Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Alginate Molecular Weight, Alginate Concentration and Gelling Solution Properties on Aerogel Beads
2.2. Aerogel Beads Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
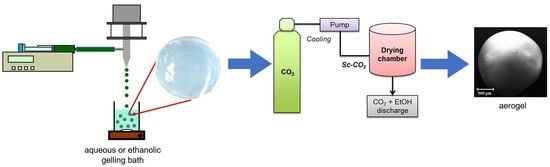
3.2.1. Alginate Gel Beads Preparation
3.2.2. Drying of the Gel Particles
3.2.3. Aerogels Characterization
Shrinkage of Gel Beads After Supercritical Drying
Textural Properties of Aerogels and Cryogels
Density and Porosity of Aerogels
Morphological Analyses
3.2.4. Aerogels Stability Tests Under Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- García-González, C.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogels in Chemical Engineering: Strategies Toward Tailor-Made Aerogels. Ann. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, A.L.; García-González, C.A. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.; Camino-Rey, M.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- García-González, C.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2018, 4, 3. [Google Scholar] [CrossRef]
- Sanz-Moral, L.M.; Rueda, M.; Mato, R.; Martín, Á. View cell investigation of silica aerogels during supercritical drying: Analysis of size variation and mass transfer mechanisms. J. Supercrit. Fluids 2014, 92, 24–30. [Google Scholar] [CrossRef]
- Del Gaudio, P.; De Cicco, F.; Sansone, F.; Aquino, R.P.; Adami, R.; Ricci, M.; Giovagnoli, S. Alginate beads as a carrier for omeprazole/SBA-15 inclusion compound: A step towards the development of personalized paediatric dosage forms. Carbohydr. Polym. 2015, 133, 464–472. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.; Subrahmanyam, R.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Palczer, A.; McCorkle, L.; Zhang, G.; Sotiriou-Leventis, C. Nanoengineered silica-polymer composite aerogels with no need for supercritical fluid drying. J. Sol-Gel Sci. Technol. 2005, 35, 99–105. [Google Scholar] [CrossRef]
- Pääkkö, M.; Vapaavuori, J.; Silvennoinen, R.; Kosonen, H.; Ankerfors, M.; Lindström, T.; Berglund, L.A.; Ikkala, O. Long and entangled native cellulose I nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 2008, 4, 2492–2499. [Google Scholar] [CrossRef]
- Tamon, H.; Ishizaka, H.; Yamamoto, T.; Suzuki, T. Influence of freeze-drying conditions on the mesoporosity of organic gels as carbon precursors. Carbon 2000, 38, 1099–1105. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; Landín, M.; Altai, A.; Russo, P.; Aquino, R.P.; Del Gaudio, P. A novel method for the production of core-shell microparticles by inverse gelation optimized with artificial intelligent tools. Int. J. Pharm. 2018, 538, 97–104. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the printer to the lungs: Inkjet-printed aerogel particles for pulmonary delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Del Gaudio, P.; De Cicco, F.; Aquino, R.P.; Picerno, P.; Russo, P.; Dal Piaz, F.; Bizzarro, V.; Belvedere, R.; Parente, L.; Petrella, A. Evaluation of in situ injectable hydrogels as controlled release device for ANXA1 derived peptide in wound healing. Carbohydr. Polym. 2015, 115, 629–635. [Google Scholar] [CrossRef]
- Pettignano, A.; Bernardi, L.; Fochi, M.; Geraci, L.; Robitzer, M.; Tanchoux, N.; Quignard, F. Alginic acid aerogel: A heterogeneous Brønsted acid promoter for the direct Mannich reaction. New J. Chem. 2015, 39, 4222–4226. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Sabra, W.; Zeng, A.-P.; Deckwer, W.-D. Bacterial alginate: Physiology, product quality and process aspects. Appl. Microbiol. Biotechnol. 2001, 56, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Alnaief, M.; Alzaitoun, M.; García-González, C.; Smirnova, I. Preparation of biodegradable nanoporous microspherical aerogel based on alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Aquino, R.P.; Auriemma, G.; d’Amore, M.; D’Ursi, A.M.; Mencherini, T.; Del Gaudio, P. Piroxicam loaded alginate beads obtained by prilling/microwave tandem technique: Morphology and drug release. Carbohydr. Polym. 2012, 89, 740–748. [Google Scholar] [CrossRef]
- Cerciello, A.; Del Gaudio, P.; Granata, V.; Sala, M.; Aquino, R.P.; Russo, P. Synergistic effect of divalent cations in improving technological properties of cross-linked alginate beads. Int. J. Biol. Macromol. 2017, 101, 100–106. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of alginate-based aerogel for nonsteroidal anti-inflammatory drugs controlled delivery systems using prilling and supercritical-assisted drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Russo, P.; Lauro, M.R.; Colombo, P.; Aquino, R.P. Encapsulation of ketoprofen and ketoprofen lysinate by prilling for controlled drug release. AAPS PharmSciTech 2009, 10, 1178–1185. [Google Scholar] [CrossRef]
- Robitzer, M.; David, L.; Rochas, C.; Di Renzo, F.; Quignard, F. Supercritically-Dried Alginate Aerogels Retain the Fibrillar Structure of the Hydrogels. In Macromolecular Symposia; Wiley Online Library, Wiley-VCH: Weinheim, Germany, 2008; pp. 80–84. [Google Scholar]
- White, R.J.; Antonio, C.; Budarin, V.L.; Bergström, E.; Thomas-Oates, J.; Clark, J.H. Polysaccharide-Derived Carbons for Polar Analyte Separations. Adv. Funct. Mater. 2010, 20, 1834–1841. [Google Scholar] [CrossRef]
- Goosen, M.F.; O’Shea, G.M.; Gharapetian, H.M.; Chou, S.; Sun, A.M. Optimization of microencapsulation parameters: Semipermeable microcapsules as a bioartificial pancreas. Biotechnol. Bioeng. 1985, 27, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, G.; Del Gaudio, P.; Barba, A.A.; d’Amore, M.; Aquino, R.P. A combined technique based on prilling and microwave assisted treatments for the production of ketoprofen controlled release dosage forms. Int. J. Pharm. 2011, 415, 196–205. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- PantiĿ, M.; Kotnik, P.; Knez, Ž.; Novak, Z. High pressure impregnation of vitamin D 3 into polysaccharide aerogels using moderate and low temperatures. J. Supercrit. Fluids 2016, 118, 171–177. [Google Scholar] [CrossRef]
- Veronovski, A.; Knez, Ž.; Novak, Z. Comparison of ionic and non-ionic drug release from multi-membrane spherical aerogels. Int. J. Pharm. 2013, 454, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, G.; Del Gaudio, P.; De Cicco, F.; Aquino, R.P.; Reverchon, E. Supercritical Drying of Alginate Beads for the Development of Aerogel Biomaterials: Optimization of Process Parameters and Exchange Solvents. Ind. Eng. Chem. Res. 2013, 52, 12003–12009. [Google Scholar] [CrossRef]
- Kommanaboyina, B.; Rhodes, C.T. Trends in Stability Testing, with Emphasis on Stability During Distribution and Storage. Drug Dev. Ind. Pharm. 1999, 25, 857–868. [Google Scholar] [CrossRef]
- Cerciello, A.; Auriemma, G.; Morello, S.; Pinto, A.; Del Gaudio, P.; Russo, P.; Aquino, R.P. Design and In Vivo Anti-Inflammatory Effect of Ketoprofen Delayed Delivery Systems. J. Pharm. Sci. 2015, 104, 3451–3458. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Auriemma, G.; Russo, P.; Mencherini, T.; Campiglia, P.; Stigliani, M.; Aquino, R.P. Novel co-axial prilling technique for the development of core–shell particles as delayed drug delivery systems. Eur. J. Pharm. Biopharm. 2014, 87, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, P.; Colombo, P.; Colombo, G.; Russo, P.; Sonvico, F. Mechanisms of formation and disintegration of alginate beads obtained by prilling. Int. J. Pharm. 2005, 302, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cerciello, A.; Auriemma, G.; Del Gaudio, P.; Cantarini, M.; Aquino, R.P. Natural polysaccharides platforms for oral controlled release of ketoprofen lysine salt. Drug Dev. Ind. Pharm. 2016, 42, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, G.; Kranvogl, R.; Perva Uzunalić, A.; Knez, Ž.; Novak, Z. Optimisation of critical parameters during alginate aerogels’ production. J. Non-Cryst. Solids 2016, 443, 112–117. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Z.; Novak, Z. Formation of polysaccharide aerogels in ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Formulation | Diameter (mm) | Shrinkage (%) | ε (%) | Diameter (mm) | Shrinkage (%) | ε (%) | ||
|---|---|---|---|---|---|---|---|---|
| Hydrogel | Aerogel | Alcogel | Aerogel | |||||
| MMW125 | 2.64 ± 0.20 | 2.52 ± 0.19 | 13.3 | 99.7 ± 0.0 | 2.31 ± 0.13 | 2.77 ± 0.23 | −75.4 * | 99.8 ± 0.0 |
| MMW150 | 2.51 ± 0.15 | 2.20 ± 0.10 | 32.5 | 99.5 ± 0.0 | 2.38 ± 0.16 | 2.51 ± 0.16 | −16.9 * | 99.7 ± 0.0 |
| MMW200 | 2.43 ± 0.09 | 2.36 ± 0.16 | 6.7 | 99.5 ± 0.0 | 2.44 ± 0.13 | 1.86 ± 0.02 | 60.9 | 97.6 ± 0.1 |
| MMW225 | 2.41 ± 0.11 | 2.33 ± 0.14 | 10.8 | 99.3 ± 0.0 | 2.37 ± 0.13 | 1.96 ± 0.10 | 43.7 | 98.7 ± 0.0 |
| HMW125 | 2.18 ± 014 | 1.93 ± 0.08 | 30.9 | 99.3 ± 0.0 | 2.46 ± 0.23 | 1.81 ± 0.02 | 60.8 | 99.2 ± 0.0 |
| HMW150 | 2.34 ± 0.14 | 1.73 ± 0.02 | 60.3 | 98.4 ± 0.0 | 2.32 ± 0.12 | 1.55 ± 0.06 | 71.2 | 98.6 ± 0.0 |
| HMW200 | 2.56 ± 0.13 | 2.03 ± 0.13 | 50.6 | 99.5 ± 0.0 | 2.33 ± 0.09 | 1.81 ± 0.03 | 53.7 | 99.5 ± 0.0 |
| HMW225 | 2.40 ± 0.13 | 1.92 ± 0.08 | 49.3 | 99.2 ± 0.0 | 2.33 ± 0.10 | 1.90 ± 0.08 | 46.3 | 99.3 ± 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics. Molecules 2019, 24, 1049. https://doi.org/10.3390/molecules24061049
Rodríguez-Dorado R, López-Iglesias C, García-González CA, Auriemma G, Aquino RP, Del Gaudio P. Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics. Molecules. 2019; 24(6):1049. https://doi.org/10.3390/molecules24061049
Chicago/Turabian StyleRodríguez-Dorado, Rosalía, Clara López-Iglesias, Carlos A. García-González, Giulia Auriemma, Rita P. Aquino, and Pasquale Del Gaudio. 2019. "Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics" Molecules 24, no. 6: 1049. https://doi.org/10.3390/molecules24061049