Synthesis of Novel Pyrazole Derivatives and Their Tumor Cell Growth Inhibitory Activity
Abstract
:1. Introduction
2. Results and Discussion
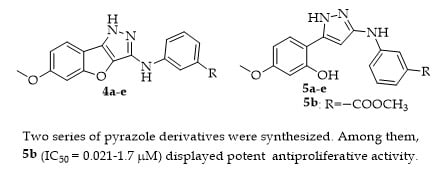
2.1. Chemistry
2.2. Tumor Cell Growth Inhibitory Activity
2.3. In Vitro Tubulin Polymerization Inhibitory Activity
3. Materials and Methods
3.1. General Chemical Experimental Procedures
3.2. MTT Assay
3.3. In Vitro Tubulin Polymerization Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| THF | Tetrahydrofuran |
| MCF-7 | Breast cancer cell |
| K562 | Human erythroleukemia cell |
| A549 | Lung cancer cell |
| NSCLC | Non-small cell lung cancer |
| KB/V | Vincristine-resistance human oral epidermoid carcinoma cell |
| LiHMDS | Lithium bis(trimethylsilyl)amide |
| PTEN | Phosphatase and tensin homolog |
| DMSO | Dimethyl sulfoxide |
| RPMI | Roswell park memorial institute |
| EGTA | Ethylene glycol-bis(2-aminoethylether)-N,N,N‘,N’-tetraacetic acid |
| GTP | Guanosine triphosphate |
References
- Duarte, C.D.; Barreiro, E.J.; Fraga, C.A. Privileged structures: A useful concept for the rational design of new lead drug candidates. Mini Rev. Med. Chem. 2007, 7, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Barlocco, D. Privileged structures as leads in medicinal chemistry. Curr. Med. Chem. 2006, 13, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Making sense of structures by utilizing mother nature’s chemical libraries as leads to potential drugs. Nat. Prod. 2014, 397–411. [Google Scholar] [CrossRef]
- Akbas, E.; Berber, I.; Sener, A.; Hasanov, B. Synthesis and antibacterial activity of 4-benzoyl-1-methyl-5- phenyl-1H-pyrazole-3-carboxylic acid and derivatives. Farmaco 2005, 60, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Prasath, R.; Bhavana, P.; Sarveswari, S.; Ng, S.W.; Tiekink, E.R.T. Efficient ultrasound-assisted synthesis, spectroscopic, crystallographic and biological investigations of pyrazole-appended quinolinyl chalcones. J. Mol. Struct. 2015, 1081, 201–210. [Google Scholar] [CrossRef]
- Kamal, A.; Shaik, A.B.; Jain, N.; Kishor, C.; Nagabhushana, A.; Supriya, B.; Kumar, G.B.; Chourasiya, S.S.; Suresh, Y.; Mishra, R.K.; et al. Design and synthesis of pyrazole–oxindole conjugates targeting tubulin polymerization as new anticancer agents. Eur. J. Med. Chem. 2015, 92, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, X.-H.; Saunders, M.; Pearce, S.; Foulks, J.M.; Parnell, K.M.; Clifford, A.; Nix, R.N.; Bullough, J.; Hendrickson, T.F.; et al. Discovery of 3-(trifluoromethyl)-1H-pyrazole-5-carboxamide activators of the M2 isoform of pyruvate kinase (PKM2). Bioorg. Med. Chem. Lett. 2014, 24, 515–519. [Google Scholar] [CrossRef]
- El-Moghazy, S.; Barsoum, F.; Abdel-Rahman, H.; Marzouk, A. Synthesis and anti-inflammatory activity of some pyrazole derivatives. Med. Chem. Res. 2012, 21, 1722–1733. [Google Scholar] [CrossRef]
- Selvam, T.P.; Kumar, P.V.; Saravanan, G.; Prakash, C.R. Microwave-assisted synthesis, characterization and biological activity of novel pyrazole derivatives. J. Saudi. Chem. Soc. 2014, 18, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Pathak, V.; Maurya, H.K.; Sharma, S.; Srivastava, K.K.; Gupta, A. Synthesis and biological evaluation of substituted 4,6-diarylpyrimidines and 3,5-diphenyl-4,5-dihydro-1H-pyrazoles as anti-tubercular agents. Bioorg. Med. Chem. Lett. 2014, 24, 2892–2896. [Google Scholar] [CrossRef]
- Jia, H.; Bai, F.; Liu, N.; Liang, X.; Zhan, P.; Ma, C.; Jiang, X.; Liu, X. Design, synthesis and evaluation of pyrazole derivatives as non-nucleoside hepatitis B virus inhibitors. Eur. J. Med. Chem. 2016, 123, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-N.; Luo, R.-H.; Zhou, Y.; Zhang, X.-J.; Li, J.; Yang, L.-M.; Zheng, Y.-T.; Liu, H. Synthesis and anti-HIV-1 activity evaluation for novel 3a,6a-dihydro-1H-pyrrolo[3,4-c]pyrazole-4,6-dione derivatives. Molecules 2016, 21, 1198. [Google Scholar] [CrossRef] [PubMed]
- Khoobi, M.; Ghanoni, F.; Nadri, H.; Moradi, A.; Hamedani, M.P.; Moghadam, F.H.; Emami, S.; Vosooghi, M.; Zadmard, R.; Foroumadi, A. New tetracyclic tacrine analogs containing pyrano[2,3-c]pyrazole: Efficient synthesis, biological assessment and docking simulation study. Eur. J. Med. Chem. 2015, 89, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Nencini, A.; Castaldo, C.; Comery, T.A.; Dunlop, J.; Genesio, E.; Ghiron, C.; Haydar, S.; Maccari, L.; Micco, I.; Turlizzi, E.; et al. Design and synthesis of a hybrid series of potent and selective agonists of α7 nicotinic acetylcholine receptor. Eur. J. Med. Chem. 2014, 78, 401–418. [Google Scholar] [CrossRef]
- Chaudhry, F.; Naureen, S.; Huma, R.; Shaukat, A.; Al-Rashida, M.; Asif, N.; Ashraf, M.; Munawar, M.A.; Khan, M.A. In search of new α-glucosidase inhibitors: Imidazolylpyrazole derivatives. Bioorg. Chem. 2017, 71, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vázquez, E.; Ocampo-Montalban, H.; Cerón-Romero, L.; Cruz, M.; Gómez-Zamudio, J.; Hiriart-Valencia, G.; Villalobos-Molina, R.; Flores-Flores, A.; Estrada-Soto, S. Antidiabetic, antidyslipidemic and toxicity profile of ENV-2: A potent pyrazole derivative against diabetes and related diseases. Eur. J. Pharmacol. 2017, 803, 159–166. [Google Scholar] [CrossRef]
- Tuha, A.; Bekhit, A.A.; Seid, Y. Screening of some pyrazole derivatives as promising antileishmanial agent. Afr. J. Pharm. Pharmacol. 2017, 11, 32–37. [Google Scholar]
- Reviriego, F.; Olmo, F.; Navarro, P.; Marín, C.; Ramírez-Macías, I.; García-España, E.; Albelda, M.T.; Gutiérrez-Sánchez, R.; Sánchez-Moreno, M.; Arán, V.J. Simple dialkyl pyrazole-3,5-dicarboxylates show in vitro and in vivo activity against disease-causing trypanosomatids. Parasitology 2017, 144, 1133–1143. [Google Scholar] [CrossRef]
- Balaji, S.N.; Ahsan, M.J.; Jadav, S.S.; Trivedi, V. Molecular modelling, synthesis, and antimalarial potentials of curcumin analogues containing heterocyclic ring. Arab. J. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Fujinaga, M.; Yamasaki, T.; Nengaki, N.; Ogawa, M.; Kumata, K.; Shimoda, Y.; Yui, J.; Xie, L.; Zhang, Y.; Kawamura, K.; et al. Radiosynthesis and evaluation of 5-methyl-N-(4-[11C]methylpyrimidin-2-yl)-4-(1H-pyrazol-4-yl)thiazol-2-amine ([11C]ADX88178) as a novel radioligand for imaging of metabotropic glutamate receptor subtype 4 (mGluR4). Bioorg. Med. Chem. Lett. 2016, 26, 370–374. [Google Scholar] [CrossRef]
- Dai, H.; Xiao, Y.-S.; Li, Z.; Xu, X.-Y.; Qian, X.-H. The thiazoylmethoxy modification on pyrazole oximes: Synthesis and insecticidal biological evaluation beyond acaricidal activity. Chin. Chem. Lett. 2014, 25, 1014–1016. [Google Scholar] [CrossRef]
- Dai, H.; Chen, J.; Li, H.; Dai, B.; He, H.; Fang, Y. Synthesis and bioactivities of novel pyrazole oxime derivatives containing a 5-trifluoromethylpyridyl moiety. Molecules 2016, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Pan, S.-L.; Su, M.-C.; Liu, Y.-M.; Kuo, C.-C.; Chang, Y.-T.; Wu, J.-S.; Nien, C.-Y.; Mehndiratta, S.; Chang, C.-Y.; et al. Furanylazaindoles: Potent anticancer agents in vitro and in vivo. J. Med. Chem. 2013, 56, 8008–8018. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.E.; Maldonado, N.V.; Khankaldyyan, V.; Shimada, H.; Song, M.M.; Maurer, B.J.; Reynolds, C.P. Reactive oxygen species mediates the synergistic activity of fenretinide combined with the microtubule inhibitor ABT-751 against multidrug-resistant recurrent neuroblastoma xenografts. Mol. Cancer Ther. 2016, 15, 2653–2664. [Google Scholar] [CrossRef]
- Dorleans, A.; Gigant, B.; Ravelli, R.B.; Mailliet, P.; Mikol, V.; Knossow, M. Variations in the colchicine-binding domain provide insight into the structural switch of tubulin. Proc. Natl. Acad. Sci. USA 2009, 106, 13775–13779. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Wang, J.-J.; Ji, Y.-T.; Zhao, G.-D.; Tang, L.-Q.; Zhang, C.-M.; Guo, X.-L.; Liu, Z.-P. Design, synthesis, and biological evaluation of 1- methyl-1,4-dihydroindeno[1,2-c]pyrazole analogues as potential anticancer agents targeting tubulin colchicine binding site. J. Med. Chem. 2016, 59, 5341–5535. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, F.-L.; Lu, Z.-N.; Wang, H.-Y.; Cheng, Y.-N.; Liu, Z.-P.; Yu, L.-G.; Zhang, H.-H.; Guo, X.-L. DHPAC, a novel synthetic microtubule destabilizing agent, possess high anti-tumor activity in vincristine-resistant oral epidermoid carcinoma in vitro and in vivo. Int. J. Biochem. Cell, B. 2017, 93, 1–11. [Google Scholar] [CrossRef]
- Luo, W.; Su, Y.B.; Hong, C.; Tian, R.-G.; Su, L.-P.; Yue-Qiao Wang, Y.-Q.; Li, Y.; Yue, J.-J.; Wang, C.-J. Design, synthesis and evaluation of novel 4-dimethylamine flavonoid derivatives as potential multi-functional anti-Alzheimer agents. Eur. J. Med. Chem. 2013, 21, 7275–7282. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Nel, J.W.; Brandt, E.V.; Bezuidenhoudt, B.; Ferreira, D. Oligomeric isoflavonoids. Part 3. Daljanelins A-D, the first pterocarpan- and isoflavanoid-neoflavonoid analogs. J. Chem. Soc. Perkin Trans. 1995, 1, 1049–1056. [Google Scholar] [CrossRef]
- Muzychka, O.V.; Kobzar, O.L.; Popova, A.V.; Frasinyuk, M.S.; Vovk, A.I. Carboxylated aurone derivatives as potent inhibitors of xanthine oxidase. Bioorg. Med. Chem. 2017, 25, 3606. [Google Scholar] [PubMed]
- Bonne, D.; Heusele, C.; Simon, C.; Pantaloni, D. 4′,6-Diamidino-2-phenylindole, a fluorescent probe for tubulin and mictrotubules. J. Biol. Chem. 1985, 260, 2819–2825. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds 4a–e and 5a–e are available from the authors. |



| GI50 (μM) | ||||
|---|---|---|---|---|
| Compound | R | K562 | MCF-7 | A549 |
| 4a | OCH2CH3 | 0.26 ± 0.04 | >20 | 0.19 ± 0.08 |
| 4b | COOCH3 | 5.46 ± 1.04 | >20 | >20 |
| 4c | CONHCH3 | 5.11 ± 0.31 | >20 | 15.11 ± 2.18 |
| 4d | CONH2 | 9.01 ± 1.81 | >20 | 10.08 ± 2.21 |
| 4e | CN | 13.53 ± 0.41 | >20 | 17.01 ± 2.76 |
| 5a | OCH2CH3 | 0.046 ± 0.007 | 16.72 ± 2.6 | 0.92 ± 0.17 |
| 5b | COOCH3 | 0.021 ± 0.004 | 1.7 ± 0.43 | 0.69 ± 0.18 |
| 5c | CONHCH3 | 7.33 ± 1.004 | 7.78 ± 0.87 | 9.46 ± 2.03 |
| 5d | CONH2 | 14.77 ± 2.62 | 5.8 ± 0.202 | 10.9 ± 0.99 |
| 5e | CN | 1.45 ± 0.047 | 2.27 ± 0.34 | 3.24 ± 0.99 |
| ABT-751 | 0.74 ± 0.078 | 0.88 ± 0.24 | 4.58 ± 0.04 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.-J.; Tang, L.-Q.; Zhang, C.-M.; Liu, Z.-P. Synthesis of Novel Pyrazole Derivatives and Their Tumor Cell Growth Inhibitory Activity. Molecules 2019, 24, 279. https://doi.org/10.3390/molecules24020279
Cui Y-J, Tang L-Q, Zhang C-M, Liu Z-P. Synthesis of Novel Pyrazole Derivatives and Their Tumor Cell Growth Inhibitory Activity. Molecules. 2019; 24(2):279. https://doi.org/10.3390/molecules24020279
Chicago/Turabian StyleCui, Ying-Jie, Long-Qian Tang, Cheng-Mei Zhang, and Zhao-Peng Liu. 2019. "Synthesis of Novel Pyrazole Derivatives and Their Tumor Cell Growth Inhibitory Activity" Molecules 24, no. 2: 279. https://doi.org/10.3390/molecules24020279