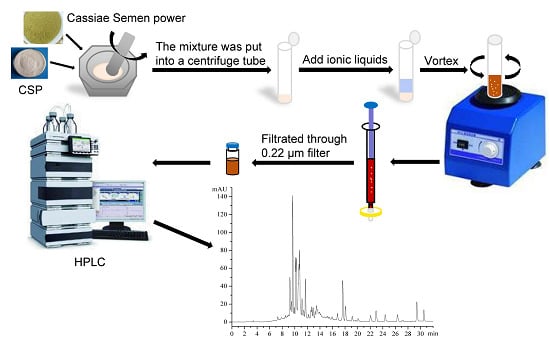
New Vortex-Synchronized Matrix Solid-Phase Dispersion Method for Simultaneous Determination of Four Anthraquinones in Cassiae Semen
Abstract
:1. Introduction
2. Results and Discussion
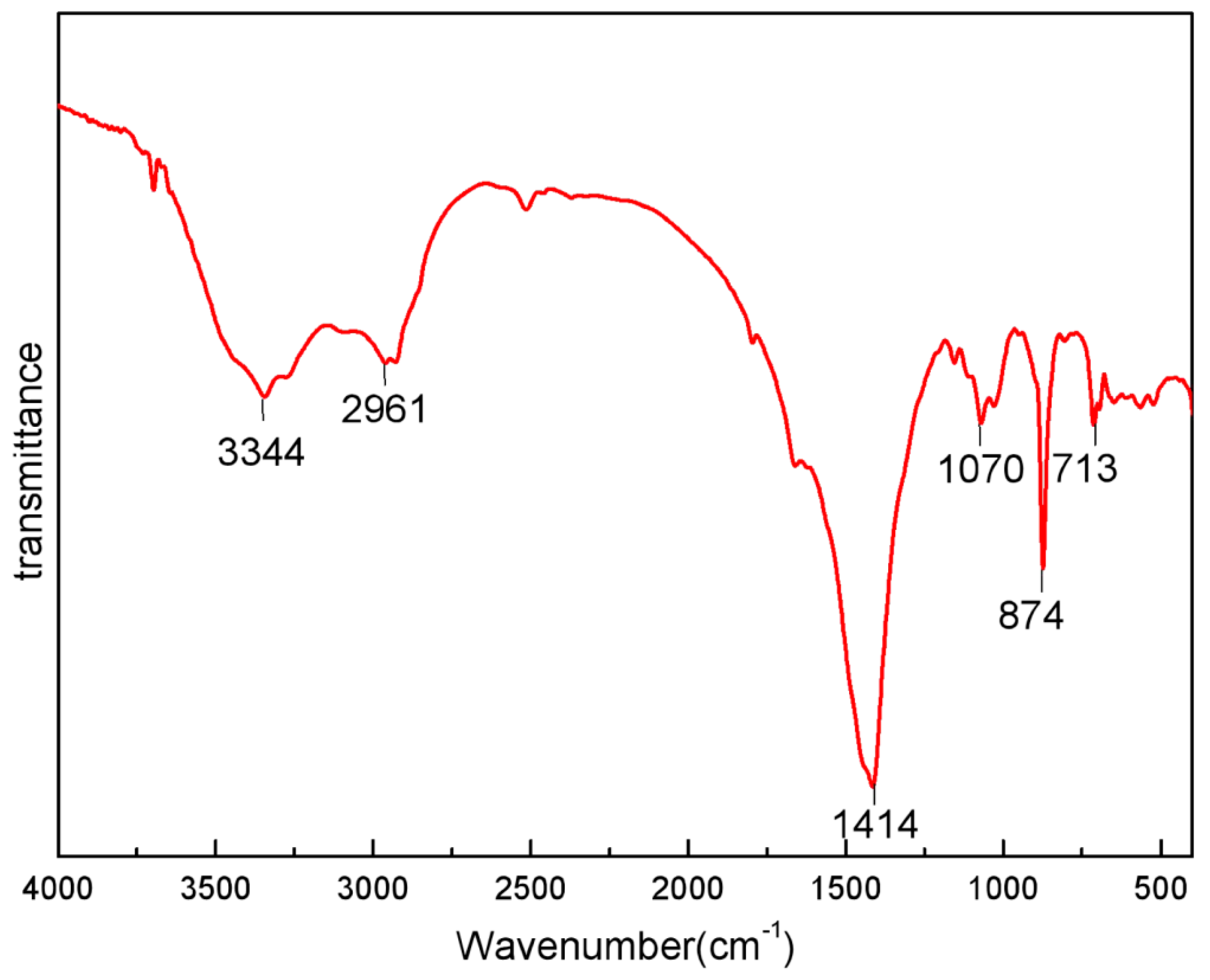
2.1. Characterization of Crab Shell Powder (CSP)
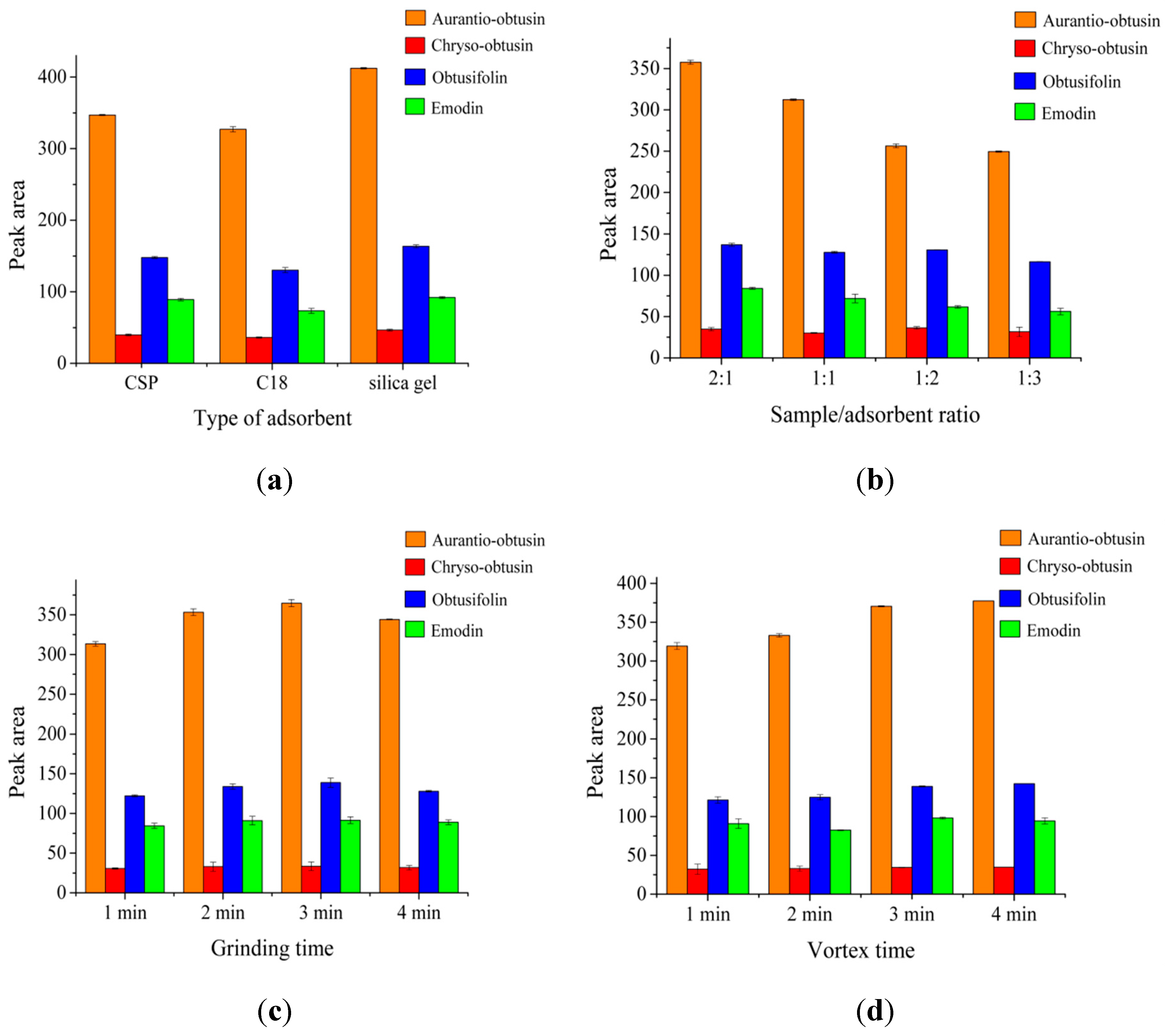
2.2. VS-MSPD Optimization
2.3. Method Validation
2.4. Application to Real Samples
2.5. Comparison with Other Methods
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation and Characterization of CSP
3.3. HPLC Analysis
3.4. Preparation of Standard Solution
3.5. VS-MSPD Procedure and Normal MSPD Procedure
3.6. Heating Reflux Extraction (HRE)
3.7. Validation of Analytical Procedure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, L.Q.; Yao, Z.T.; Yang, W.Y.; Xia, M.S.; Ye, Y. Crab shell: A potential high-efficiency and low-cost adsorbent for dye wastewater. Fresen. Environ. Bull. 2017, 26, 4991–4998. [Google Scholar]
- Tamtam, M.R.; Vudata, V.B.R. Biosorption of Congo Red from aqueous solution by crab shell residue: A comprehensive study. Springerplus 2016, 5, 537. [Google Scholar] [CrossRef]
- Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetesopilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Wang, S.L.; Shih, I.L.; Liang, T.W.; Wang, C.H. Purification and characterization of two antifungal chitinases extracellularly produced by Bacillus amyloliquefaciens V656 in a shrimp and crab shell powder medium. J. Agric. Food Chem. 2002, 50, 2241–2248. [Google Scholar] [CrossRef]
- Wang, S.L.; Hsiao, W.J.; Chang, W.T. Purification and characterization of an antimicrobial chitinase extracellularly produced by Monascuspurpureus CCRC31499 in a shrimp and crab shell powder medium. J. Agric. Food Chem. 2002, 50, 2249–2255. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Zhu, Y.; Kumar, R. Recycled chitosan nanofibril as an effective Cu(II), Pb(II) and Cd(II) ionic chelating agent: Adsorption and desorption performance. Carbohyd. Polym. 2014, 111, 469–476. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Palanivelu, K.; Velan, M. Crab shell-based biosorption technology for the treatment of nickel-bearing electroplating industrial effluents. J. Hazard. Mater. 2005, 119, 251–254. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Zhang, Z.; Zhao, Y. Adsorptive characteristics of chromium (VI) ion from aqueous solutions using the modified crab shell. In Proceedings of the 2015 4th International Conference on Sensors, Measurement and Intelligent Materials, AtlantisPress, Paris, France, January 2016; pp. 732–735. [Google Scholar]
- Niu, H.; Volesky, B. Characteristics of anionic metal species biosorption with waste crab shells. Hydrometallurgy 2003, 71, 209–215. [Google Scholar] [CrossRef]
- Cadogan, E.I.; Lee, C.H.; Popuri, S.R.; Lin, H.Y. Efficiencies of chitosan nanoparticles and crab shell particles in europium uptake from aqueous solutions through biosorption: Synthesis and characterization. Int. Biodeter. Biodegr. 2014, 95, 232–240. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Palanivelu, K.; Velan, M. Biosorption of copper(II) and cobalt(II) from aqueous solutions by crab shell particles. Bioresource Technol. 2006, 97, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Balasubramanian, R. Single and binary biosorption of cerium and europium onto crab shell particles. Chem. Eng. J. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Jeon, D.J.; Yeom, S.H. Recycling wasted biomaterial, crab shells, as an adsorbent for the removal of high concentration of phosphate. Bioresource Technol. 2009, 100, 2646. [Google Scholar] [CrossRef]
- Jeon, C. Removal of As(V) from aqueous solutions by waste crab shells. Korean J. Chem. Eng. 2011, 28, 813–816. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Yu, S.; Zhang, Y.; Wang, N.; Sun, C. Kinetic and thermodynamic analysis of adsorption of arsenic (III) with waste crab shells. J. Water Supply Res. T. 2014, 63, 642–649. [Google Scholar] [CrossRef]
- Bu, Z.; Lv, L.; Li, X.; Chu, C.; Tong, S. pH-zone-refining elution-extrusion countercurrent chromatography: Separation of hydroxyanthraquinones from Cassiae semen. J. Sep. Sci. 2017, 40, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Geng, X.M.; Jiang, H.X.; Zhang, H.Y. Microwave assisted micellar extraction-HPLC determination of anthraquinone derivatives in Cassiae Semen. J. Liq. Chromatogr. R. T. 2010, 33, 1369–1380. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wang, Y.; Wang, Q.; Yang, W.J.; Gu, Y.; Wang, R. Quality evaluation of Semen Cassiae (Cassia obtusifolia L.) by using ultra-high performance liquid chromatography coupled with mass spectrometry. J. Sep. Sci. 2015, 35, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wu, Y.; Wu, X.; Liang, S.; Sun, H. A novel nonaqueous capillary electrophoresis method for effective separation and simultaneous determination of aurantio-obtusin, emodin and rhein in semen cassiae and cassia seed tea. Anal. Methods 2014, 6, 5133–5139. [Google Scholar] [CrossRef]
- Wang, N.; Su, M.; Liang, S.; Sun, H. Investigation of six bioactive anthraquinones in slimming tea by accelerated solvent extraction and high performance capillary electrophoresis with diode-array detection. Food Chem. 2016, 199, 1–7. [Google Scholar] [CrossRef]
- Chen, Q.D.; Xu, R.; Xu, Z.N.; Cen, P.L. Progress in studies of active constituents of anthraquinones and their biological activities from Semen Cassiae. Chin. J. Mod. Appl. Pharm. 2003, 20, 120–124. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China (1); Medical Science and Technology Press: Beijing, Chna, 2015; p. 145. [Google Scholar]
- Zhang, J.; Zhang, Z.; Mi, B.; Qu, Y. Determination of nine constituents in Cassiae Semen from different sources by HPLC. Chin. J. Pharm. Anal. 2013, 33, 1665–1671. [Google Scholar] [CrossRef]
- Guo, R.; Wu, H.; Yu, X.; Xu, M.; Zhang, X.; Tang, L. Simultaneous determination of seven anthraquinone aglycones of crude and processed semen cassiae extracts in rat plasma by UPLC-MS/MS and its application to a comparative pharmacokinetic study. Molecules 2017, 22, 1803. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.; Chen, X.; Hu, Z. Identification and determination of active anthraquinones in Chinese teas by micellar electrokinetic capillary chromatography. Biomed. Chromatogr. 2004, 18, 167–172. [Google Scholar] [CrossRef]
- Soares, K.L.; Caldas, S.S.; Primel, E.G. Evaluation of alternative environmentallyfriendly matrix solid phase dispersion solid supports for the simultaneous extraction of 15 pesticides of different chemical classes from drinking water treatment sludge. Chemosphere 2017, 182, 547–554. [Google Scholar] [CrossRef]
- Vieira, A.A.; Caldas, S.S.; Escarrone, A.L.V.; Jlo, A.; Primel, E.G. Environmentally friendly procedure based on VA-MSPD for the determination of booster biocides in fish tissue. Food Chem. 2018, 242, 475–480. [Google Scholar] [CrossRef]
- Caldas, S.S.; Bolzan, C.M.; Menezes, E.J.D.; Escarrone, A.L.V.; Martins, C.D.M.G.; Bianchini, A.; Primel, E.G. A vortex-assisted MSPD method for the extraction of pesticide residues from fish liver and crab hepatopancreas with determination by GC-MS. Talanta 2013, 112, 63–68. [Google Scholar] [CrossRef]
- Hertzog, G.I.; Soares, K.L.; Caldas, S.S.; Primel, E.G. Study of vortex-assisted MSPD and LC-MS/MS using alternative solid supports for pharmaceutical extraction from marketed fish. Anal. Bioanal. Chem. 2015, 407, 4793–4803. [Google Scholar] [CrossRef]
- Escarrone, A.L.V.; Caldas, S.S.; Soares, B.M.; Martins, S.E.; Primel, E.G.; Nery, L.E.M. A vortex-assisted MSPD method for triclosan extraction from fish tissues with determination by LC-MS/MS. Anal. Methods 2014, 6, 8306–8313. [Google Scholar] [CrossRef]
- León-González, M.E.; Rosales-Conrado, N. Determination of ibuprofen enantiomers in breast milk using vortex-assisted matrix solid-phase dispersion and direct chiral liquid chromatography. J. Chromatogr. A 2017, 1514, 88–94. [Google Scholar] [CrossRef]
- Caldas, S.S.; Soares, B.M.; Abreu, F.; Castro, Í.B.; Fillmann, G.; Primel, E.G. Antifouling booster biocide extraction from marine sediments: A fast and simple method based on vortex-assisted matrix solid-phase extraction. Environ. Sci. Pollut. R. 2018, 25, 7553–7565. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Xu, B.; Li, X.; Wang, X.; Zhang, H.; Song, D. Matrix solid-phase dispersion coupled with magnetic ionic liquid dispersive liquid–liquid microextraction for the determination of triazine herbicides in oilseeds. Anal. Chim. Acta 2015, 888, 67–74. [Google Scholar] [CrossRef]
- He, Z.; Yang, H. Colourimetric detection of swine-specific DNA for halal authentication using gold nanoparticles. Food Control. 2018, 88, 9–14. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H. Pyrethroid residue determination in organic and conventional vegetables using liquid-solid extraction coupled with magnetic solid phase extraction based on polystyrene-coated magnetic nanoparticles. Food Chem. 2017, 217, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Yang, R.; Ye, L.H.; Cao, J.; Cao, W.; Hu, S.S.; Peng, L.Q. Application of ionic liquids for elution of bioactive flavonoid glycosides from lime fruit by miniaturized matrix solid-phase dispersion. Food Chem. 2016, 204, 167–175. [Google Scholar] [CrossRef]
- Du, K.Z.; Li, J.; Bai, Y.; An, M.R.; Gao, X.M.; Chang, Y.X. A green ionic liquid-based vortex-forced MSPD method for the simultaneous determination of 5-HMF and iridoid glycosides from Fructus Corni by ultra-high performance liquid chromatography. Food Chem. 2018, 244, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Z.; Chen, Y. Adsorption behavior of Zn(II) on calcinated Chinese loess. J. Hazard. Mater. 2009, 161, 824–834. [Google Scholar] [CrossRef]
- Njoku, V.O.; Foo, K.Y.; Asif, M.; Hameed, B.H. Preparation of activated carbons from rambutan (Nepheliumlappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem. Eng. J. 2014, 250, 198–204. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as alpha-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Huang, Y.S.; Yu, S.H.; Sheu, Y.R.; Huang, K.S. Preparation and thermal andanti-UV properties of Chitosan/Mica Copolymer. J. Nanomater. 2010, 5, 65. [Google Scholar] [CrossRef]
- Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Sawicki, J.; Staniak, M.; Dresler, S. Silica Modified with polyaniline as a potential sorbent for matrix solid phase dispersion (MSPD) and dispersive solid phase extraction (d-SPE) of plant samples. Materials 2018, 11, 467. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





| Analytes | Linear Regression Data | Range (μg mL−1) | LOD (μg mL−1) | LOQ (μg mL−1) | Precision (RSD%) | Reproducibility (RSD%) | Stability (RSD%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Calibration Curve | R | Intra-Day (n = 6) | Inter-Day (n = 3) | ||||||
| Aurantio-obtusin | y = 10.048x + 6.8409 | 0.9997 | 3.09–123.60 | 1.40 | 4.00 | 1.26 | 2.50 | 1.79 | 2.47 |
| Chryso-obtusin | y = 2.5094x − 1.4986 | 0.9997 | 2.00–40.00 | 2.00 | 13.30 | 3.59 | 2.90 | 4.60 | 2.75 |
| Obtusifolin | y = 13.724x + 0.7768 | 0.9999 | 1.04–41.60 | 1.04 | 3.50 | 1.17 | 2.90 | 2.41 | 3.15 |
| Emodin | y = 35.053x + 1.3954 | 0.9998 | 1.32–13.20 | 0.44 | 2.20 | 1.91 | 2.90 | 3.03 | 2.00 |
| Analytes | Original (µg) | Spiked (µg) | Found (µg) | Recoveries (%) | RSD (%) |
|---|---|---|---|---|---|
| Aurantio-obtusin | 18.22 | 9.11 | 27.33 | 94.34 | 3.78 |
| 18.22 | 36.44 | 103.68 | 2.85 | ||
| 27.33 | 45.55 | 99.67 | 2.67 | ||
| Chryso-obtusin | 8.40 | 4.20 | 12.60 | 92.90 | 3.97 |
| 8.40 | 16.80 | 100.00 | 3.98 | ||
| 12.60 | 21.00 | 98.22 | 3.88 | ||
| Obtusifolin | 5.86 | 2.93 | 8.79 | 91.30 | 2.54 |
| 5.86 | 11.72 | 102.72 | 1.48 | ||
| 8.79 | 14.65 | 106.40 | 2.93 | ||
| Emodin | 1.54 | 0.77 | 2.31 | 94.60 | 3.72 |
| 1.54 | 3.08 | 105.19 | 0.98 | ||
| 2.31 | 3.85 | 99.60 | 3.39 |
| Sample Number | Aurantio-Obtusin | Chryso-Obtusin | Obtusifolin | Emodin |
|---|---|---|---|---|
| 1 a | 1.48 ± 0.18 | 0.11 ± 0.01 | 0.23 ± 0.03 | 0.03 ± 0.01 |
| 2 b | 2.93 ± 0.03 | 0.30 ± 0.04 | 0.45 ± 0.03 | 0.06 ± 0.01 |
| 3 a | 0.42 ± 0.02 | 0.21 ± 0.02 | 0.12 ± 0.01 | 0.02 ± 0.00 |
| 4 b | 2.92 ± 0.02 | 0.23 ± 0.04 | 0.47 ± 0.02 | 0.06 ± 0.00 |
| 5 a | 1.69 ± 0.20 | 0.11 ± 0.02 | 0.27 ± 0.04 | 0.02 ± 0.01 |
| No. | Extraction Method | Analytes | Sample Amounts (g) | Type of Solvent | Solvent Volume (mL) | Extraction Time (min) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Heating reflux extraction | chrysophanol; aurantio-obtusin | 0.5 | methanol | 50 | 120 | [22] |
| 2 | Heating reflux extraction | aurantio-obtusin; obtusin; chrysophanol; emodin; chrysophanol; physcion | 0.5 | 90% methanol | 50 | 120 | [23] |
| 3 | Ultrasonic extraction | emodin; rhein; autrantio-obtusin | 0.2 | chloroform | 60 | 40 | [19] |
| 4 | Heating reflux extraction | aurantio-obtusin; obtusifolin; questin; SC-1; rhein; emodin; SC-2 | 50 | 75% methanol | 500 | 60 | [24] |
| 5 | Accelerated solvent extraction (ASE) | aurantio-obtusin; aloe-emodin; rhein; emodin; physcion; chrysophanol | 0.2 | acetonitrile | 11 | 8 | [20] |
| 6 | Ultrasonic extraction | emodin; aloe-emodin; rhein | 2.0 | ethanol-chloroform (1:1) | 75 | 90 | [25] |
| 7 | Microwave assisted extraction | aloe-emodin; rhein; emodin; chrysophanol; physcion | 0.1 | 10% Genapol X-080 | 6 | 3 | [17] |
| 8 | Heating reflux extraction | 2-gluco-aurantioobtusin; casside; rhein; torachrysoneglucosides; physcion; 2-gluco-chrysoobtusin; aurantio-obtusin; chryso-obtusin; 1-desmethylobtusin; obtusin; aloe-emodin; emodin; chrysophanol | 0.5 | 70% ethanol | 50 | 180 | [18] |
| 9 | VS-MSPD | aurantio-obtusin; chryso-obtusin; obtusifolin; emodin | 0.02 | 250 mM [Domim]HSO4 | 1 | 10 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wang, J.; Zhang, H.; Liu, C.; Tang, Y.; Chu, C. New Vortex-Synchronized Matrix Solid-Phase Dispersion Method for Simultaneous Determination of Four Anthraquinones in Cassiae Semen. Molecules 2019, 24, 1312. https://doi.org/10.3390/molecules24071312
Jiang L, Wang J, Zhang H, Liu C, Tang Y, Chu C. New Vortex-Synchronized Matrix Solid-Phase Dispersion Method for Simultaneous Determination of Four Anthraquinones in Cassiae Semen. Molecules. 2019; 24(7):1312. https://doi.org/10.3390/molecules24071312
Chicago/Turabian StyleJiang, Luyi, Jie Wang, Huan Zhang, Caijing Liu, Yiping Tang, and Chu Chu. 2019. "New Vortex-Synchronized Matrix Solid-Phase Dispersion Method for Simultaneous Determination of Four Anthraquinones in Cassiae Semen" Molecules 24, no. 7: 1312. https://doi.org/10.3390/molecules24071312