A Vortex-Assisted Dispersive Liquid-Liquid Microextraction Followed by UPLC-MS/MS for Simultaneous Determination of Pesticides and Aflatoxins in Herbal Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Instruments and Equipment
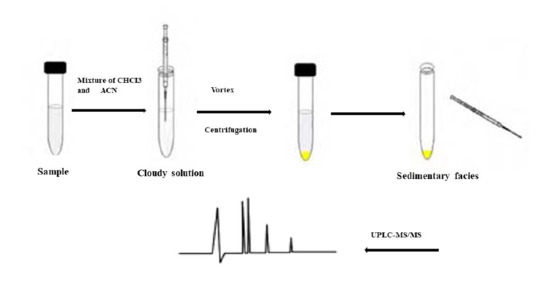
2.3. Sample Preparation
2.4. Optimization of the Vortex Assisted DLLME Process
2.5. Experimental Design and Data Analysis
3. Results and Discussion
3.1. DLLME Procedure Optimization
3.2. Extraction Solvent Selection
3.3. Effect of the Extraction Solvent Volume (Chloroform)
3.4. Effect of Dispersive Solvent
3.5. Effect of Dispersive Solvent Volume (Acetonitrile)
3.6. Effect of pH
3.7. Experimental Design
3.8. Method Evaluation
3.9. Application of the Developed DLLME Method to Real Herbal Tea Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jin, B.; Liu, Y.; Xie, J.; Luo, B.; Long, C. Ethnobotanical survey of plant species for herbal tea in a Yao autonomous county (Jianghua, China): Results of a 2-year study of traditional medicinal markets on the Dragon Boat Festival. J. Ethnobiol. Ethnomed. 2018, 14, 58–78. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, J.; Cunningham, A.B.; Towns, A.M.; Zhang, Y.; Yang, H.Y.; Li, J.W.; Yang, X.F. A billion cups: The diversity, traditional uses, safety issues and potential of Chinese herbal teas. J. Ethnopharmacol. 2018, 222, 217–228. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Long, C. Ethnobotanical survey of cooling herbal drinks from southern China. J. Ethnobiol. Ethnomed. 2013, 9, 82–89. [Google Scholar] [CrossRef] [PubMed]
- De Santayana, M.P.; Blanco, E.; Morales, R. Plants known as té in Spain: An ethno-pharmaco-botanical review. J. Ethnopharmacol. 2005, 98, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Han, B.Q.; Peng, Y.; Xiao, P.G. Systematic Research on Chinese Non-Camellia Tea. Mod. Chin. Med. 2013, 15, 259–269. [Google Scholar]
- Li, S.; Li, S.K.; Li, H.B.; Xu, X.R.; Deng, G.F.; Xu, D.P. Antioxidant Capacities of Herbal Infusions; South China Sea Institute of Oceanology, Chinese Academy of Sciences: Guangzhou, China, 2014; pp. 41–51. [Google Scholar]
- Ranasinghe, S.; Ansumana, R.; Lamin, J.M.; Bockarie, A.S.; Bangura, U.; Jacob, A.G.; Stenger, D.A.; Jacobsen, K.H. Herbs and herbal combinations used to treat suspected malaria in Bo, Sierra Leone. J. Ethnopharmacol. 2015, 166, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Paniagua-Zambrana, N.; Sifuentes, R.Y.C.; Velazco, Y.A.P.; Mandujano, J. Health in a Pot-The Ethnobotany of Emolientes and Emolienteros in Peru. Econ. Bot. 2015, 69, 83–88. [Google Scholar] [CrossRef]
- Obón, C.; Rivera, D.; Alcaraz, F.; Attieh, L. Beverage and culture. “Zhourat”, a multivariate analysis of the globalization of an herbal tea from the Middle East. Appetite 2014, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pardo, D.S.M.; Blanco, E.R. Plants known as té in Spain: an ethno-pharmaco-botanical review. J. Ethnopharmacol. 2005, 98, 1–19. [Google Scholar] [CrossRef]
- Chi, L.; Li, Z.; Dong, S.; He, P.; Wang, Q.; Fang, Y. Simultaneous determination of flavonoids and phenolic acids in Chinese herbal tea by beta-cyclodextrin based capillary zone electrophoresis. Microchim. Acta 2009, 167, 179–185. [Google Scholar] [CrossRef]
- Hajjo, R.M.; Battah, F.U.A.A. Multiresidue pesticide analysis of the medicinal plant Origanum syriacum. Food Addit. Contam. 2007, 24, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.Y.; Chan, K.; Chan, C.L.; Lu, G.H. Systematic evaluation of organochlorine pesticide residues in Chinese materia medica. Phytother. Res. 2010, 19, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, D.; Wu, J.; Qin, J.; Hu, J.; Huang, W.; Wang, Z.; Xiao, W.; Wang, Y. Simultaneous determination of 35 ultra-trace level organophosphorus pesticide residues in Sanjie Zhentong capsules of traditional Chinese medicine using ultra high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2017, 40, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, M.; Kosanović, M. Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2010, 29, 195–201. [Google Scholar] [CrossRef]
- Wei, J.; Hu, J.; Cao, J.L.; Wan, J.B.; He, C.W.; Hu, Y.J.; Hu, H.; Li, P. Sensitive detection of organophosphorus pesticides in medicinal plants using ultrasound-assisted dispersive liquid–liquid microextraction combined with sweeping micellar electrokinetic chromatography. J. Agric. Food Chem. 2016, 64, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yao, K.; Zhao, S.; Zheng, P.; Wang, S.; Zeng, Y.; Liang, D.; Ke, Y.; Jiang, H. Determination of aflatoxin and zearalenone analogs in edible and medicinal herbs using a group-specific immunoaffinity column coupled to ultra-high-performance liquid chromatography with tandem mass spectrometry. J. Chromatogr. B 2018, 1092, 228–236. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Working Group Evaluation Carcinogenic Risks Humans I; IARC: Lyon, France, 2010; Volume 96, pp. 27–338. [Google Scholar]
- International Agency for Research on Cancer (IARC). Aflatoxins: B1, B2, G1, G2, M1, 245–395. In IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans; IARC: Lyon, France, 2010; Volume 56. [Google Scholar]
- Locatelli, M.; Sciascia, F.; Cifelli, R.; Malatesta, L.; Bruni, P.; Croce, F. Analytical methods for the endocrine disruptor compounds determination in environmental water samples. J. Chromotogr. A 2016, 1434, 1–18. [Google Scholar] [CrossRef]
- Chen, L.; Xing, W. Simple one-step preconcentration and cleanup with a micellar system for high performance liquid chromatography determination of pyrethroids in traditional Chinese medicine. Anal. Methods 2015, 7, 1691–1700. [Google Scholar] [CrossRef]
- Guo, Q.; Lv, X.; Tan, L.; Yu, B.Y. Simultaneous determination of 26 pesticide residues in 5 Chinese medicinal materials using solid-phase extraction and GC-ECD Method. Chin. J. Nat. Med. 2009, 7, 210–216. [Google Scholar] [CrossRef]
- Rutkowska, E.; łozowicka, B.; Kaczyński, P. Three approaches to minimize matrix effects in residue analysis of multiclass pesticides in dried complex matrices using gas chromatography tandem mass spectrometry. Food Chem. 2019, 279, 20–29. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.; Yang, X.; Li, S.; Wang, Y.; Tang, Y.; Liu, C. Multi-residue method for determination of selected neonicotinoid insecticides in traditional Chinese medicine using modified dispersive solid-phase extraction combined with ultra-performance liquid chromatography tandem mass spectrometry. Anal. Sci. 2015, 31, 823–830. [Google Scholar] [CrossRef]
- Burmistrova, N.A.; Rusanova, T.Y.; Yurasov, N.A.; De Saeger, S.; Goryacheva, I. Simultaneous determination of several mycotoxins by rapid immunofiltration assay. J. Anal. Chem. 2014, 69, 525–534. [Google Scholar] [CrossRef]
- Mohamadi, S.A.; Nikpooyan, H. Determination of aflatoxin M1 in milk by high-performance liquid chromatography in Mashhad (North East of Iran). Toxicol. Ind. Health 2012, 29, 334. [Google Scholar]
- Han, Z.; Zheng, Y.; Luan, L.; Cai, Z.; Ren, Y.; Ren, Y. An ultra-high-performance liquid chromatography-tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in traditional Chinese medicines. Anal. Chim. Acta 2010, 664, 165–171. [Google Scholar] [CrossRef]
- Boulanouar, S.; Combès, A.; Mezzache, S.; Pichon, V. Synthesis and application of molecularly imprinted silica for the selective extraction of some polar organophosphorus pesticides from almond oil. Anal. Chim. Acta 2018, 1018, 35–44. [Google Scholar] [CrossRef]
- Notardonato, I.; Russo, M.V.; Vitali, M.; Protano, C.; Avino, P. Analytical method validation for determining organophosphorus pesticides in baby foods by a modified liquid–liquid microextraction method and gas chromatography–ion trap/mass spectrometry analysis. Food Anal. Method 2019, 12, 41–50. [Google Scholar] [CrossRef]
- Prosen, H. Applications of Liquid-Phase Microextraction in the Sample Preparation of Environmental Solid Samples. Molecules 2014, 19, 6776–6808. [Google Scholar] [CrossRef] [Green Version]
- Kissoudi, M.; Samanidou, V. Recent advances in applications of ionic liquids in miniaturized microextraction techniques. Molecules 2018, 23, 1437. [Google Scholar] [CrossRef]
- Diuzheva, A.; Carradori, S.; Andruch, V.; Locatelli, M.; De Luca, E.; Tiecco, M.; Germani, R.; Menghini, L.; Nocentini, A.; Gratteri, P.; et al. Use of innovative (Micro)Extraction techniques to CharacteriseHarpagophytum procumbens root and its commercial food supplements. Phytochem. Anal. 2018, 29, 233–241. [Google Scholar] [CrossRef]
- Moyakao, K.; Santaladchaiyakit, Y.; Srijaranai, S.; Vichapong, J. Preconcentration of trace neonicotinoid insecticide residues using vortex-assisted dispersive micro solid-phase extraction with montmorillonite as an efficient sorbent. Molecules 2018, 23, 883. [Google Scholar] [CrossRef]
- De Souza, G.; Mithöfer, A.; Daolio, C.; Schneider, B.; Rodrigues-Filho, E. Identification of Alternaria alternata mycotoxins by LC-SPE-NMR and their cytotoxic effects to soybean (Glycine max) cell suspension culture. Molecules 2013, 18, 2528–2538. [Google Scholar] [CrossRef]
- Ho, Y.; Tsoi, Y.; Leung, K.S. Highly sensitive and selective organophosphate screening in twelve commodities of fruits, vegetables and herbal medicines by dispersive liquid–liquid microextraction. Anal. Chim. Acta 2013, 775, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luan, C.; Wang, L.; Wang, S.; Shao, L. Simultaneous determination of six mycotoxins in peanut by high-performance liquid chromatography with a fluorescence detector. J. Sci. Food Agric. 2017, 97, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, H. Recent development and applications of dispersive liquid–liquid microextraction. J. Chromatogr. A 2013, 1295, 1–15. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M. Dispersive liquid-liquid microextraction for determination of organic analytes. TrAC Trends Anal. Chem. 2010, 29, 728–751. [Google Scholar] [CrossRef]
- Chen, B.; Wu, F.; Wu, W.; Jin, B.; Xie, L.; Feng, W.; Ouyang, G. Determination of 27 pesticides in wine by dispersive liquid–liquid microextraction and gas chromatography–mass spectrometry. Microchem. J. 2016, 126, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Dispersive liquid–liquid microextraction using non-chlorinated, lighter than water solvents for gas chromatography–mass spectrometry determination of fungicides in wine. J. Chromotogr. A 2011, 1218, 6603–6611. [Google Scholar] [CrossRef] [PubMed]
- Zgoła-Grześkowiak, A.; Grześkowiak, T. Dispersive liquid-liquid microextraction. TrAC Trends Anal. Chem. 2011, 30, 1382–1399. [Google Scholar] [CrossRef]
- Chaiyamate, P.; Seebunrueng, K.; Srijaranai, S. Vortex-assisted low density solvent and surfactant based dispersive liquid-liquid microextraction for sensitive spectrophotometric determination of cobalt. RSC Adv. 2018, 8, 7243–7251. [Google Scholar] [CrossRef]
- Lai, X.; Ruan, C.; Li, R.; Liu, C. Application of ionic liquid-based dispersive liquid–liquid microextraction for the analysis of ochratoxin A in rice wines. Food Chem. 2014, 161, 317–322. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y. Temperature-assisted ionic liquid dispersive liquid–liquid microextraction combined with high performance liquid chromatography for the determination of anthraquinones in Radix et Rhizoma Rhei samples. Talanta 2010, 82, 1010–1016. [Google Scholar] [CrossRef]
- Hendriks, G.; Uges, D.R.A.; Franke, J.P. Reconsideration of sample pH adjustment in bioanalytical liquid–liquid extraction of ionisable compounds. J. Chromatogr. B 2007, 853, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, A. Matrix effect in gas chromatographic determination of insecticides and fungicides in vegetables. Int. J. Environ. Chem. 2004, 84, 15–27. [Google Scholar]
- Commission, E. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Analytes | Adduct On | Retention Time | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy/eV | Cone Voltage/V |
|---|---|---|---|---|---|---|
| dichlorvos | [M + H]+ | 2.89 | 221 | 79/109 | 34/22 | 30 |
| phoxim | [M + H]+ | 5.41 | 299 | 129/153 | 13/7 | 30 |
| Chlorpyrifos-methyl | [M + H]+ | 5.61 | 321.8 | 125/289.9 | 20/16 | 30 |
| chlorpyrifos | [M + H]+ | 6.18 | 349.9 | 97/198 | 32/20 | 30 |
| tolcofos-methyl | [M + H]+ | 5.47 | 263.9 | 79/109 | 36/22 | 30 |
| ediphenphos | [M + H]+ | 5.24 | 311 | 109/111 | 32/26 | 30 |
| ethion | [M + H]+ | 6.09 | 385 | 199.1/143 | 10/20 | 30 |
| profenofos | [M + H]+ | 5.89 | 372.9 | 127.9/302.6 | 40/20 | 30 |
| AFB1 | [M + H]+ | 2.87 | 313.2 | 241.1/285.1 | 36/24 | 40 |
| AFB2 | [M + H]+ | 2.64 | 315.2 | 259.1/287.1 | 30/26 | 40 |
| AFG1 | [M + H]+ | 2.43 | 329.2 | 243.1/283.1 | 30/30 | 40 |
| AFG2 | [M + H]+ | 2.18 | 331.2 | 243.1/257.1 | 25/25 | 35 |
| variable | Parameter | Variable Levels | ||||
|---|---|---|---|---|---|---|
| −α(low) | −1 | 0 | 1 | +α(high) | ||
| A | pH | 4 | 4.4 | 5 | 5.6 | 6 |
| B | Volume of CHCI3 (μL) | 310 | 326 | 350 | 374 | 400 |
| C | Volume of ACN (μL) | 1500 | 1541 | 1600 | 1659 | 1700 |
| Source | Sum of Squares | d.f a | Mean Square | F-Value b | p-Value c | Prof > F |
|---|---|---|---|---|---|---|
| Model | 0.86 | 9 | 0.095 | 77.74 | <0.0001 | significant |
| A-pH | 0.035 | 1 | 0.035 | 28.73 | 0.0003 | |
| B-VE | 0.016 | 1 | 0.016 | 13.33 | 0.0045 | |
| C-VD | 0.28 | 1 | 0.28 | 226.20 | <0.0001 | |
| AB | 0.011 | 1 | 0.011 | 9.31 | 0.0122 | |
| AC | 3.306 × 10−3 | 1 | 3.306 × 10−3 | 2.48 | 0.14666 | |
| BC | 0.037 | 1 | 0.037 | 30.27 | 0.0003 | |
| A2 | 0.25 | 1 | 0.25 | 203.26 | <0.0001 | |
| B2 | 0.12 | 1 | 0.12 | 94.77 | <0.0001 | |
| C2 | 0.20 | 1 | 0.20 | 164.98 | <0.0001 | |
| Redisual | 0.012 | 10 | 1.205 × 10−3 | |||
| Lack of fit | 0.012 | 5 | 2.393 × 10−3 | 38.76 | 0.0005 | significant |
| Pure Error | 3.082 × 10−4 | 5 | 6.165 × 10−5 | |||
| Cor Total | 0.87 | 19 |
| Samples | Analytes | Linearity (μg·L−1) | S(Sa) a | R2(Ra2) b | Ratio (%) | Matrix Effect |
|---|---|---|---|---|---|---|
| Wang Laoji | dichlorvos | 0.1–25 | 519,146(485,390) | 0.9992(0.9994) | 6.50 | Mild |
| phoxim | 0.1–25 | 192,788(190,297) | 0.9995(0.9995) | 1.30 | Mild | |
| Chlorpyrifos-methyl | 0.1–25 | 89,333(85,254) | 0.9996(0.9994) | 4.57 | Mild | |
| chlorpyrifos | 0.1–25 | 183,791(172,781) | 0.9997(0.9995) | 5.99 | Mild | |
| tolcofos-methyl | 0.1–25 | 39,699 (38,327) | 0.9990(0.9996) | 3.46 | Mild | |
| ediphenphos | 0.1–25 | 750,665(761,053) | 0.9993(0.9991) | 1.38 | Mild | |
| profenofos | 0.1–25 | 160,427(157,929) | 0.9990(0.9998) | 3.72 | Mild | |
| ethion | 0.1–25 | 387,602(383,630) | 0.9996(0.9995) | 1.03 | Mild | |
| AFB1 | 0.1–25 | 213,455(207,545) | 0.9998(0.9998) | 2.77 | Mild | |
| AFB2 | 0.1–25 | 7042.5(7133.2) | 0.9995(0.9995) | 1.27 | Mild | |
| AFG1 | 0.1–25 | 155,448(135,238) | 0.9994(0.9996) | 13.00 | Mild | |
| AFG2 | 0.1–25 | 55,640(51,872) | 0.9995(0.9995) | 6.77 | Mild | |
| Jia Duo Bao | dichlorvos | 0.1–25 | 519,146(470,095) | 0.9992(0.9989) | 9.45 | Mild |
| phoxim | 0.1–25 | 192,788(185,570) | 0.9995(0.9987) | 3.74 | Mild | |
| Chlorpyrifos-methyl | 0.1–25 | 89,333(81,727) | 0.9994(0.9989) | 8.51 | Mild | |
| chlorpyrifos | 0.1–25 | 183,791(162,751) | 0.9995(0.9991) | 11.45 | Mild | |
| tolcofos-methyl | 0.1–25 | 39,699(36,143) | 0.9996(0.9999) | 8.96 | Mild | |
| ediphenphos | 0.1–25 | 750,665(760,249) | 0.999(0.9991) | 1.28 | Mild | |
| profenofos | 0.1–25 | 164,027(152,981) | 0.9997(0.9998) | 6.73 | Mild | |
| ethion | 0.1–25 | 387,602(370,279) | 0.9996(0.9996) | 4.35 | Mild | |
| AFB1 | 0.1–25 | 213,455(189,305) | 0.9998(0.9990) | 11.31 | Mild | |
| AFB2 | 0.1–25 | 7133.2(7457.9) | 0.9995(0.9996) | 4.55 | Mild | |
| AFG1 | 0.1–25 | 155,448(136,653) | 0.9994(0.9998) | 12.09 | Mild | |
| AFG2 | 0.1–25 | 55,640(50,823) | 0.9995(0.9998) | 8.66 | Mild |
| Analytes | Spiked Level μg/L | Wang Laoji | Jia Duo Bao | ||
|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| dichlorvos | 500 | 75.34 | 8.14 | 72.48 | 7.77 |
| 100 | 70.12 | 5.57 | 71.61 | 6.83 | |
| 10 | 70.27 | 2.28 | 72.45 | 1.53 | |
| 1 | 70.06 | 2.95 | 70.44 | 3.86 | |
| phoxim | 500 | 97.81 | 5.12 | 90.97 | 7.29 |
| 100 | 79.78 | 5.33 | 74.50 | 4.85 | |
| 10 | 78.35 | 1.30 | 88.06 | 7.49 | |
| 1 | 71.19 | 4.43 | 70.21 | 4.54 | |
| Chlorpyrifos-methyl | 500 | 82.39 | 5.17 | 89.05 | 8.26 |
| 100 | 72.65 | 7.31 | 83.22 | 3.69 | |
| 10 | 77.15 | 3.14 | 79.53 | 7.45 | |
| 1 | 77.05 | 8.50 | 70.32 | 4.05 | |
| chlorpyrifos | 500 | 84.84 | 3.00 | 83.11 | 5.92 |
| 100 | 72.94 | 4.98 | 78.19 | 5.90 | |
| 10 | 74.63 | 2.39 | 86.64 | 5.03 | |
| 1 | 74.17 | 4.45 | 71.68 | 2.52 | |
| Tolcofos-methyl | 500 | 75.70 | 8.28 | 104.34 | 5.03 |
| 100 | 73.64 | 7.68 | 79.59 | 6.41 | |
| 10 | 76.23 | 5.92 | 76.23 | 5.92 | |
| 1 | 73.29 | 5.50 | 86.65 | 5.11 | |
| ediphenphos | 500 | 71.04 | 3.63 | 72.28 | 2.51 |
| 100 | 70.64 | 6.23 | 72.39 | 4.99 | |
| 10 | 71.80 | 6.96 | 70.44 | 4.69 | |
| 1 | 95.25 | 4.63 | 107.73 | 2.12 | |
| ethion | 500 | 101.12 | 5.35 | 106.38 | 2.29 |
| 100 | 78.08 | 4.68 | 82.26 | 4.05 | |
| 10 | 94.12 | 2.17 | 86.59 | 2.86 | |
| 1 | 115.65 | 2.40 | 114.09 | 2.21 | |
| profenofos | 500 | 102.36 | 3.40 | 102.55 | 6.62 |
| 100 | 81.89 | 5.28 | 78.78 | 3.29 | |
| 10 | 99.49 | 4.00 | 91.24 | 2.73 | |
| 1 | 81.88 | 5.29 | 83.59 | 4.72 | |
| AFB1 | 10 | 94.59 | 5.25 | 92.14 | 7.62 |
| 1 | 83.28 | 3.83 | 77.44 | 2.22 | |
| 0.2 | 72.68 | 2.88 | 71.04 | 1.90 | |
| AFB2 | 10 | 101.19 | 5.22 | 97.07 | 5.12 |
| 1 | 74.87 | 8.41 | 72.61 | 8.54 | |
| 0.2 | 73.56 | 4.76 | 75.81 | 3.42 | |
| AFG1 | 10 | 99.39 | 1.25 | 91.03 | 2.33 |
| 1 | 70.67 | 4.07 | 70.67 | 2.32 | |
| 0.2 | 72.36 | 7.53 | 70.36 | 7.84 | |
| AFG2 | 10 | 102.99 | 2.62 | 92.15 | 2.46 |
| 1 | 72.59 | 6.17 | 70.68 | 3.24 | |
| 0.2 | 70.10 | 2.70 | 72.40 | 1.89 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Tan, Z.-C.; Huang, K.-C.; Wen, Y.; Li, X.-Y.; Zhao, J.-L.; Liu, C.-L. A Vortex-Assisted Dispersive Liquid-Liquid Microextraction Followed by UPLC-MS/MS for Simultaneous Determination of Pesticides and Aflatoxins in Herbal Tea. Molecules 2019, 24, 1029. https://doi.org/10.3390/molecules24061029
Zhang R, Tan Z-C, Huang K-C, Wen Y, Li X-Y, Zhao J-L, Liu C-L. A Vortex-Assisted Dispersive Liquid-Liquid Microextraction Followed by UPLC-MS/MS for Simultaneous Determination of Pesticides and Aflatoxins in Herbal Tea. Molecules. 2019; 24(6):1029. https://doi.org/10.3390/molecules24061029
Chicago/Turabian StyleZhang, Rui, Zhen-Chao Tan, Ke-Cheng Huang, Yan Wen, Xiang-Ying Li, Jun-Long Zhao, and Cheng-Lan Liu. 2019. "A Vortex-Assisted Dispersive Liquid-Liquid Microextraction Followed by UPLC-MS/MS for Simultaneous Determination of Pesticides and Aflatoxins in Herbal Tea" Molecules 24, no. 6: 1029. https://doi.org/10.3390/molecules24061029