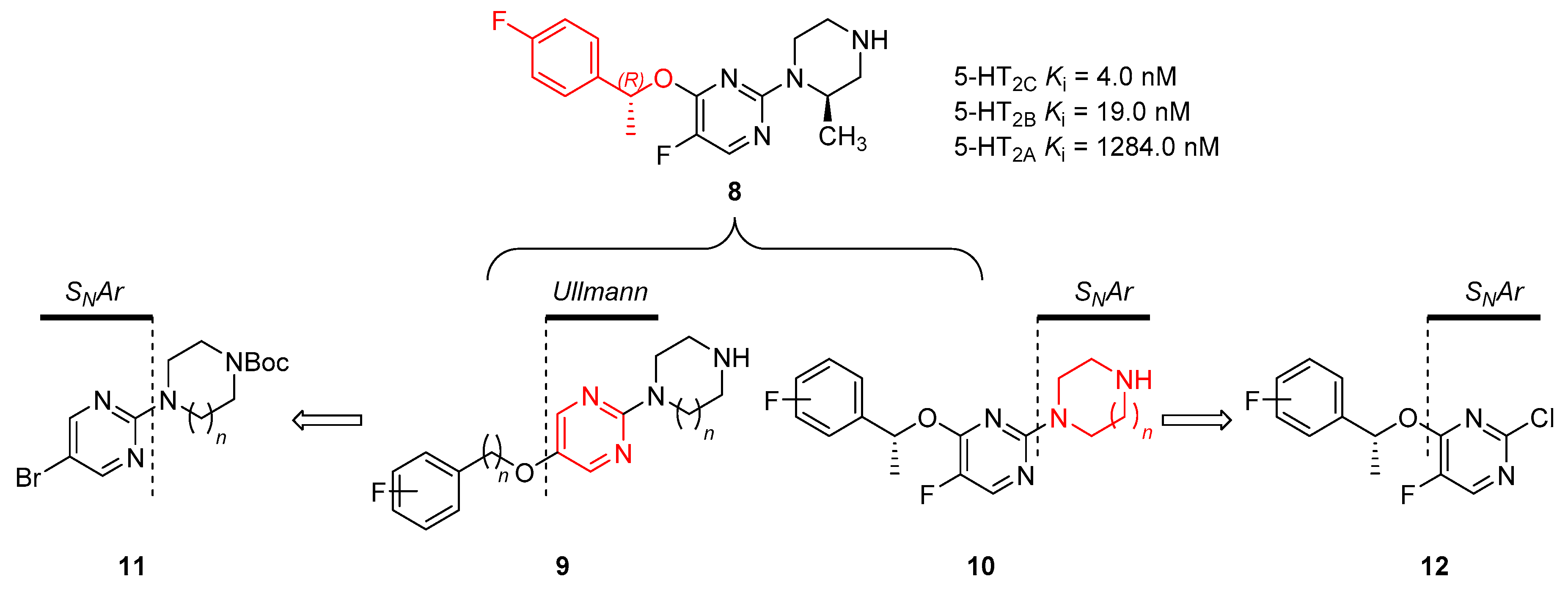
2. Results and Discussion
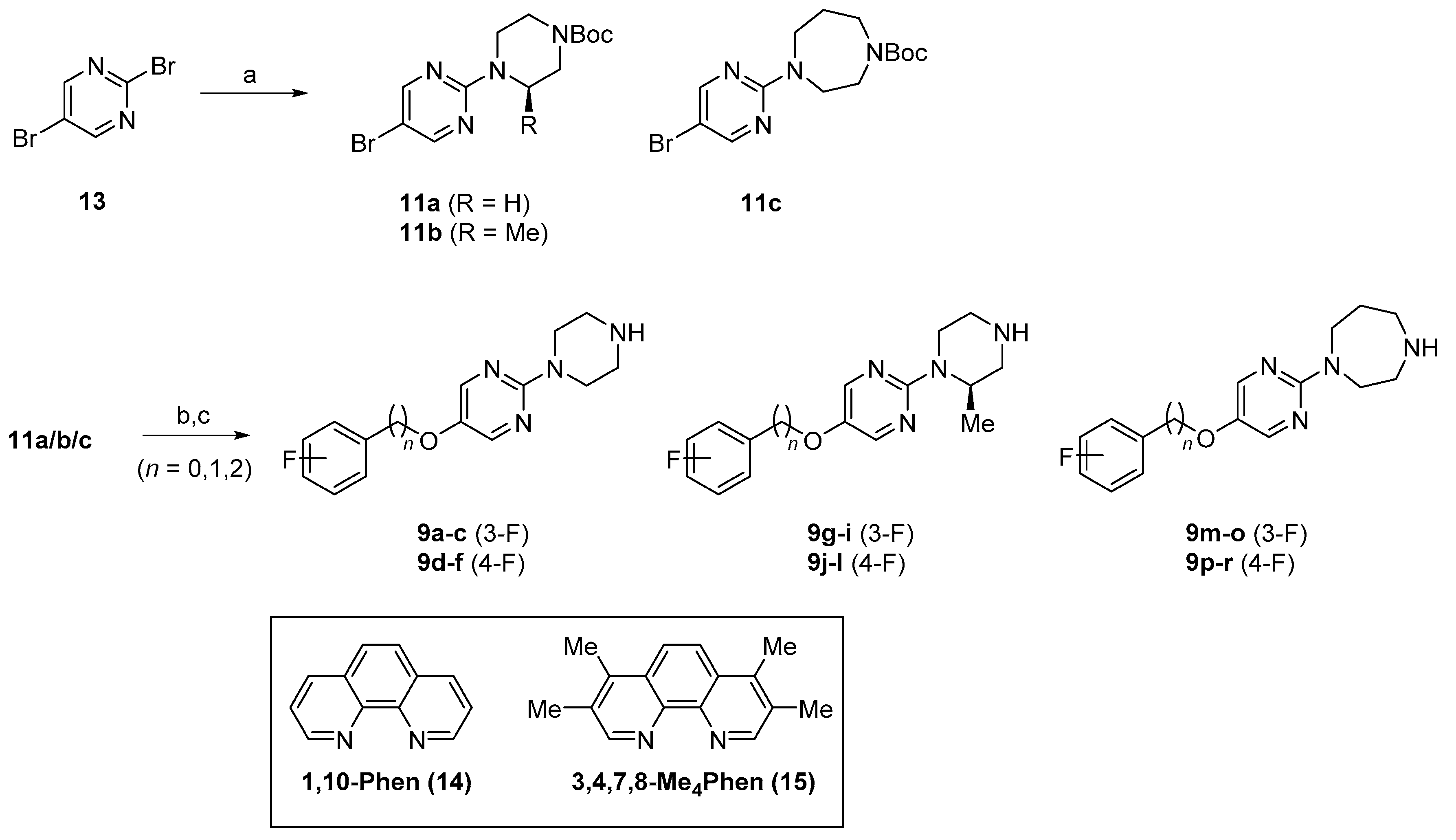
2.1. Synthesis of 2,5-Disubstituted Pyrimidine Derivatives
2.2. Synthesis of 2,4-Disubstituted Pyrimidine Derivatives
2.3. In Vitro Evaluation of Pyrimidine Derivatives
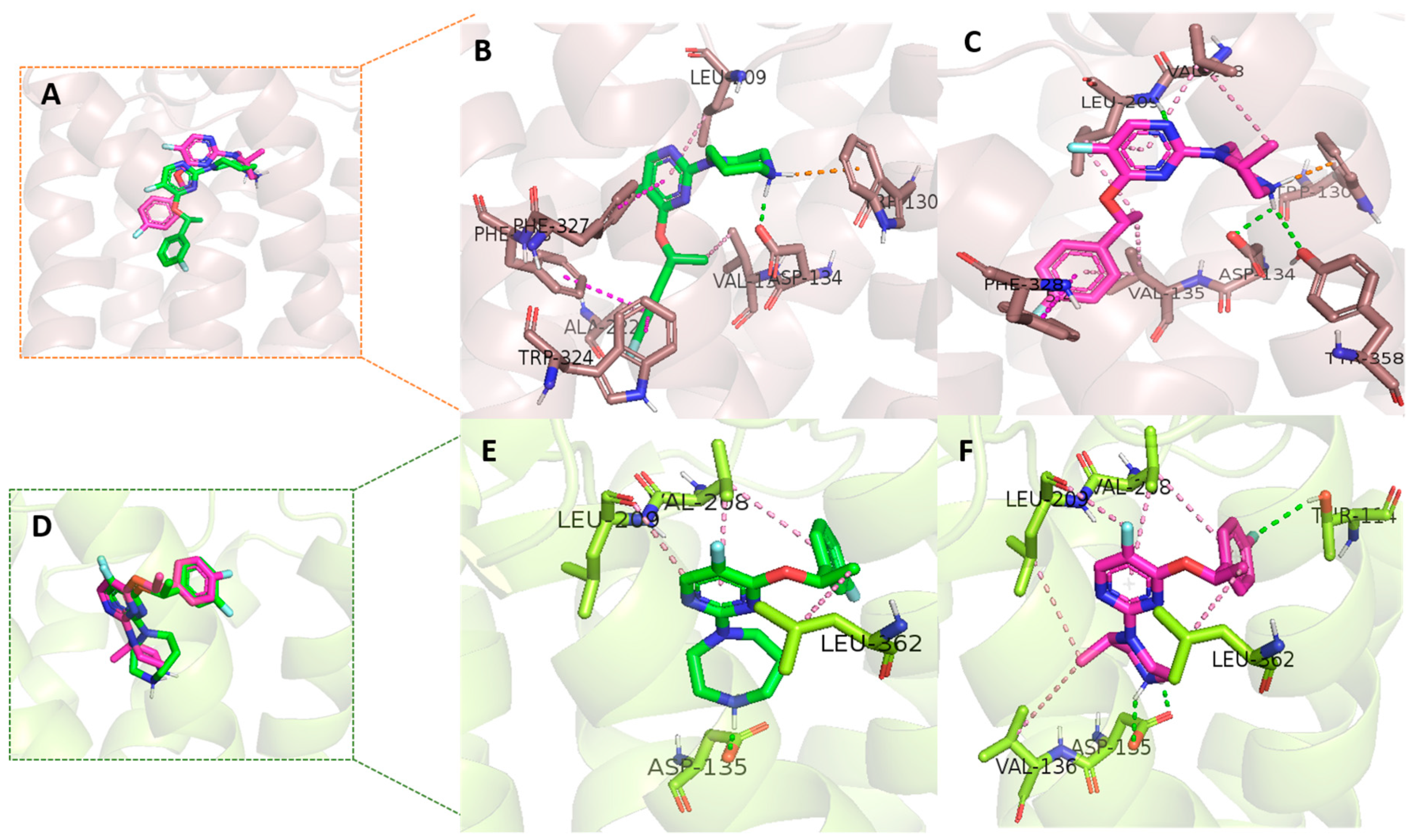
2.4. Molecular Docking Study
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of 2,5-Disubstituted Pyrimidines
3.2.1. General Procedure for Preparing Compounds 11a–c
3.2.2. General Procedure for Preparing Compounds 9a–r
3.3. Synthesis of 2,4-Disubstituted Pyrimidines 10a–j
3.3.1. General Procedure for Preparing Compound (R)-(+)-16
3.3.2. General Procedure for Preparing Compound 17
3.3.3. General Procedure for Preparing Compounds pre-10b–e and pre-10g–j
3.3.4. General Procedure for Preparing Compounds 10a and 10f
3.3.5. General Procedure for Preparing Compounds 10b–e and 10g–j
3.4. Molecular Docking Study
3.5. In Vitro Assay
3.5.1. Serotonin Receptor Cell-Based Functional Assays
3.5.2. Serotonin Receptor Binding Affinity Assays
3.6. Drug-Like Properties
3.6.1. Plasma Stability
3.6.2. Microsomal Stability
3.6.3. CYP Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brummelte, S.; MC Glanaghy, E.; Bonnin, A.; Oberlander, F. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 2017, 342, 212–231. [Google Scholar] [CrossRef]
- Amato, D. Serotonin in antipsychotic drugs action. Behav. Brain Res. 2015, 277, 125–135. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Li, Z.; Kaneda, Y.; Ichikawa, J. Serotonin receptors: Their key role in drugs to treat schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2003, 27, 1159–1172. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P.A. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Chou-Green, J.M.; Holscher, T.D.; Dallman, M.F.; Akana, S.F. Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol. Behav. 2003, 78, 641–649. [Google Scholar] [CrossRef]
- Lee, M.A.; Jayathilake, K.; Sim, M.Y.; Meltzer, H.Y. Decreased serotonin2C receptor responses in male patients with schizophrenia. Psychiatry Res. 2015, 226, 308–315. [Google Scholar] [CrossRef]
- Chagraoui, A.; Thibaut, F.; Skiba, M.; Thuillez, C.; Bourin, M. 5-HT2C receptors in psychiatric disorders: A review. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2016, 66, 120–135. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T.A. Review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Berg, K.A.; Stout, B.D.; Cropper, J.D.; Maayani, S.; Clarke, W.P. Novel Actions of Inverse Agonists on 5-HT2C Receptor Systems. Mol. Pharmacol. 1999, 55, 863–872. [Google Scholar]
- Thomsen, W.J.; Grottick, A.J.; Menzaghi, F.; Reyes-Saldana, H.; Espitia, S.; Yuskin, D.; Whelan, K.; Martin, M.; Morgan, M.; Chen, W.; et al. Lorcaserin, a Novel Selective Human 5-Hydroxytryptamine2C Agonist: In Vitro and in Vivo Pharmacological Characterization. JPET 2008, 325, 577–587. [Google Scholar] [CrossRef]
- Dunlop, J.; Watts, S.W.; Barrett, J.E.; Coupet, J.; Harrison, B.; Mazandarani, H.; Nawoschik, S.; Pangalos, M.N.; Ramamoorthy, S.; Schechter, L.; et al. Characterization of Vabicaserin (SCA-136), a Selective 5-Hydroxytryptamine 2C Receptor Agonist. JPET 2011, 337, 673–680. [Google Scholar] [CrossRef]
- Jensen, A.A.; Plath, N.; Pedersen, M.H.F.; Isberg, V.; Krall, J.; Wellendorph, P.; Stensboel, T.B.; Gloriam, D.E.; Krogsgaard-Larsen, P.; Froelund, B. Design, Synthesis, and Pharmacological Characterization of N- and O-Substituted 5,6,7,8-Tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol Analogues: Novel 5-HT2A/5-HT2C Receptor Agonists with Pro-Cognitive Properties. J. Med. Chem. 2013, 56, 1211–1227. [Google Scholar] [CrossRef]
- Cheng, J.; Giguere, P.M.; Lv, W.; Roth, B.L.; Kozikowski, A.P. Design and synthesis of (2-(5-chloro-2,2-dimethyl-2,3-dihydrobenzofuran-7-yl)cyclopropyl)methanamine as a selective serotonin 2C agonist. Tetrahedron Lett. 2015, 56, 3420–3422. [Google Scholar] [CrossRef] [Green Version]
- Siuciak, J.A.; Chapin, D.S.; McCarthy, S.A.; Guanowsky, V.; Brown, J.; Chiang, P.; Marala, R.; Patterson, T.; Seymour, P.A.; Swick, A.; et al. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 2007, 52, 279–290. [Google Scholar] [CrossRef]
- Cheng, J.; Giguere, P.M.; Onajole, O.K.; Lv, W.; Gaisin, A.; Gunosewoyo, H.; Schmerberg, C.M.; Pogorelov, V.M.; Rodriguiz, R.M.; Vistoli, G.; et al. Optimization of 2-Phenylcyclopropylmethylamines as Selective Serotonin 2C Receptor Agonists and Their Evaluation as Potential Antipsychotic Agents. J. Med. Chem. 2015, 58, 1992–2002. [Google Scholar] [CrossRef] [Green Version]
- Rouquet, G.; Moore, D.E.; Spain, M.; Allwood, D.M.; Battilocchio, C.; Blakemore, D.C.; Fish, P.V.; Jenkinson, S.; Jessiman, A.S.; Ley, S.V.; et al. Design, Synthesis, and Evaluation of Tetra substituted Pyridines as Potent 5-HT2C Receptor Agonists. ACS Med. Chem. Lett. 2015, 6, 329–333. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Cho, S.J.; Jensen, N.H.; Allen, J.A.; Svennebring, A.M.; Roth, B.L. HTS and Rational Drug Design to Generate a Class of 5-HT2C-Selective Ligands for Possible Use in Schizophrenia. ChemMedChem 2010, 5, 1221–1225. [Google Scholar] [CrossRef]
- Green, M.P.; McMurray, G.; Storer, R.I. Selective 5-HT2C receptor agonists: Design and synthesis of pyridazine-fused azepines. Bioorg. Med. Chem. Lett. 2016, 26, 4117–4121. [Google Scholar] [CrossRef]
- Granda, M.L.; Carlin, S.M.; Moseley, C.K.; Neelamegam, R.; Mandeville, J.B.; Hooker, J.M. Synthesis and Evaluation of Methylated Arylazepine Compounds for PET Imaging of 5-HT2c Receptors. ACS Chem. Neurosci. 2013, 4, 261–265. [Google Scholar] [CrossRef]
- Kamlet, A.S.; Neumann, C.N.; Lee, E.; Carlin, S.M.; Moseley, C.K.; Stephenson, N.; Hooker, J.M.; Ritter, T. Application of palladium-mediated 18F-fluorination to PET radiotracer development: Overcoming hurdles to translation. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Kim, J.; Jo, H.; Lee, H.; Choo, H.; Kim, H.-J.; Pae, A.N.; Cho, Y.S.; Min, S.-J. Identification of Optically Active Pyrimidine Derivatives as Selective 5-HT2C Modulators. Molecules 2017, 22, 1416. [Google Scholar] [CrossRef]
- Kim, j.; Moon, B.S.; Lee, B.C.; Lee, H.Y.; Kim, H.-J.; Choo, H.; Pae, A.N.; Cho, Y.S.; Min, S.-J. A Potential PET Radiotracer for the 5-HT2C Receptor: Synthesis and in Vivo Evaluation of 4-(3-[18F]fluorophenethoxy) pyrimidine. ACS Chem. Neurosci. 2017, 8, 996–1003. [Google Scholar] [CrossRef]
- Prabhakaran, J.; Underwood, M.D.; Kumar, J.S.D.; Simpson, N.R.; Kassir, S.A.; Bakalian, M.J.; Mann, J.J.; Arango, V. Synthesis and in vitro evaluation of [18F]FECIMBI-36: A potential agonist PET ligand for 5-HT2A/2C receptors. Bioorg. Med. Chem. Lett. 2015, 25, 3933–3936. [Google Scholar] [CrossRef]
- Neelamegam, R.; Hellenbrand, T.; Schroeder, F.K.A.; Wang, C.; Hooker, J.M. Imaging Evaluation of 5HT2C Agonists, [11C]WAY-163909 and [11C]Vabicaserin, Formed by Pictet−Spengler Cyclization. J. Med. Chem. 2014, 57, 1488–1494. [Google Scholar] [CrossRef]
- Zeng, F.; Nye, J.A.; Voll, R.J.; Howell, L.; Goodman, M.M. Synthesis and Evaluation of Pyridyloxypyridyl Indole Carboxamides as Potential PET Imaging Agents for 5-HT2C Receptors. ACS Med. Chem. Lett. 2018, 9, 188–192. [Google Scholar] [CrossRef]
- Heifetz, A.; Storer, R.I.; McMurray, G.; James, T.; Morao, I.; Aldeghi, M.; Bodkin, M.J.; Biggin, P.C. Application of an Integrated GPCR SAR-Modeling Platform To Explain the Activation Selectivity of Human 5-HT2C over 5-HT2B. ACS Chem. Biol. 2016, 11, 1372–1382. [Google Scholar] [CrossRef]
- Peng, Y.; McCorvy, J.D.; Harpsoe, K.; Lansu, K.; Yuan, S.; Popov, P.; Qu, L.; Pu, M.; Che, T.; Nikolajsen, L.F.; et al. 5-HT2C Receptor Structures Reveal the Structural Basis of GPCR Polypharmacology. Cell 2018, 172, 719–730. [Google Scholar] [CrossRef]
- Maiti, D.; Buchwald, S.L. Cu-Catalyzed Arylation of Phenols: Synthesis of Sterically Hindered and Heteroaryl Diaryl Ethers. J. Org. Chem. 2010, 75, 1791–1794. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Chen, X.; Zhou, W.; Fan, M.; Lai, Y.; Ma, D. Copper-Catalyzed Diaryl Ether Formation from (Hetero)aryl Halides at Low Catalytic Loadings. J. Org. Chem. 2017, 82, 4964–4969. [Google Scholar] [CrossRef]
- Wolter, M.; Nordmann, G.; Job, G.E.; Buchwald, S.L. Copper-Catalyzed Coupling of Aryl Iodides with Aliphatic Alcohols. Org. Lett. 2002, 4, 973–976. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.S.; Min, S.-J. Facile synthesis of 2-amino-4-alkoxypyrimidines via consecutive nucleophilic aromatic substitution (SNAr) reactions. Bull. Korean Chem. Soc. 2016, 37, 1998–2008. [Google Scholar] [CrossRef]
- Wacker, D.; Wang, C.; Katritch, V.; Han, G.W.; Huang, X.P.; Vardy, E.; McCorvy, J.D.; Jiang, Y.; Chu, M.; Siu, F.Y.; et al. Structural features for functional selectivity at serotonin receptors. Science 2013, 340, 615–619. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment, Release 2019; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Porter, R.H.P.; Benwell, K.R.; Lamb, H.; Malcolm., C.S.; Allen, N.H.; Revell, D.F.; Adams, D.R.; Sheardown, M.J. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Brit. J. Pharmacol. 1999, 128, 13–20. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 9a–9r, 10a–10j, and 20a/b are available from the authors. |



| Entry | Compds | n | R1 | R2 | %activation (10 μM) |
|---|---|---|---|---|---|
| 1 | 9a | 0 | 3-F |  | 7 |
| 2 | 9b | 1 | 3-F | 61 | |
| 3 | 9c | 2 | 3-F | 15 | |
| 4 | 9d | 0 | 4-F | 5 | |
| 5 | 9e | 1 | 4-F | 18 | |
| 6 | 9f | 2 | 4-F | 4 | |
| 7 | 9g | 0 | 3-F | 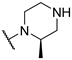 | 35 |
| 8 | 9h | 1 | 3-F | 48 | |
| 9 | 9i | 2 | 3-F | 40 | |
| 10 | 9j | 0 | 4-F | 26 | |
| 11 | 9k | 1 | 4-F | 48 | |
| 12 | 9l | 2 | 4-F | 32 | |
| 13 | 9m | 0 | 3-F | 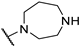 | 16 |
| 14 | 9n | 1 | 3-F | −1 | |
| 15 | 9o | 2 | 3-F | 3 | |
| 16 | 9p | 0 | 4-F | 14 | |
| 17 | 9q | 1 | 4-F | 16 | |
| 18 | 9r | 2 | 4-F | −1 | |
| 19 | Lorcaserin (1) | 94a |

| Entry | Compds | R1 | R2 | %activationa | %binding | Ki (nM) |
|---|---|---|---|---|---|---|
| 1 | 10a |  |  | 81.0 | 81.3 | 7.9 |
| 2 | 10b | 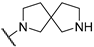 | 78.2 | 47.6 | ND | |
| 3 | 10c | 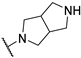 | 29.5 | 73.6 | 295.0 | |
| 4 | 10d | 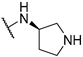 | 16.4 | 88.6 | 119.0 | |
| 5 | 10e |  | 7.7 | 61.4 | 1255.0 | |
| 6 | 10f | 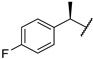 |  | 52.4 | 93.9 | 19.0 |
| 7 | 10g |  | ND | 78.1 | 232.0 | |
| 8 | 10h | 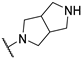 | 10.1 | 50.8 | 466.0 | |
| 9 | 10i | 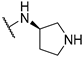 | ND | 85.9 | 120.0 | |
| 10 | 10j | 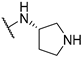 | 0.1 | 26.2 | ND | |
| 11 | 20a | 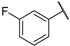 | 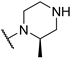 | 56.0 | 64.8 | 660.0 |
| 12 | 20b |  | 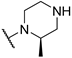 | 34.0 | 54.8 | 1107.0 |
| 13 | 8 | N/Ab | 89.0 | 5.9 |
| 5-HT Subtypes (%Binding at 10 μM/Ki (nM)) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | 1D | 1E | 2A | 2B | 2C | 3 | 5A | 6 | 7 | |
| 10a | 74.1 /578.0 | 56.1 /3237.0 | 71.4 /679.0 | 43.1 /NDb | 75.5 /1284.0 | 98.0 /83.0 | 81.3 /7.9 | 89.0 /323.0 | 22.5 /ND | 50.7 /348.0 | 61.3 /1016.0 |
| SI | 73.2 | 409.7 | 85.9 | - | 162.5 | 10.5 | 1.0 | 40.9 | 23.4 | 44.1 | 128.6 |
| 10f | 67.6 /1066.0 | 19.2 /NDb | 81.3 /937.0 | 31.3 /NDb | 52.9 /2863.0 | 95.0 /118.0 | 98.9 /19.0 | 88.0 /415.0 | 15.2 /ND | 56.5 /467.0 | 55.4 /616.0 |
| SI | 56.1 | - | 49.3 | - | 150.7 | 6.2 | 1.0 | 21.8 | 10.3 | 24.6 | 32.4 |
| 8c | 89.1 /271.0 | 4.9 /NDb | 83.4 /1052.0 | 48.0 /NDb | 85.3 /511.0 | 98.1 /38.0 | 89.0 /5.9 | 83.4 /543.0 | 18.5 /NDb | 56.7 /30.0 | 63.4 /528.0 |
| SI | 45.9 | - | 178.3 | - | 86.6 | 6.4 | 1.0 | 92.0 | - | 5.1 | 89.5 |
| Compd | Plasma Stability %Remaining @ 10 μM after 0.5 and 2 h | HLM %Remaining @ 1 μM after 0.5 h | CYP Isozymes (%Remaining @ 10 μM) | ||||
|---|---|---|---|---|---|---|---|
| 1A2 | 2D6 | 2C9 | 3A4 | 2C19 | |||
| 10a | 99.9/98.0 | 96.6 | 86.5 | 63.6 | >100 | 86.2 | 86.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, Y.J.; Londhe, A.M.; Pae, A.N.; Choo, H.; Kim, H.J.; Min, S.-J. Synthesis and Biological Evaluation of Disubstituted Pyrimidines as Selective 5-HT2C Agonists. Molecules 2019, 24, 3234. https://doi.org/10.3390/molecules24183234
Kim J, Kim YJ, Londhe AM, Pae AN, Choo H, Kim HJ, Min S-J. Synthesis and Biological Evaluation of Disubstituted Pyrimidines as Selective 5-HT2C Agonists. Molecules. 2019; 24(18):3234. https://doi.org/10.3390/molecules24183234
Chicago/Turabian StyleKim, Juhyeon, Yoon Jung Kim, Ashwini M. Londhe, Ae Nim Pae, Hyunah Choo, Hak Joong Kim, and Sun-Joon Min. 2019. "Synthesis and Biological Evaluation of Disubstituted Pyrimidines as Selective 5-HT2C Agonists" Molecules 24, no. 18: 3234. https://doi.org/10.3390/molecules24183234





