Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress
Abstract
:1. Introduction
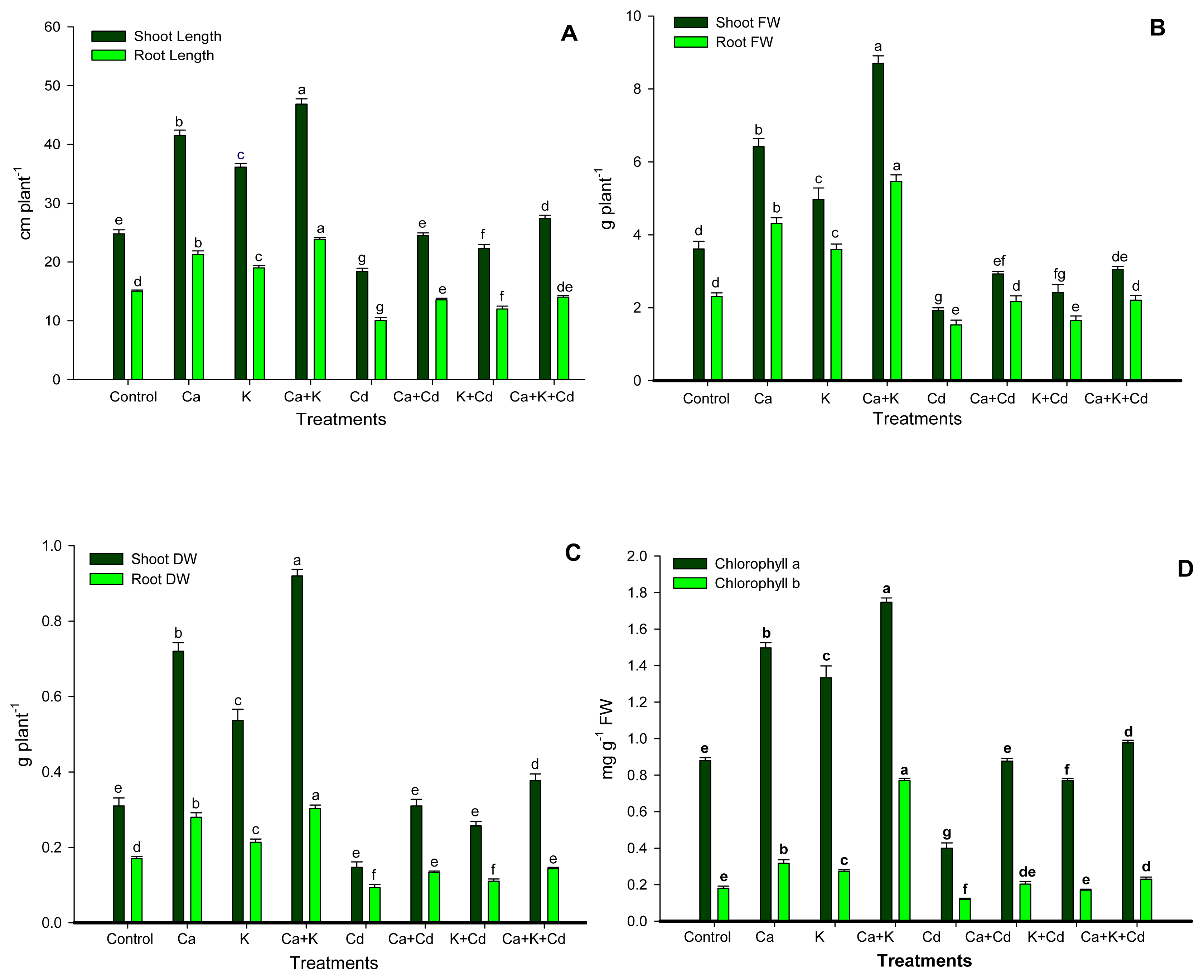
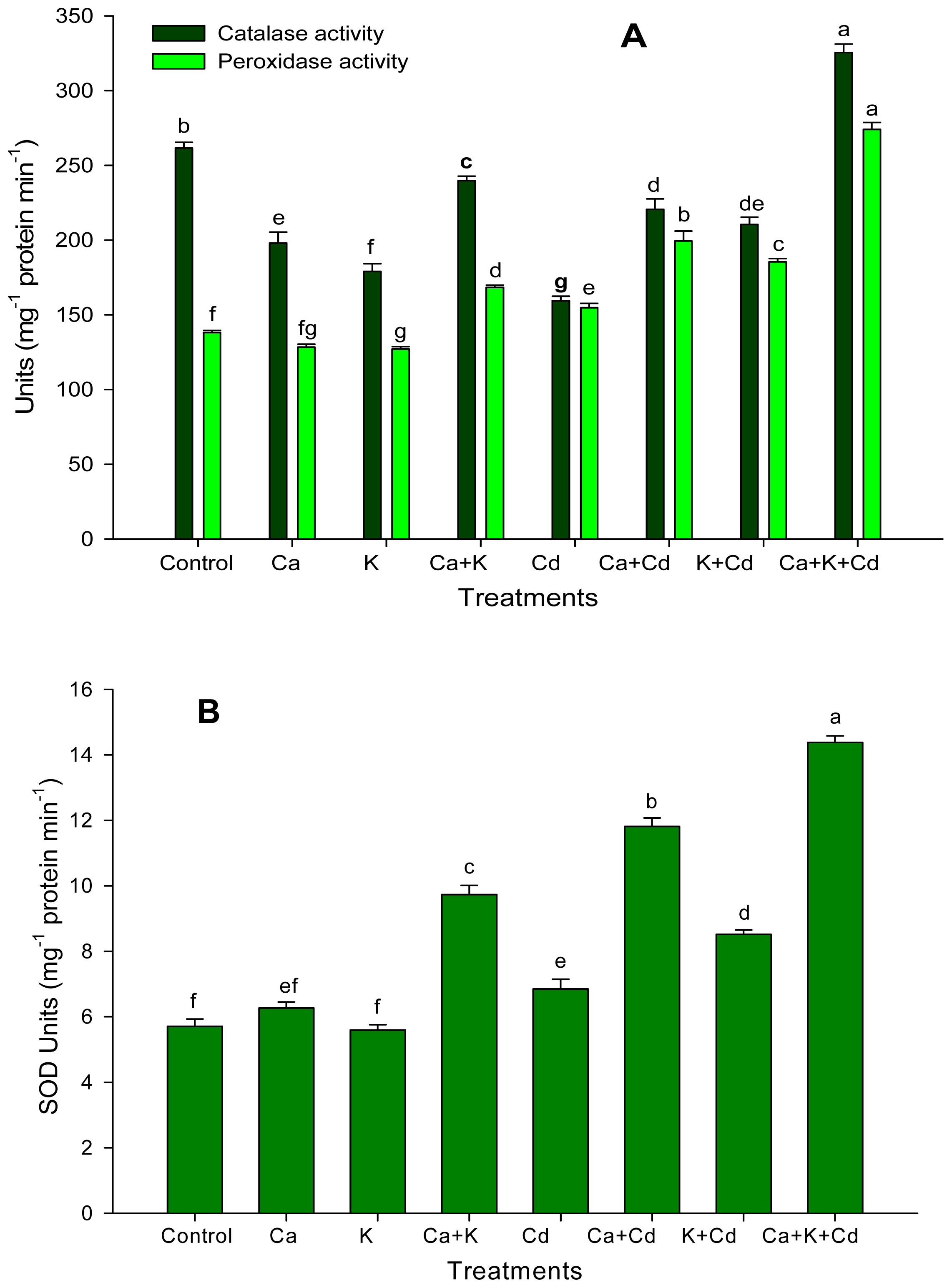
2. Results
3. Discussion
4. Experimental Section
4.1. Plant Cultures and Treatments
4.2. Plant Growth Characteristics
4.3. Physiological and Biochemical Parameters
4.3.1. Chemical Content of Leaves
4.3.2. Enzyme Activity
4.4. Statistical Analysis
5. Conclusions
Acknowledgements
- Conflict of InterestThe authors declare that they have no conflict of interest.
References
- Sanitá di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot 1999, 41, 105–130. [Google Scholar]
- Yang, X.E.; Long, X.X.; Ye, H.B.; He, Z.L.; Calvert, D.V.; Stoffella, P.J. Cadmium tolerance and hyperaccumulation in a new Zn hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 2004, 259, 181–189. [Google Scholar]
- Leita, L.; de Nobili, M.; Cesco, C.; Mondini, C. Analysis of intercellular cadmium forms in roots and leaves of bush bean. J. Plant Nutr 1996, 19, 527–533. [Google Scholar]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Govindarajan, R.; Kuriakose, S.V.; Prasad, M.N.V. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol. Biochem 2006, 44, 25–37. [Google Scholar]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut 1997, 98, 29–36. [Google Scholar]
- Clarkson, D.T.; Luttge, U. Mineral nutrition: Divalent cations, transport and compartmentation. Progr. Bot 1989, 51, 93–112. [Google Scholar]
- Rivetta, A.; Negrini, N.; Cocucci, M. Involvement of Ca2+-calmodulin in Cd2+ toxicity during the early phases of radish (Raphanus sativus L.) seed germination. Plant Cell Environ 1997, 20, 600–608. [Google Scholar]
- Sandalio, L.M.; Dalurzo, H.C.; Gómez, M.; Romero-Puertas, M.C.; del Río, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot 2001, 52, 2115–2126. [Google Scholar]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmiño, D.M.; Testillano, P.S.; Risueño, M.C.; del Río, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 2009, 150, 229–243. [Google Scholar]
- Chen, S.; Sun, L.; Sun, T.; Chao, L.; Guo, G. Interaction between cadmium, lead and potassium fertilizer (K2SO4) in a soil-plant system. Environ. Geochem. Health 2007, 29, 435–446. [Google Scholar]
- Leigh, R.A.; Jones, R.G.W. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant-cell. New Phytol 1984, 97, 1–13. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed; Academic Press: London, UK, 2002. [Google Scholar]
- Mengel, K. Potassium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 91–120. [Google Scholar]
- Bhandal, I.S.; Malik, C.P. Potassium estimation, uptake and its role in the physiology and metabolism of flowering plants. Int. Rev. Cytol 1988, 110, 205–254. [Google Scholar]
- Suzuki, N. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotech 2005, 22, 19–25. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma 2011, 248, 503–511. [Google Scholar]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M.; Khan, M.M.A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant 2010, 32, 121–132. [Google Scholar]
- Antosiewicz, D.M.; Hennig, J. Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environ. Pollut 2004, 129, 237–245. [Google Scholar]
- Jáuregui-Zùñiga, D.; Ferrer, M.A.; Calderón, A.A.; Muñoz, R.; Moreno, A. Heavy metal stress reduces the deposition of calcium oxalate crystals in leaves of Phaseolus vulgaris. J. Plant Physiol 2005, 162, 1183–1187. [Google Scholar]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 2004, 136, 2438–2442. [Google Scholar]
- Plieth, C. Plant calcium signaling and monitoring: Pros and cons and recent experimental approaches. Protoplasma 2001, 218, 1–23. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot 2003, 92, 487–511. [Google Scholar]
- Handley, R.; Metwally, A.; Overstreet, R. Effects of Ca upon metabolic and nonmetabolic uptake of Na and Rb by root segments of Zea mays. Plant Physiol 1965, 40, 513–520. [Google Scholar]
- Elzam, O.E.; Hodges, T.K. Calcium inhibition of potassium absorption in corn roots. Plant Physiol 1967, 42, 1483–1488. [Google Scholar]
- Rains, D.W.; Epstein, E. Sodium absorption by barley roots: Its mediation by mechanism 2 of alkali cation transport. Plant Physiol 1967, 42, 319–323. [Google Scholar]
- Van Steveninck, R.F.M. The significance of calcium on the apparent permeability of cell membranes and the effects of substitution with other divalent ions. Physiol. Plant 1965, 18, 54–69. [Google Scholar]
- Epstein, E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol 1961, 36, 437–444. [Google Scholar]
- Jakobsen, S.T. Interaction between plant Nutrients: III. Antagonism between potassium, magnesium and calcium. Acta Agric. Scand. Sect. B Soil Plant 1993, 43, 1–5. [Google Scholar]
- Siddiqui, M.H.; Khan, M.N.; Mohammad, F.; Khan, M.M.A. Role of nitrogen and gibberellins (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci 2008, 194, 214–224. [Google Scholar]
- Malvi, U.R. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci 2011, 24, 106–109. [Google Scholar]
- Oliver, D.P.; Schultz, J.E.; Tiller, K.G.; Merry, R.H. The effect of crop rotations and tillage practices on cadmium concentration in wheat grain. Agric. Res 1993, 44, 1221–1234. [Google Scholar]
- Nie, J.H.; Liu, X.M.; Wang, Q.R. Effects of nutrient elements on the lead uptake by hyperaccumulators. Ecol. Environ 2004, 13, 306–309. [Google Scholar]
- Tu, C.; Zheng, C.R.; Chen, H.M. Effect of applying chemical fertilizers on forms of lead and cadmium in red soil. Chemosphere 2000, 41, 133–138. [Google Scholar]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.M.A.; Al-Whaibi, M.H. Cumulative effect of nitrogen and sulphur on Brassica juncea L. genotypes under NaCl stress. Protoplasma 2012, 249, 139–153. [Google Scholar]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J 2002, 32, 539–548. [Google Scholar]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M. Cadmium toxicity in plants. Braz. J. Plant Physiol 2005, 17, 21–34. [Google Scholar]
- Dong, J.; Wu, F.; Zhang, G. Effect of cadmium on growth and photosynthesis of tomato seedlings. Zhejiang Univ. Sci. B 2005, 6, 974–980. [Google Scholar]
- Kurtyka, R.; Małkowski, E.; Kita, A.; Karcz, W. Effect of calcium and cadmium on growth and accumulation of cadmium, calcium, potassium and sodium in maize seedlings. Polish J. Environ. Stud 2008, 17, 51–56. [Google Scholar]
- Parmelee, J.T.; Beebe, D.C. Decreased membrane permeability to potassium is responsible for the cell volume increase that drives lens fiber cell elongation. J. Cell Physiol 1988, 134, 491–496. [Google Scholar]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci 2005, 168, 521–530. [Google Scholar]
- Vassilev, A.; Iordanov, I.; Chakalova, E.; Kerin, V. Effect of cadmium stress on growth and photosynthesis of young barley (H. vulgare L.) plants. 2. Structural and functional changes in the photosynthetic apparatus. Bujg. J. Plant Physiol 1995, 21, 12–21. [Google Scholar]
- Mohanty, N.; Vass, I.; Demeter, S. Impairment of photosystem II activity at the level of secondary quinine acceptor in chloroplasts treated with cobalt, nickel and zinc ions. Physiol. Plant 1989, 76, 386–390. [Google Scholar]
- Shalygo, N.V.; Kolensikova, N.V.; Voronetskaya, V.V.; Averina, N.G. Effects of Mn2+, Fe2+, Co2+ and Ni2+ on chlorophyll accumulation and early stages of chlorophyll formation of greening barley seedling. Russ. J. Plant Physiol 1999, 46, 496–501. [Google Scholar]
- Pandey, S.; Gupta, K.; Mukherjee, A.K. Impact of cadmium and lead on Catharanthus roseus—A phytoremediation study. J. Environ. Biol 2007, 28, 655–662. [Google Scholar]
- Lechowski, Z.; Bialczyk, J. Calcium mediated cytokinin action on chlorophyll synthesis in isolated embryo of Scots pine. Biol. Plant 1993, 35, 53–62. [Google Scholar]
- Tanaka, A.; Tsuji, H. Effects of calcium on chlorophyll synthesis and stability in the early phase of greening in cucumber cotyledons. Plant Physiol 1980, 65, 1211–1215. [Google Scholar]
- Kórzyńska-Polit, E.; Drążkiewicz, M.; Krupa, Z. Lipid peroxidation and antioxidative response in Arabidopsis thaliana exposed to cadmium and copper. Acta Physiol. Plant 2010, 32, 169–175. [Google Scholar]
- Liu, Z.; Chen, W.; He, X. Cadmium-induced changes in growth and antioxidative mechanisms of a medicine plant (Lonicera japonica Thunb.). J. Med. Plants Res 2011, 5, 1411–1417. [Google Scholar]
- McAinsh, M.R.; Clayton, H.; Mansfield, T.A.; Hetherington, A.M. Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 1996, 111, 1031–1042. [Google Scholar]
- Umar, S.; Diva, I.; Anjum, N.A.; Iqbal, M. Potassium nutrition reduces cadmium accumulation and oxidative burst in mustard (Brassica campestris L.). Electron. Int. Fertil. Corresp 2008, 16, 6–10. [Google Scholar]
- Choi, Y.E.; Harada, E.; Wada, M.; Tsuboi, H.; Morita, Y.; Kusano, T.; Sano, H. Detoxification of cadmium in tobacco plants: Formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 2001, 213, 45–50. [Google Scholar]
- Matysik, J.; Alia, B.B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci 2002, 82, 525–532. [Google Scholar]
- Zhao, Y. Cadmium accumulation and antioxidative defenses in leaves of Triticum aestivum L. and Zea mays L. African J. Biotech 2011, 10, 2936–2943. [Google Scholar]
- Dinakar, N.; Nagajyothi, P.C.; Suresh, S.; Udaykiran, Y.; Damodharam, T. Phytotoxicity of cadmium on protein, proline and antioxidant enzyme activities in growing Arachis hypogaea L. seedlings. J. Environ. Sci 2008, 20, 199–206. [Google Scholar]
- Bandurska, H. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiol. Plant 2001, 23, 483–490. [Google Scholar]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar]
- Jones, R.G.; Pollard, A. Proteins, Enzymes and Inorganic Ions. In Inorganic Plant Nutrition; Läuchli, A., Bieleski, R.L., Eds.; Springer: New York, NY, USA, 1983; pp. 528–562. [Google Scholar]
- Tripathi, B.N.; Bhatt, I.; Dietz, K.J. Peroxiredoxins: A less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 2009, 235, 3–15. [Google Scholar]
- Yang, T.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci 2003, 8, 505–512. [Google Scholar]
- Park, H.Y.; Kim, S.A.; Korlach, J.; Rhoades, E.; Kwok, L.W.; Zipfel, W.R.; Waxham, M.N.; Webb, W.W.; Pollack, L. Conformational changes of calmodulin upon Ca2+ binding studied with a microfluidic mixer. Proc. Nat. Acad. Sci. USA 2008, 105, 542–547. [Google Scholar]
- DalCorso, G.; Farinati, S.; Furini, A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav 2010, 5, 663–667. [Google Scholar]
- Smith, G.S.; Johnston, C.M.; Cornforth, I.S. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol 1983, 94, 537–548. [Google Scholar]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot 1992, 32, 85–100. [Google Scholar]
- Zheljazkov, V.D.; Nielson, N.E. Effect of heavy metals on peppermint and cornmint. Plant Soil 1996, 178, 59–66. [Google Scholar]
- Hseu, Z.Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol 2004, 95, 53–59. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys 1968, 125, 189–198. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol 1955, 2, 764–775. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol 1984, 105, 121–126. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 1977, 59, 309–314. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Basalah, M.O.; Ali, H.M. Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress. Int. J. Mol. Sci. 2012, 13, 6604-6619. https://doi.org/10.3390/ijms13066604
Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM. Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress. International Journal of Molecular Sciences. 2012; 13(6):6604-6619. https://doi.org/10.3390/ijms13066604
Chicago/Turabian StyleSiddiqui, Manzer H., Mohamed H. Al-Whaibi, Ahmed M. Sakran, Mohammed O. Basalah, and Hayssam M. Ali. 2012. "Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress" International Journal of Molecular Sciences 13, no. 6: 6604-6619. https://doi.org/10.3390/ijms13066604
APA StyleSiddiqui, M. H., Al-Whaibi, M. H., Sakran, A. M., Basalah, M. O., & Ali, H. M. (2012). Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress. International Journal of Molecular Sciences, 13(6), 6604-6619. https://doi.org/10.3390/ijms13066604




