Fabrication and Characterization of MSQ Aerogel Coating on ePTFE Thin Films for Cable Sheaths
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Monolithic MSQ Aerogel
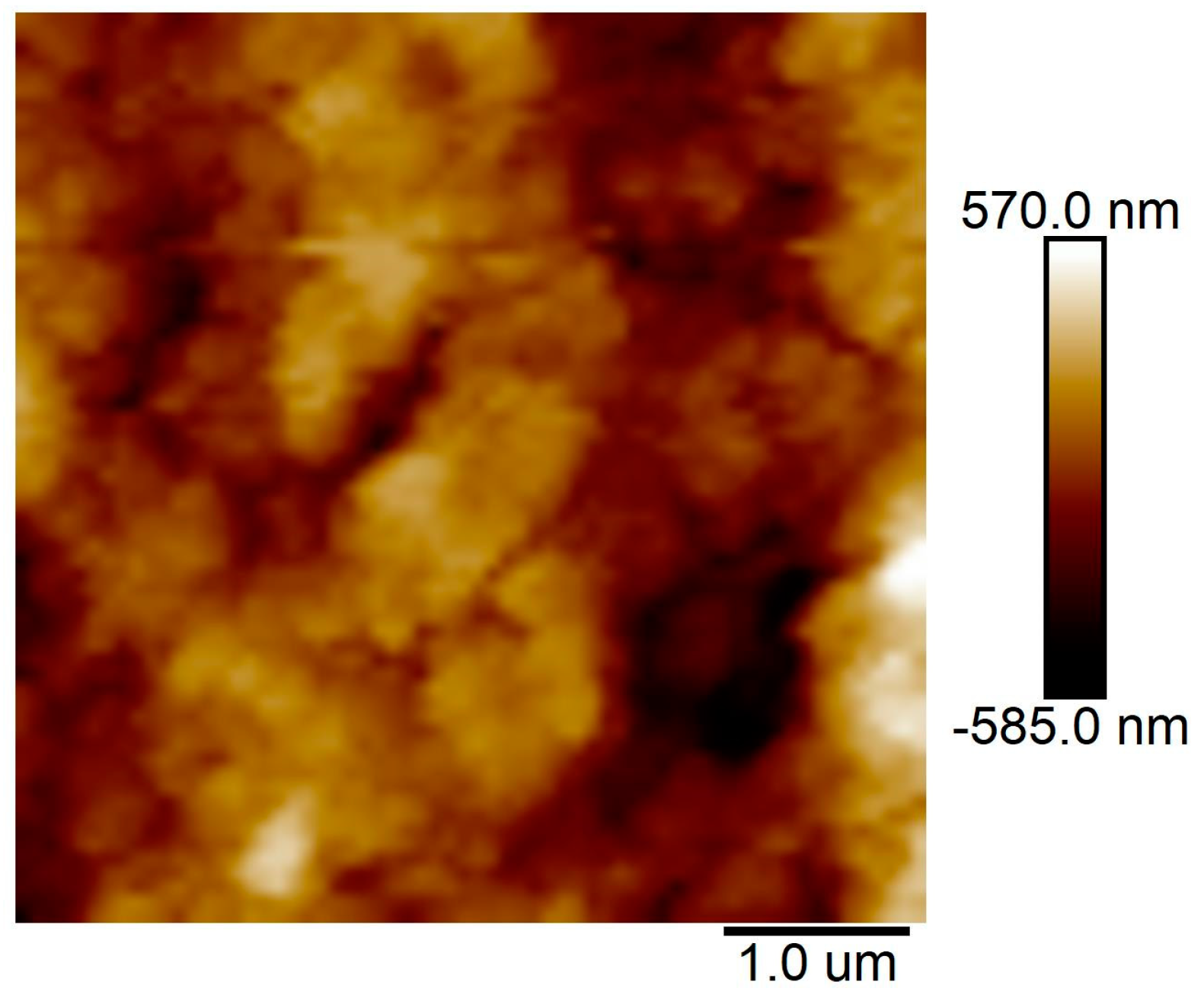
2.2. Characteristics and Microstructure of the MSQ Aerogel Coating Layer on ePTFE Thin Film
2.3. Mechanical, Thermal, and Dielectric Properties of ePTFE Thin Film Coated with an MSQ Aerogel Coating Layer
3. Materials and Methods
3.1. Synthesis of the MSQ Aerogel Monolith
3.2. Fabrication of an MSQ Aerogel Coating Layer on ePTFE Thin Film
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ledergerber, C.; Kornowski, R.R.; Kramer, W. Rugged RF Coaxial Cable is Environmentally Friendly. Microw. RF 1999, 38, 99–104. [Google Scholar]
- Tang, H.; Pan, M.; Jiang, S.P.; Wang, X.; Ruan, Y. Fabrication and Characterization of PFSI/ePTFE Composite Proton Exchange Membranes of Polymer Electrolyte Fuel Cells. Electrochim. Acta 2007, 52, 5304–5311. [Google Scholar] [CrossRef]
- Takahashi, N.; Ujiie, H.; Suzuki, Y.; Iwaki, M.; Hori, T. Biocompatibility of ePTFE Modified by Ion Beam Irradiation. Neurol. Surg. 2004, 32, 339–344. [Google Scholar]
- Lassus, C. Expanded Ptfe in the Treatment of Facial Wrinkles. Aesthet. Plast. Surg. 1991, 15, 167–174. [Google Scholar] [CrossRef]
- Huang, J.; Lee, Y.H. Evaluation of Uni-Axially Expanded PTFE as a Gasket Material for Fluid Sealing Applications. Mater. Chem. Phys. 2001, 70, 197–207. [Google Scholar] [CrossRef]
- Huang, J.; Lee, W. Sealing and Mechanical Behaviors of Expanded PTFE Gasket Sheets Characterized by PVRC Room Temperature Tightness Tests. Mater. Chem. Phys. 2001, 68, 180–196. [Google Scholar] [CrossRef]
- Vail, J.R.; Krick, B.A.; Marchman, K.R.; Sawyer, W.G. Polytetrafluoroethylene (PTFE) Fiber Reinforced Polyetheretherketone (PEEK) Composites. Wear 2011, 270, 737–741. [Google Scholar] [CrossRef]
- Anjana, P.S.; Sebastian, M.T.; Suma, M.N.; Mohanan, P. Low Dielectric Loss PTFE/CeO2 Ceramic Composites for Microwave Substrate Applications. Int. J. Appl. Ceram. Technol. 2008, 5, 325–333. [Google Scholar] [CrossRef]
- Rajesh, S.; Murali, K.P.; Rajani, K.V.; Ratheesh, R. SrTiO3-Filled PTFE Composite Laminates for Microwave Substrate Applications. Int. J. Appl. Ceram. Technol. 2009, 6, 553–561. [Google Scholar] [CrossRef]
- Rajesh, S.; Murali, K.P.; Ratheesh, R. Preparation and Characterization of High Permittivity and Low Loss PTFE/CaTiO3 Microwave Laminates. Polym. Compos. 2009, 30, 1480–1485. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Lee, K.; Choe, Y.; Kim, Y.H.; Lee, J.K.; Hwang, H. Fabrication of Silica Aerogel Composite Blankets from an Aqueous Silica Aerogel Slurry. Ceram. Int. 2018, 44, 2204–2208. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, Q.; Wang, T. Synthesis and Property of Alumina Aerogel. J. Inorg. Mater. 2018, 33, 259–265. [Google Scholar] [CrossRef]
- Ward, D.A.; Ko, E.I. Synthesis and Structural Transformation of Zirconia Aerogels. Chem. Mater. 1993, 5, 956–969. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, J.C.; Hong, Z.S.; Deng, J.F.; Fan, K.N. Characterization of High-Surface-Area Zirconia Aerogel Synthesized from Combined Alcohothermal and Supercritical Fluid Drying Techniques. Catal. Lett. 2002, 81, 107–112. [Google Scholar] [CrossRef]
- Hu, J.C.; Cao, Y.; Deng, J.F. A Simple Alcohothermal Synthetic Route to High Surface Area Zirconia Aerogel. Chem. Lett. 2001, 30, 398–399. [Google Scholar] [CrossRef]
- Bangi, U.K.H.; Jung, H.; Park, C.; Mahadik, D.B.; Park, H. Effect of Thermal Treatment on the Textural Properties and Thermal Stability of Surface Modified Zirconia Aerogel Powders. Int. J. Nanotechnol. 2016, 13, 452–462. [Google Scholar] [CrossRef]
- Jiang, T.; Bu, F.; Feng, X.; Shakir, I.; Hao, G.; Xu, Y. Porous Fe2O3 Nanoframeworks Encapsulated within Three-Dimensional Graphene as High-Performance Flexible Anode for Lithium-Ion Battery. ACS Nano 2017, 11, 5140–5147. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Li, L.; Wei, X. A Versatile Biomass Derived Carbon Material for Oxygen Reduction Reaction, Supercapacitors and Oil/Water Separation. Nano Energy 2017, 33, 334–342. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W.; et al. High-Performance Energy Storage and Conversion Materials Derived from a Single Metal Organic Framework/Graphene Aerogel Composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Peng, Q.; Wang, Q.; Zhao, G. Catalytic Activity of MOF(2Fe/Co)/carbon Aerogel for Improving H2O2 and (OH)-O-center Dot Generation in Solar photo-electro-Fenton Process. Appl. Catal. B Environ. 2017, 203, 127–137. [Google Scholar] [CrossRef]
- Oschatz, M.; Boukhalfa, S.; Nickel, W.; Hofmann, J.P.; Fischer, C.; Yushin, G.; Kaskel, S. Carbide-Derived Carbon Aerogels with Tunable Pore Structure as Versatile Electrode Material in High Power Supercapacitors. Carbon 2017, 113, 283–291. [Google Scholar] [CrossRef]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S.; Chook, S.W.; Khiew, P.S. CaCO3-decorated Cellulose Aerogel for Removal of Congo Red from Aqueous Solution. Cellulose 2015, 22, 2683–2691. [Google Scholar] [CrossRef]
- Zheng, T.; Li, A.; Li, Z.; Hu, W.; Shao, L.; Lu, L.; Cao, Y.; Chen, Y. Mechanical Reinforcement of a Cellulose Aerogel with Nanocrystalline Cellulose as Reinforcer. RSC Adv. 2017, 7, 34461–34465. [Google Scholar] [CrossRef]
- Ma, S.; Mi, Q.; Yu, J.; He, J.; Zhang, J. Aerogel Materials Based on Cellulose. Prog. Chem. 2014, 26, 796–809. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Geng, B.; Ru, J.; Du, C.; Jin, C.; Han, J. Research Progress in the Cellulose Based Aerogel-type Oil Sorbents. Acta Polym. Sin. 2016, 545–559. [Google Scholar] [CrossRef]
- Guo, X.; Shan, J.; Lai, Z.; Lei, W.; Ding, R.; Zhang, Y.; Yang, H. Facile Synthesis of Flexible Methylsilsesquioxane Aerogels with Surface Modifications for Sound-Absorbance, Fast Dye Adsorption and Oil/Water Separation. Molecules 2018, 23, 945. [Google Scholar] [CrossRef]
- Borba, A.; Almangano, M.; Portugal, A.A.; Patrício, R.; Simões, P.N. Methylsilsesquioxane-Based Aerogel Systems—Insights into the Role of the Formation of Molecular Clusters. J. Phys. Chem. A 2016, 120, 4079–4088. [Google Scholar] [CrossRef]
- Kanamori, K.; Aizawa, M.; Nakanishi, K.; Hanada, T. New Transparent Methylsilsesquioxane Aerogels and Xerogels with Improved Mechanical Properties. Adv. Mater. 2007, 19, 1589–1593. [Google Scholar] [CrossRef]
- Kurahashi, M.; Kanamori, K.; Takeda, K.; Kaji, H.; Nakanishi, K. Role of Block Copolymer Surfactant on the Pore Formation in Methylsilsesquioxane Aerogel Systems. RSC Adv. 2012, 2, 7166. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Luo, J.; Wang, D.; Gao, H.; Zhang, J.; Xing, Y.; Yang, Z.; Cao, H.; He, W. Silica Aerogel Films Via Ambient Pressure Drying for Broadband Reflectors. New J. Chem. 2018, 42, 6525–6531. [Google Scholar] [CrossRef]
- Bauer, M.L.; Bauer, C.M.; Fish, M.C.; Matthews, R.E.; Garner, G.T.; Litchenberger, A.W.; Norris, P.M. Thin-Film Aerogel Thermal Conductivity Measurements Via 3 Omega. J. Non-Cryst. Solids 2011, 357, 2960–2965. [Google Scholar] [CrossRef]
- Xiao, K.; Wu, G.; Shen, J.; Xie, D.; Zhou, B. Preparation and Electrochemical Properties of Vanadium Pentoxide Aerogel Film Derived at the Ambient Pressure. Mater. Chem. Phys. 2006, 100, 26–30. [Google Scholar] [CrossRef]
- Jung, S.B.; Park, H.H.; Kim, H. Investigation of the Bonding States of the SiO2 Aerogel Film/Metal Interface. Thin Solid Films 2004, 447, 575–579. [Google Scholar] [CrossRef]
- On, N.K.; Rashid, A.A.; Nazlan, M.M.M.; Hamdan, H. Thermal and Mechanical Behavior of Natural Rubber Latex-Silica Aerogel Film. J. Appl. Polym. Sci. 2012, 124, 3108–3116. [Google Scholar] [CrossRef]
- Yang, H.S.; Choi, S.Y.; Hyun, S.H.; Park, H.H.; Hong, J.K. Ambient-Dried Low Dielectric SiO2 Aerogel Thin Film. J. Non-Cryst Solids 1997, 221, 151–156. [Google Scholar] [CrossRef]
- Fan, H.Y.; Bentley, H.R.; Kathan, K.R.; Clem, P.; Lu, Y.F.; Brinker, C.J. Self-Assembled Aerogel-Like Low Dielectric Constant Films. J. Non-Cryst Solids 2001, 285, 79–83. [Google Scholar] [CrossRef]
- Kim, G.S.; Hyun, S.H. Synthesis of Window Glazing Coated with Silica Aerogel Films Via Ambient Drying. J. Non-Cryst Solids 2003, 320, 125–132. [Google Scholar] [CrossRef]
Sample Availability: Samples of the MSQ aerogel and MSQ aerogel layer coated ePTFE film are available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Bai, S.; Shan, J.; Lei, W.; Ding, R.; Zhang, Y.; Yang, H. Fabrication and Characterization of MSQ Aerogel Coating on ePTFE Thin Films for Cable Sheaths. Molecules 2019, 24, 1246. https://doi.org/10.3390/molecules24071246
Guo X, Bai S, Shan J, Lei W, Ding R, Zhang Y, Yang H. Fabrication and Characterization of MSQ Aerogel Coating on ePTFE Thin Films for Cable Sheaths. Molecules. 2019; 24(7):1246. https://doi.org/10.3390/molecules24071246
Chicago/Turabian StyleGuo, Xingzhong, Shengchi Bai, Jiaqi Shan, Wei Lei, Ronghua Ding, Yun Zhang, and Hui Yang. 2019. "Fabrication and Characterization of MSQ Aerogel Coating on ePTFE Thin Films for Cable Sheaths" Molecules 24, no. 7: 1246. https://doi.org/10.3390/molecules24071246





