Bioavailability and Brain-Targeting of Geniposide in Gardenia-Borneol Co-Compound by Different Administration Routes in Mice
Abstract
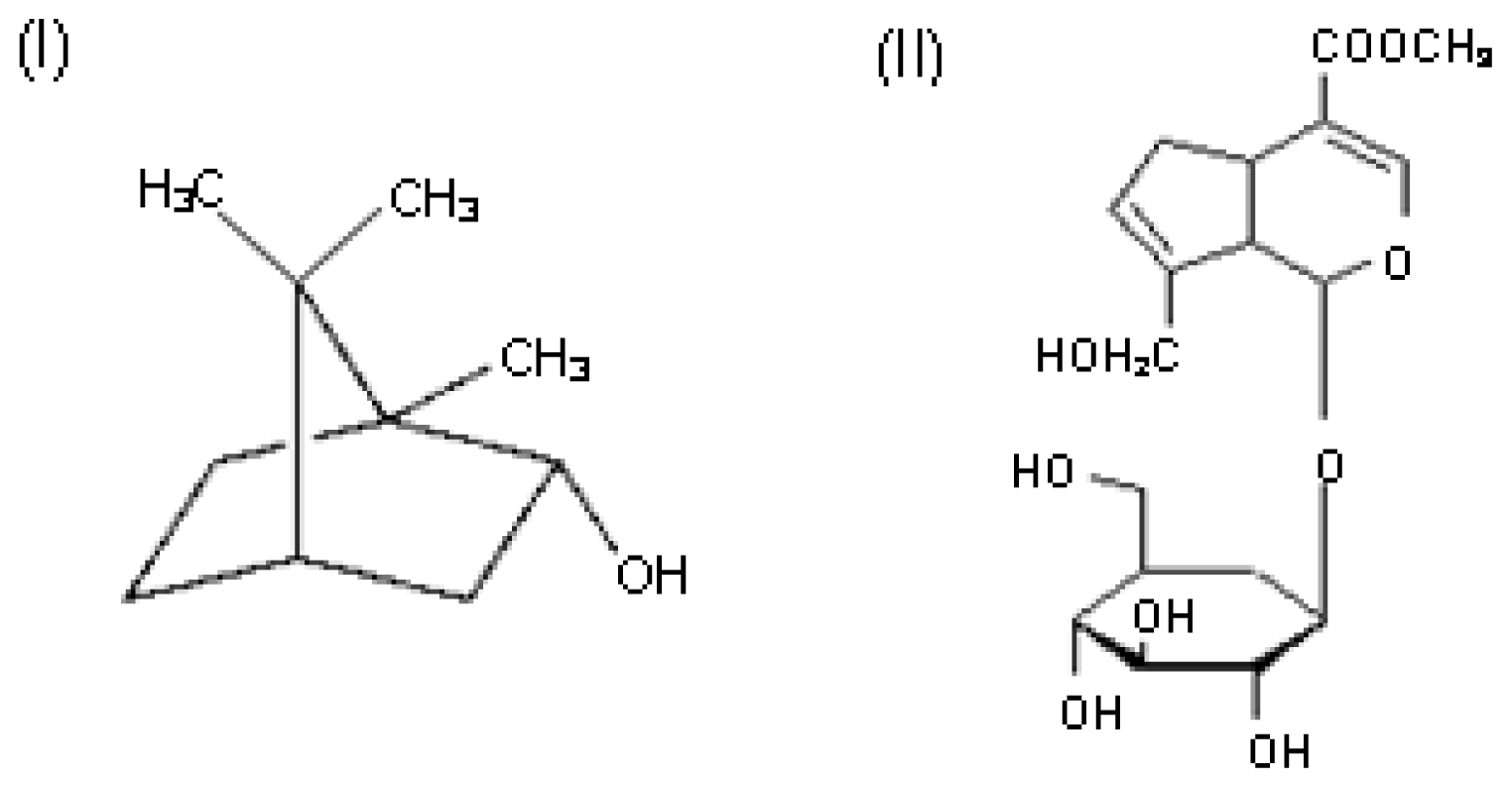
:1. Introduction
2. Results and Discussion
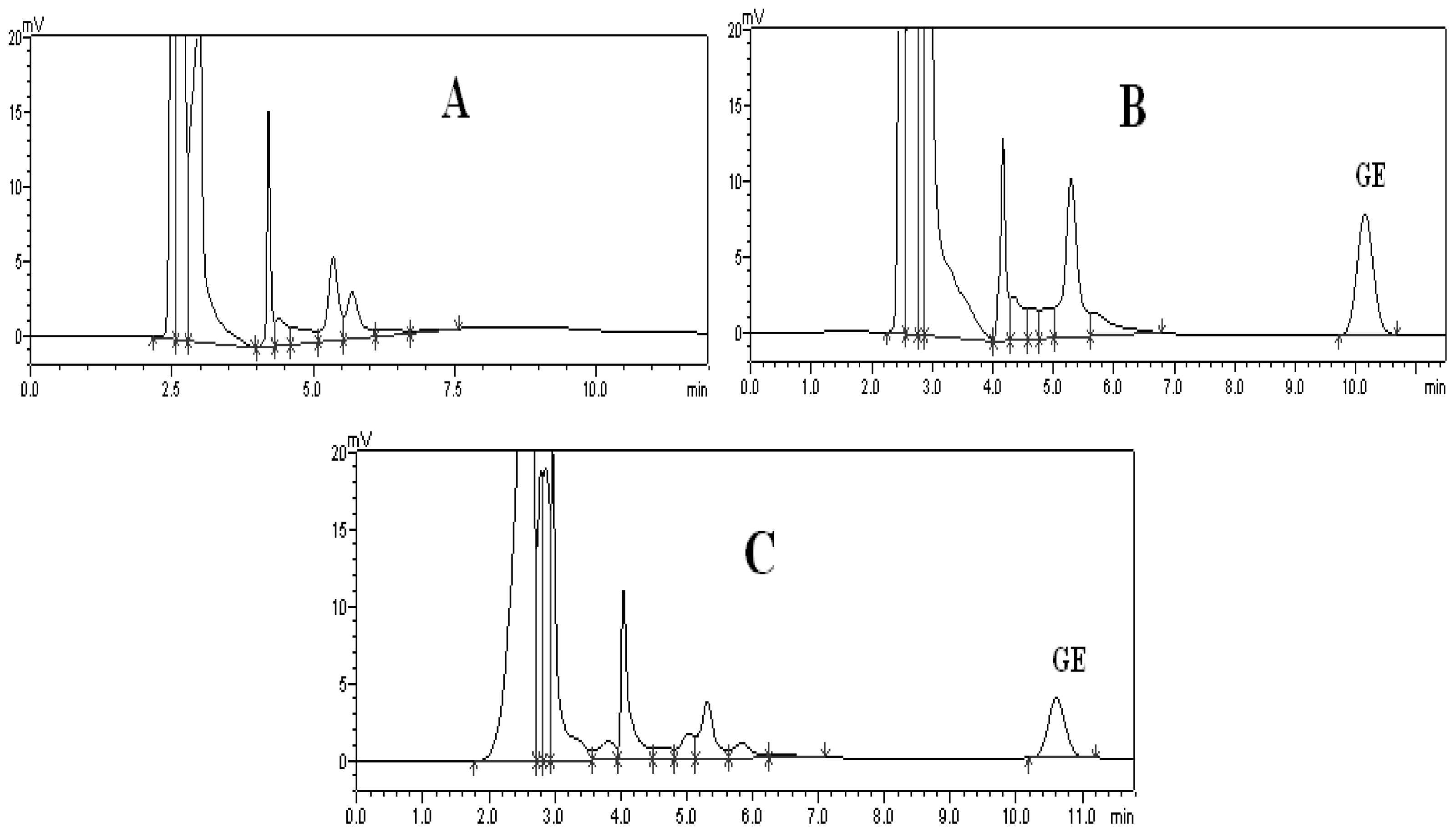
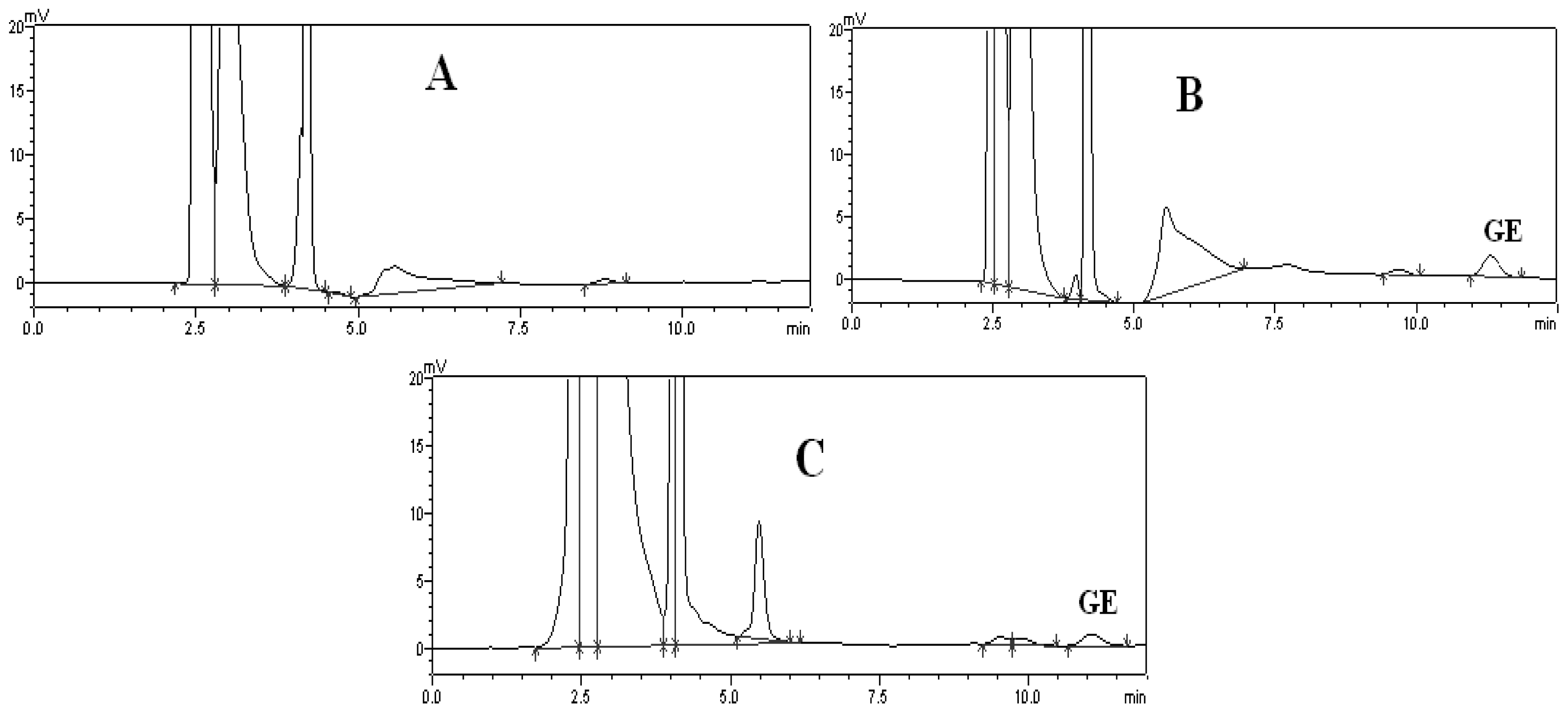
2.1. Method of Plasma Samples Qualification
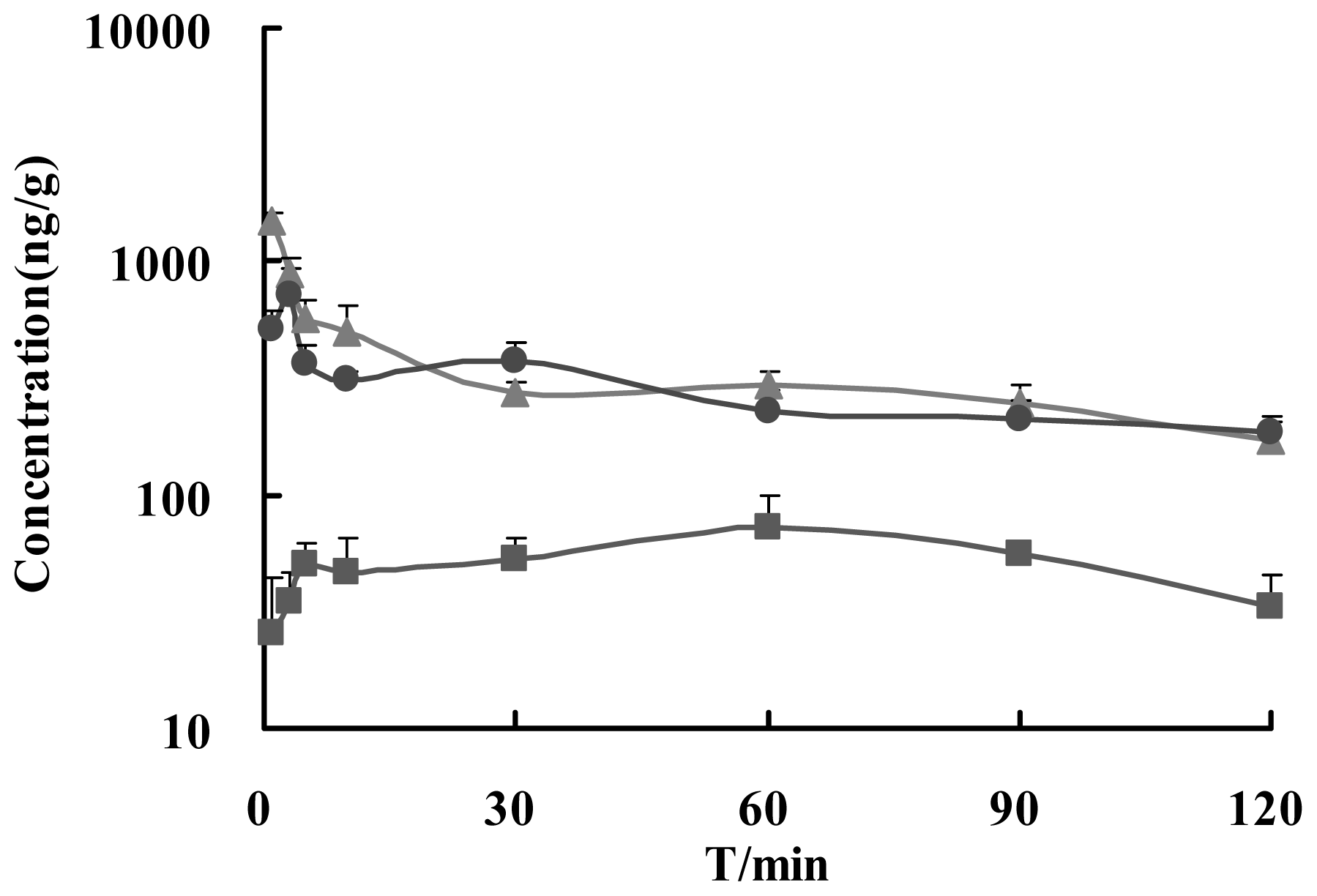
2.2. Method of Brain Samples Qualification
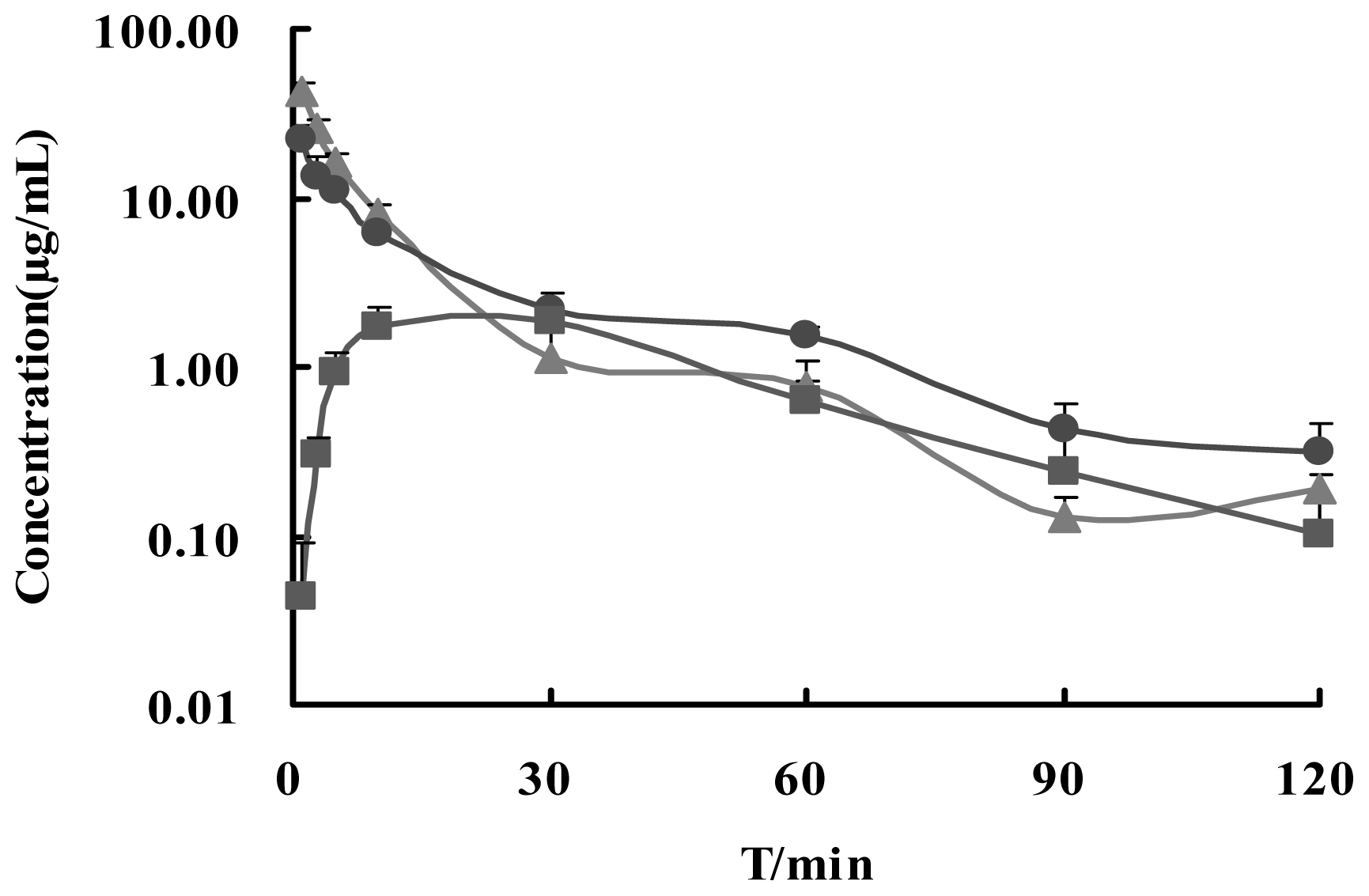
2.3. Statistical Analysis and Pharmacokinetics
3. Experimental
3.1. Chemicals, Reagents and Animals
3.2. Instrumentation
3.3. Chromatographic Conditions
3.4. Sample Preparation
3.4.1. Solution Preparation
3.4.2. In Vivo Experiments
3.5. Statistical Evaluation
4. Conclusions
Acknowledgements
References
- Suzuki, Y.; Kondo, K.; Ikeda, Y. Antithrombotic effect of genipeside and genipin in the mouse thrombosis model. Planta Med 2001, 67, 807–810. [Google Scholar]
- Koo, H.J.; Lim, K.H.; Jung, H.J.; Park, E.H. Anti-inflammatory evaluation of gardenia extract geniposide and genipin. J. Ethnopharmacol 2006, 103, 496–500. [Google Scholar]
- Lu, Y.; Chen, X.; Du, S.; Wu, Q.; Yao, Z.; Zhai, Y. The in situ and in vivo study on enhancing effect of borneol in nasal absorption of geniposide in rats. Arch. Pharm. Res 2010, 33, 691–696. [Google Scholar]
- Lu, Y.; Du, S.; Chen, X.; Wu, Q.; Song, X.; Xu, B.; Zhai, Y. The enhancing effect of natural borneol on the absorption of geniposide in rat via intranasal administration. J. Zhejiang Univ. Sci. B 2011, 12, 143–148. [Google Scholar]
- Li, F.; Feng, J.; Cheng, Q.; Zhu, W.; Jin, Y. Delivery of 125I-cobrotoxin after intranasal administration to the brain: A microdialysis study in freely moving rats. Int. J. Pharm 2007, 328, 161–167. [Google Scholar]
- Cai, Z.; Hou, S.; Li, Y.; Zhao, B.; Yang, Z.; Xu, S.; Pu, J. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J. Drug Target 2008, 16, 178–184. [Google Scholar]
- Cai, Z.; Hou, S.; Li, Y.; Zhao, B.; Yang, Z.; Xu, S.; Pu, J. Literature analysis of 20 ADR cases induced by XINGNAOJING injection. Mod. Prev. Med 2011, 38, 4991–4994. [Google Scholar]
- Albrecht, J.; Kopietz, R.; Frasnelli, J.; Wiesmann, M.; Hummel, T.; Lundstrom, J.N. The neuronal correlates of intranasal trigeminal function—An ALE meta-analysis of human functional brain imaging data. Brain Res. Rev 2010, 62, 183–196. [Google Scholar]
- Chen, X.; Lu, Y.; Du, S.; Xu, B.; Wang, S.; Zhai, Y.; Song, X.; Li, P. In situ and in vivo study of nasal absorption of paeonol in rats. Int. J. Mol. Sci 2010, 11, 4882–4890. [Google Scholar]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar]
- Du, S.; Zhang, Q.; Lu, Y.; Zhai, Y.; Wu, Q. Study of components in Xingnaojing affecting intestine absorption of gardenia extract. China J. Chin. Mater. Med 2010, 35, 297–300. [Google Scholar]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., II. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci 2010, 99, 1654–1673. [Google Scholar]
| Group | Parameters | ||||
|---|---|---|---|---|---|
| Cmax (μg/mL) | Tmax (min) | AUC0–120 (μg/mL·min) | MRT0–120 (min) | F (%) | |
| i.v. | 42.410 ± 6.268 | - | 324.88 ± 37.62 | 15.01 ± 1.49 | 100 |
| i.n. | 21.881 ± 5.398 ** | 1 | 277.39 ± 22.65 * | 31.70 ± 5.68 ** | 85.38 |
| i.g. | 1.914 ± 0.327 ** | 30 | 93.44 ± 9.71 ** | 42.03 ± 6.63 ** | 28.76 |
| Group | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Cmax (ng/g) | Tmax (min) | AUC0–120 (ng/g·min) | MRTlast (min) | Re (%) | Te (%) | DTI | |
| i.v. | 1476.4 ± 145.1 | 1 | 37270.5 ± 4160.6 | 48.3 ± 2.0 | 100 | 11.47 | 1 |
| i.n. | 746.7 ± 174.8 ** | 3 | 32413.6 ± 4573.9 | 51.2 ± 2.6 | 86.97 | 11.69 | 1.02 |
| i.g. | 76.2 ± 22.1 ** | 60 ** | 6440.1 ± 863.7 ** | 60.0 ± 3.4 ** | 17.28 ** | 6.89 ** | 0.60 ** |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, Y.; Du, S.; Bai, J.; Li, P.; Wen, R.; Zhao, X. Bioavailability and Brain-Targeting of Geniposide in Gardenia-Borneol Co-Compound by Different Administration Routes in Mice. Int. J. Mol. Sci. 2012, 13, 14127-14135. https://doi.org/10.3390/ijms131114127
Lu Y, Du S, Bai J, Li P, Wen R, Zhao X. Bioavailability and Brain-Targeting of Geniposide in Gardenia-Borneol Co-Compound by Different Administration Routes in Mice. International Journal of Molecular Sciences. 2012; 13(11):14127-14135. https://doi.org/10.3390/ijms131114127
Chicago/Turabian StyleLu, Yang, Shouying Du, Jie Bai, Pengyue Li, Ran Wen, and Xuejiao Zhao. 2012. "Bioavailability and Brain-Targeting of Geniposide in Gardenia-Borneol Co-Compound by Different Administration Routes in Mice" International Journal of Molecular Sciences 13, no. 11: 14127-14135. https://doi.org/10.3390/ijms131114127




