Syntheses and Self-assembling Behaviors of Pentagonal Conjugates of Tryptophane Zipper-Forming Peptide
Abstract
:1. Introduction
2. Results and Discussion
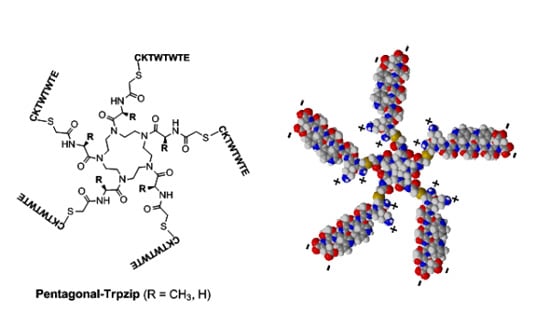
2.1. Synthesis of Pentagonal Conjugates of Tryptophane Zipper-Forming Peptide
2.2. Secondary Structure of Pentagonal Peptide Conjugates
2.3. Self-Assembly of Pentagonal Peptide Conjugates in Water
3. Experimental Section
3.1. General
3.2. Synthesis of Peptide Conjugates
3.3. CD Spectrum Measurements
3.4. Transmission Electron Microscopy (TEM)
4. Conclusions
Acknowledgments
References
- Mammen, M; Choi, S; Whitesides, GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed 1998, 37, 2754–2794. [Google Scholar]
- Kiessling, LL; Gestwicki, JE; Strong, LE. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. Int. Ed 2006, 45, 2348–2368. [Google Scholar]
- Dam, TK; Brewer, CF. Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry 2008, 47, 8470–8476. [Google Scholar]
- Mulder, A; Huskens, J; Reinhoudt, DN. Multivalency in supramolecular chemistry and nanofabrication. Organ. Biomol. Chem 2004, 2, 3409–3424. [Google Scholar]
- Kitov, PI; Sadowska, JM; Mulvey, G; Armstrong, GD; Ling, H; Pannu, NS; Read, RJ; Bundle, DR. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 2000, 403, 669–672. [Google Scholar]
- Zhang, Z; Merritt, EA; Ahn, M; Roach, C; Hou, Z; Verlinde, CLMJ; Hol, WGJ; Fan, E. Solution and crystallographic studies of branched multivalent ligands that inhibit the receptor-binding of cholera toxin. J. Am. Chem. Soc 2002, 124, 12991–12998. [Google Scholar]
- Mutter, M; Vuilleumier, S. A Chemical approach to protein design. Template-assembled synthetic proteins (TASP). Angew. Chem. Int. Ed 1989, 28, 535–676. [Google Scholar]
- Crespo, L; Sanclimens, G; Pons, M; Giralt, E; Royo, M; Albericio, F. Peptide and amide bond-containing dendrimers. Chem. Rev 2005, 105, 1663–1681. [Google Scholar]
- Darbre, T; Reymond, J-L. Peptide dendrimers as artificial enzymes, receptors, and drug-delivery agents. Acc. Chem. Res 2006, 39, 925–934. [Google Scholar]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotech 2003, 21, 1171–1178. [Google Scholar]
- Gao, X; Matsui, H. Peptide-based nanotubes and their application in bionanotechnology. Adv. Mater 2005, 17, 2037–2050. [Google Scholar]
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev 2007, 36, 1263–1269. [Google Scholar]
- Ryadnov, MG; Woolfson, DN. Introducing branches into a self-assembling peptide fiber. Angew. Chem. Int. Ed 2003, 42, 3021–3023. [Google Scholar]
- Ryadnov, MG; Woolfson, DN. Engineering the morphology of a self-assembling protein fibre. Nat. Mater 2003, 2, 329–332. [Google Scholar]
- Smith, AM; Acquah, SFA; Bone, N; Kroto, HW; Ryadnov, MG; Stevens, MSP; Walton, DRM; Woolfson, DN. Polar assembly in a designed protein fiber. Angew. Chem. Int. Ed 2005, 44, 325–328. [Google Scholar]
- Boato, F; Thomas, RM; Ghasparian, A; Freund-Renard, A; Moehle, K; Robinson, JA. Synthetic virus-like particles from self-assembling coiled-coil lipopeptides and their use in antigen display to the immune system. Angew. Chem. Int. Ed 2007, 46, 9015–9018. [Google Scholar]
- Marini, DM; Hwang, W; Lauffenburger, A; Zhang, S; Kamm, RD. Left-handed helical ribbon intermediates in the self-assembly of a β-sheet peptide. Nano Lett 2002, 2, 295–299. [Google Scholar]
- Mihara, H; Matsumura, S; Takahashi, T. Construction and control of self-assembly of amyloid and fibrous peptides. Bull. Chem. Soc. Jpn 2005, 78, 572–590. [Google Scholar]
- Lim, Y-B; Lee, E; Lee, M. Cell-penetrating-peptide-coated nanoribbons for intracellular nanocarriers. Angew. Chem. Int. Ed 2007, 46, 3475–3478. [Google Scholar]
- Lim, Y-B; Park, S; Lee, E; Jeong, H; Ryu, J-H; Lee, MS; Lee, M. Glycoconjugate nanoribbons from the self-assembly of carbohydrate-peptide block molecules for controllable bacterial cell cluster formation. Biomacromolecules 2007, 8, 1404–1408. [Google Scholar]
- Kwon, S; Jeon, A; Yoo, SH; Chung, IS; Lee, H-S. Unprecedented molecular architectures by the controlled self-assembly of a β-peptide foldamer. Angew. Chem. Int. Ed 2010, 49, 8232–8236. [Google Scholar]
- Zhou, M; Bentley, D; Ghosh, I. Helical supramolecules and fibers utilizing leucine zipper-displaying dendrimers. J. Am. Chem. Soc 2004, 126, 734–735. [Google Scholar]
- Matsuura, K; Murasato, K; Kimizuka, N. Artificial peptide-nanospheres self-assembled from three-way junctions of β-sheet-forming peptides. J. Am. Chem. Soc 2005, 127, 10148–10149. [Google Scholar]
- Murasato, K; Matsuura, K; Kimizuka, N. Self-assembly of nanofiber with uniform width from wheel-type trigonal-β-sheet forming peptide. Biomacromolecules 2008, 9, 913–918. [Google Scholar]
- Matsuura, K; Watanabe, K; Sakurai, K; Matsuzaki, T; Kimizuka, N. Self-assembled synthetic viral capsids from a 24-mer viral peptide fragment. Angew. Chem. Int. Ed 2010, 49, 9662–9665. [Google Scholar]
- Matsuura, K; Matsuyama, H; Fukuda, T; Teramoto, T; Watanabe, K; Murasato, K; Kimizuka, N. Spontaneous self-assembly of nano-spheres from trigonal conjugate of glutathione in water. Soft Matter 2009, 5, 2463–2470. [Google Scholar]
- Matsuura, K; Fujino, K; Teramoto, T; Murasato, K; Kimizuka, N. Glutathione nanospheres: Self-assembly of conformation-regulated trigonal-glutathiones in water. Bull Chem Soc Jpn 2010, 83, 880–886. [Google Scholar]
- Matsuura, K; Tochio, K; Watanabe, K; Kimizuka, N. Controlled release of guest molecules from spherical assembly of trigonal-gultathione by a disulfide recombination. Chem. Lett 2011, 40, 711–713. [Google Scholar]
- Cochran, AG; Skelton, NJ; Starovasnik, MA. Tryptophan zippers: Stable, monomeric β-hairpins. Proc. Natl. Acad. Sci. USA 2001, 98, 5578–5583. [Google Scholar]
- Richardson, JS; Richardson, DC. Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc. Natl. Acad. Sci. USA 2002, 99, 2754–2759. [Google Scholar]
- Dempsey, CE; Piggot, TJ; Mason, PE. Dissecting contributions to the denaturant sensitivities of proteins. Biochemistry 2005, 44, 775–781. [Google Scholar]
- Yang, WY; Pitera, JW; Swope, WC; Gruebele, M. Heterogeneous folding of the Trpzip hairpin: Full atom simulation and experiment. J. Mol. Biol 2004, 336, 241–251. [Google Scholar]
- Streicher, WW; Makhatadze, GI. Calorimetric evidence for a two-state unfolding of the β-hairpin peptide Trpzip4. J. Am. Chem. Soc 2006, 128, 30–31. [Google Scholar]
- Chetal, P; Chauhan, VS; Sahal, D. A meccano set approach of joining trpzip a water soluble β-hairpin peptide with a didehydrophenylalanine containing hydrophobic helical peptide. J. Peptide Res 2005, 65, 475–484. [Google Scholar]
- Matsuura, K; Hayashi, H; Murasato, K; Kimizuka, N. Trigonal tryptophane-zipper as a novel building block for pH-responding peptide nano-assemblies. Chem. Commun 2011, 47, 265–267. [Google Scholar]
- Harrison, SC. Multiple modes of subunit association in the structures of simple spherical viruses. Trends Biochem. Sci 1984, 9, 345–351. [Google Scholar]
- Harrison, SC. The familiar and the unexpected in structures of icosahedral viruses. Curr. Opin. Struct. Biol 2001, 11, 195–199. [Google Scholar]
- Olson, AJ; Hu, YHE; Kelnan, E. Chemical mimicry of viral capsid self-assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 20731–20736. [Google Scholar]
- Bencini, A; Fabbrizzi, L; Poggi, A. Formation of nickel(III) complexes with n-dentate amine macrocycles (n = 4, 5, 6). ESR and electrochemical studies. Inorg. Chem 1981, 20, 2544–2549. [Google Scholar]
- Extension of the incubation time (96 h) minimally affected to the results of CD spectra and TEM images.
- The measurement was repeated for three times to confirm data reproducibility. The standard deviation was about 22%.







© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsuura, K.; Murasato, K.; Kimizuka, N. Syntheses and Self-assembling Behaviors of Pentagonal Conjugates of Tryptophane Zipper-Forming Peptide. Int. J. Mol. Sci. 2011, 12, 5187-5199. https://doi.org/10.3390/ijms12085187
Matsuura K, Murasato K, Kimizuka N. Syntheses and Self-assembling Behaviors of Pentagonal Conjugates of Tryptophane Zipper-Forming Peptide. International Journal of Molecular Sciences. 2011; 12(8):5187-5199. https://doi.org/10.3390/ijms12085187
Chicago/Turabian StyleMatsuura, Kazunori, Kazuya Murasato, and Nobuo Kimizuka. 2011. "Syntheses and Self-assembling Behaviors of Pentagonal Conjugates of Tryptophane Zipper-Forming Peptide" International Journal of Molecular Sciences 12, no. 8: 5187-5199. https://doi.org/10.3390/ijms12085187
APA StyleMatsuura, K., Murasato, K., & Kimizuka, N. (2011). Syntheses and Self-assembling Behaviors of Pentagonal Conjugates of Tryptophane Zipper-Forming Peptide. International Journal of Molecular Sciences, 12(8), 5187-5199. https://doi.org/10.3390/ijms12085187