Oxidative Stress/Angiotensinogen/Renin-Angiotensin System Axis in Patients with Diabetic Nephropathy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Subjects’ Profiles and Laboratory Data
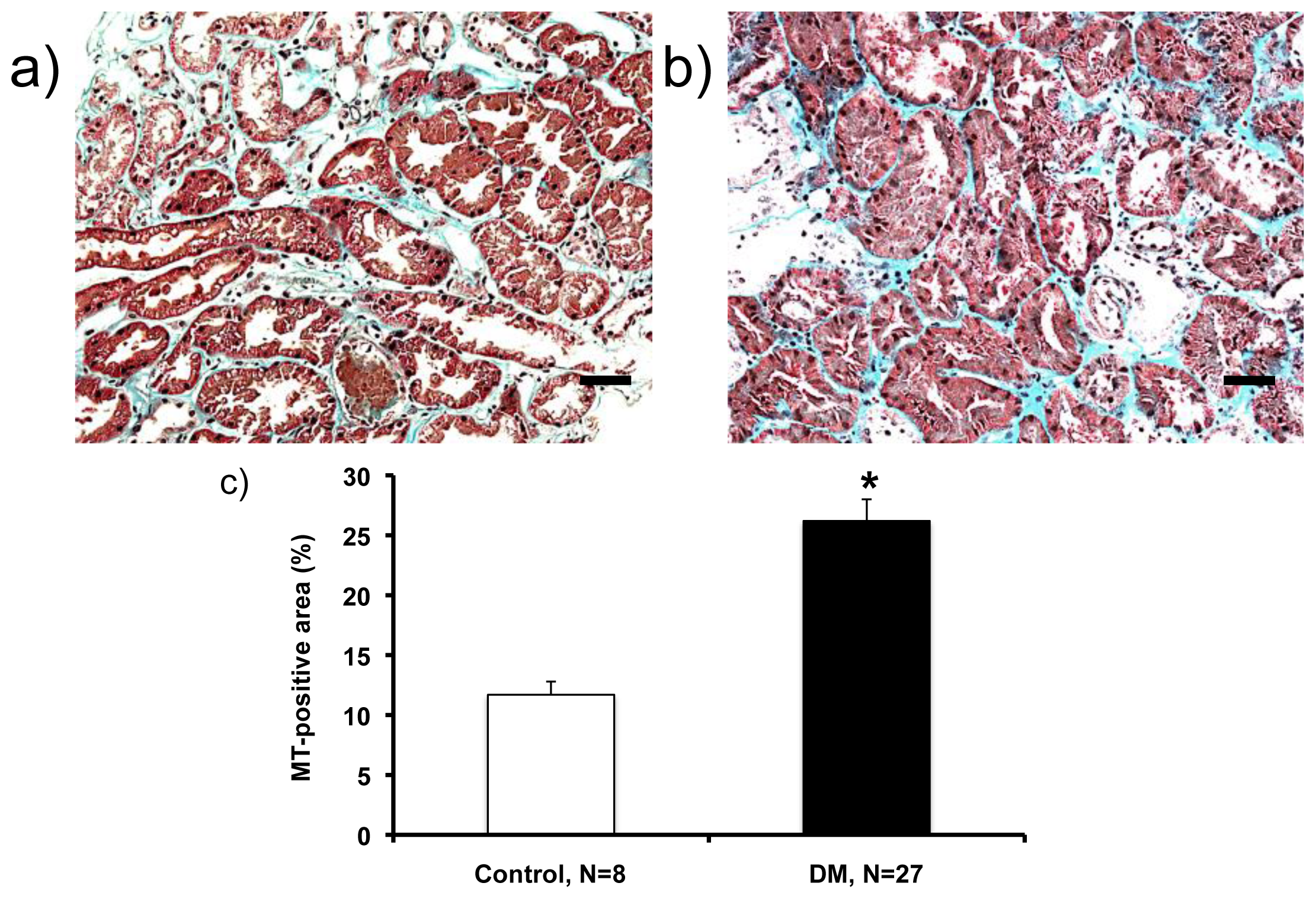
2.2. Histological Analysis (Masson’s Trichrome (MT)-Staining)
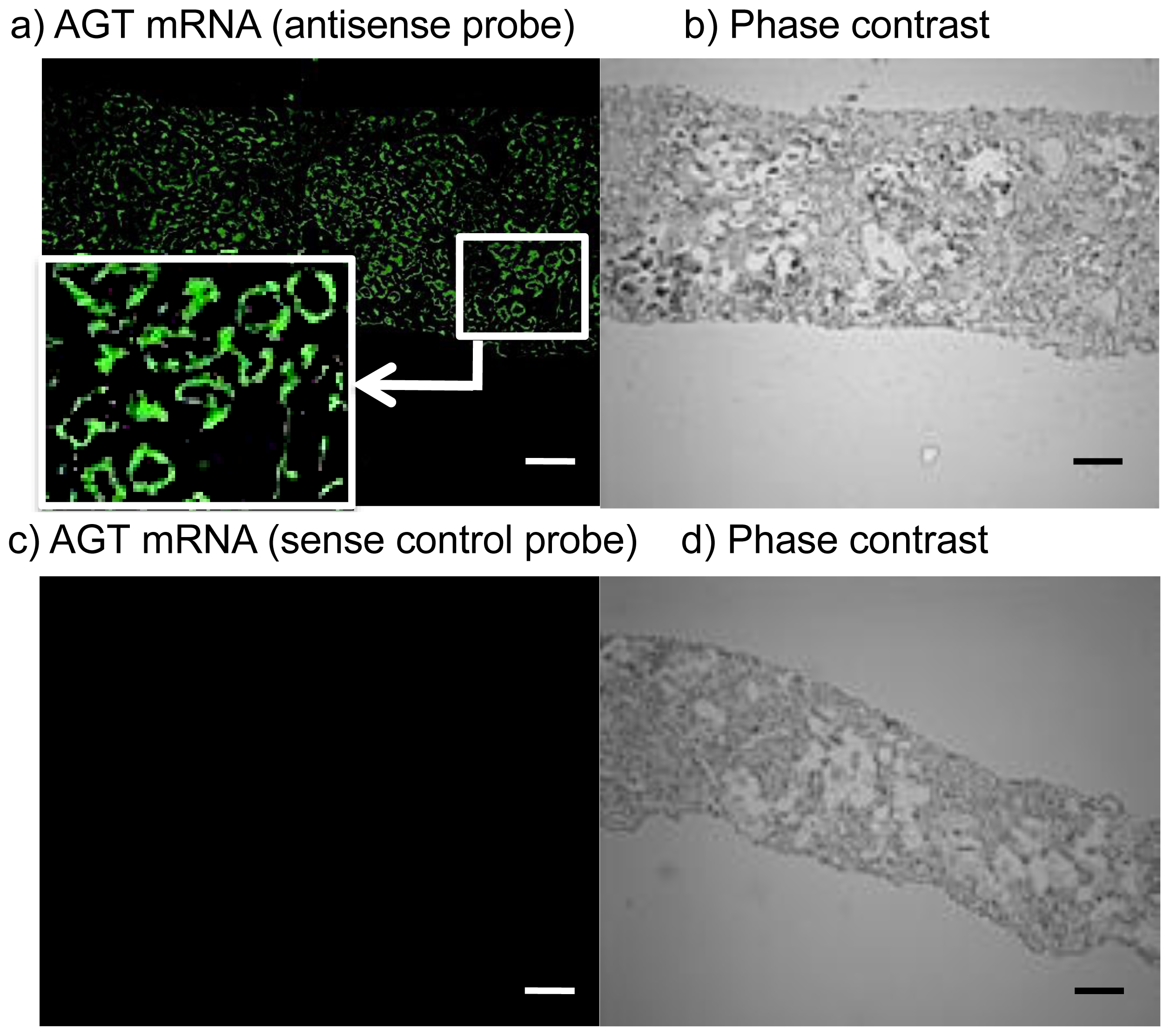
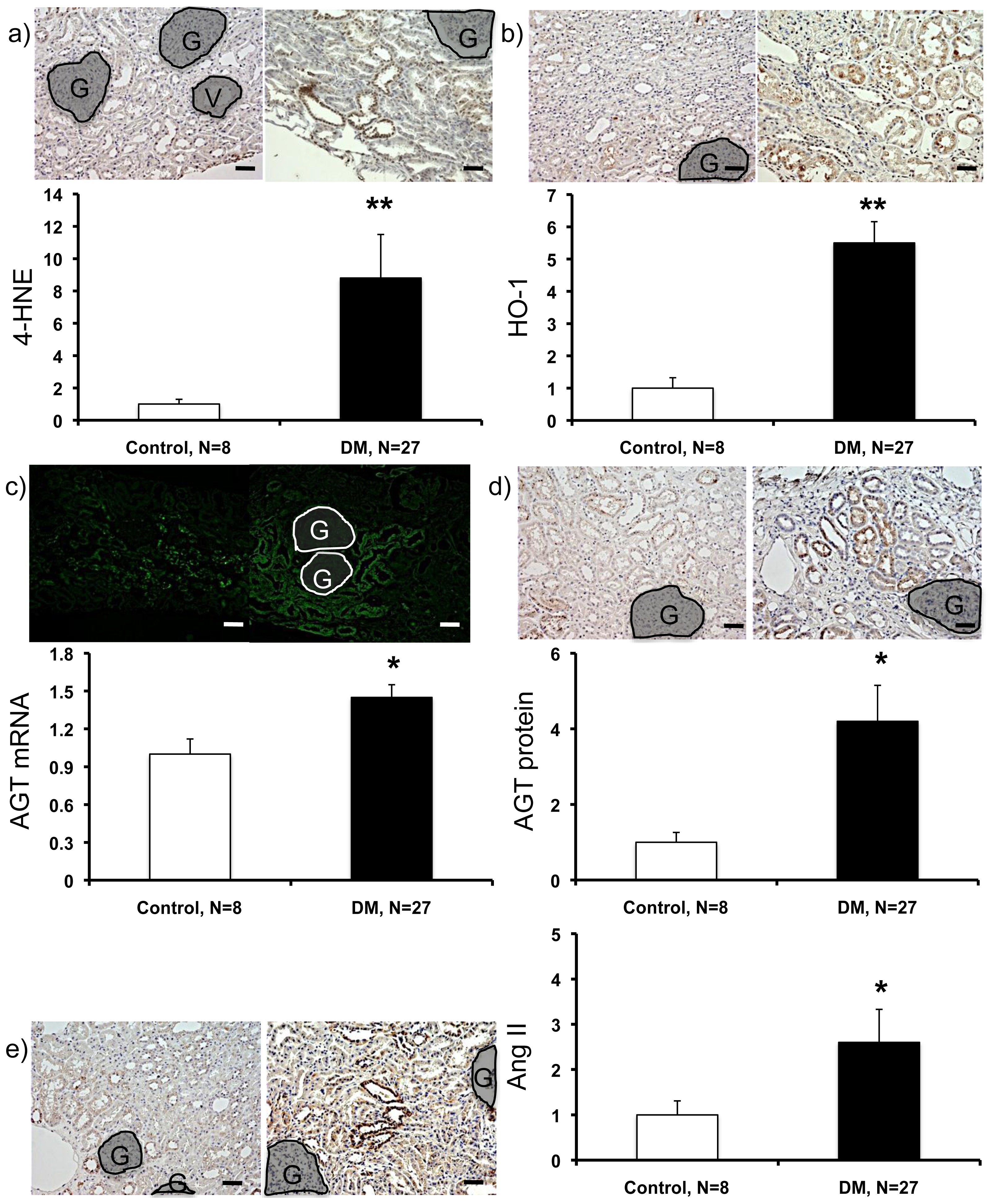
2.3. Expression Levels of ROS- and RAS-Related Factors in the Kidneys of Control Subjects and Patients with Diabetes
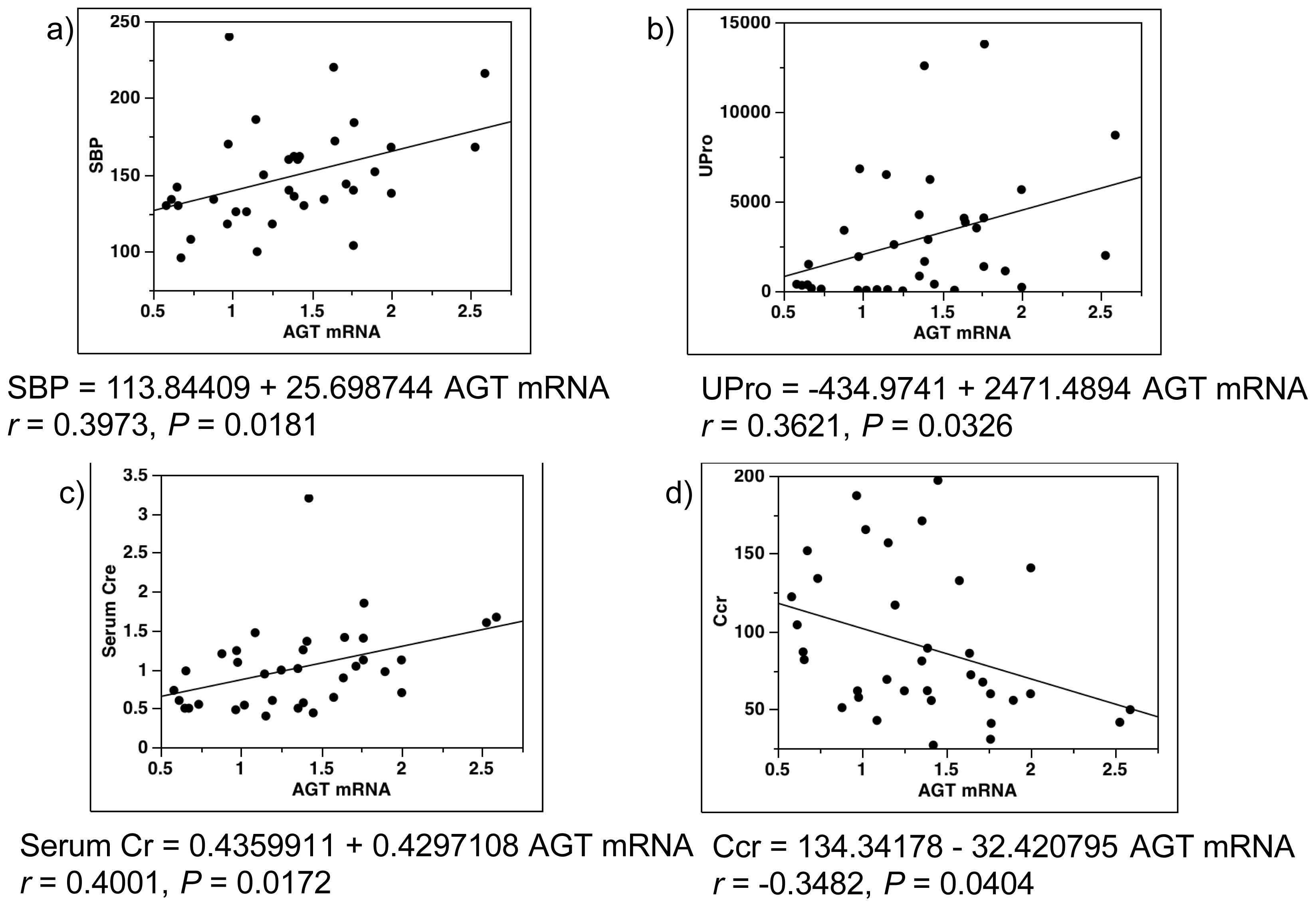
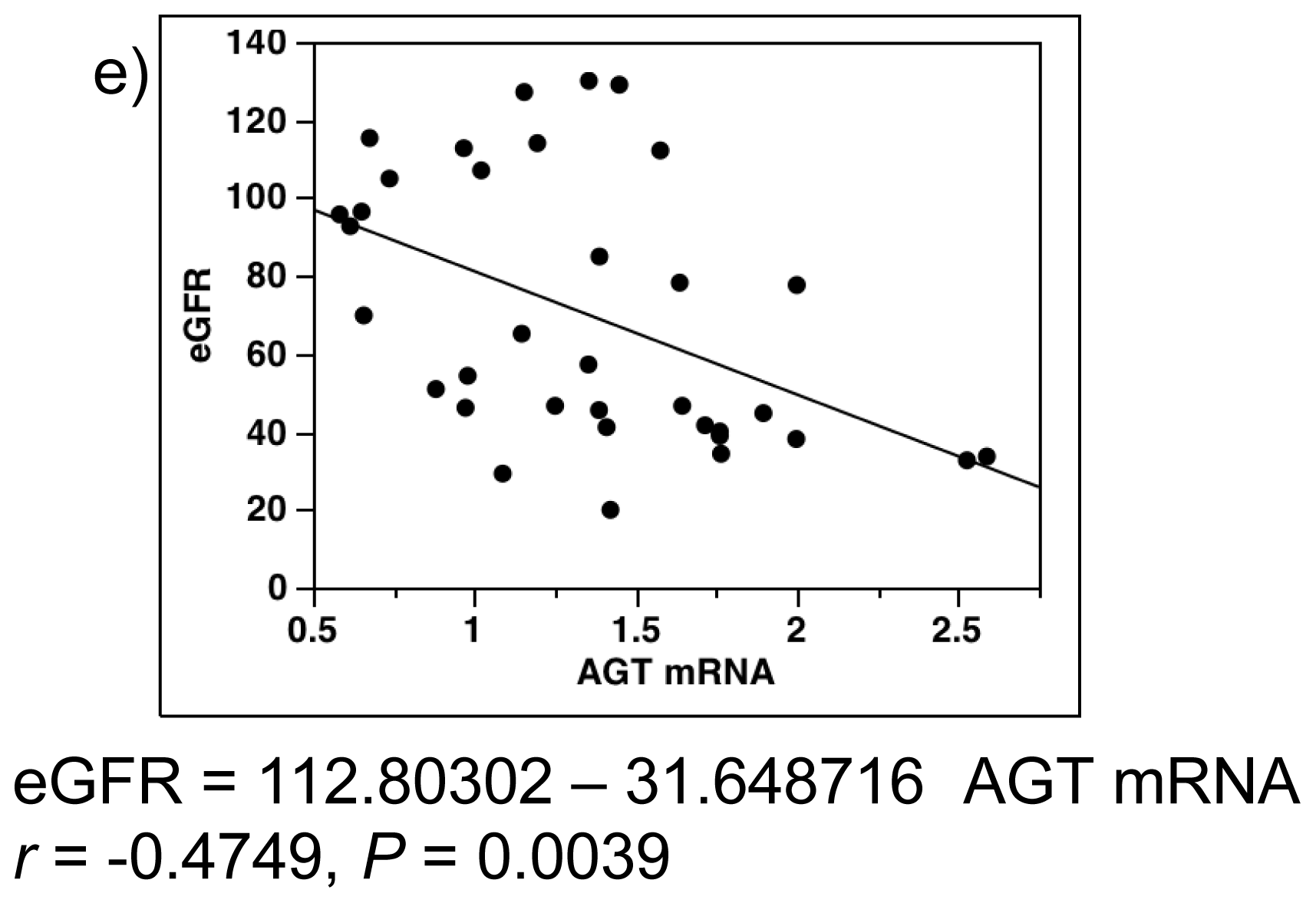
2.4. Single-Regression Analysis for AGT mRNA Levels with Clinical Parameters in All Subjects (Control and Diabetes)
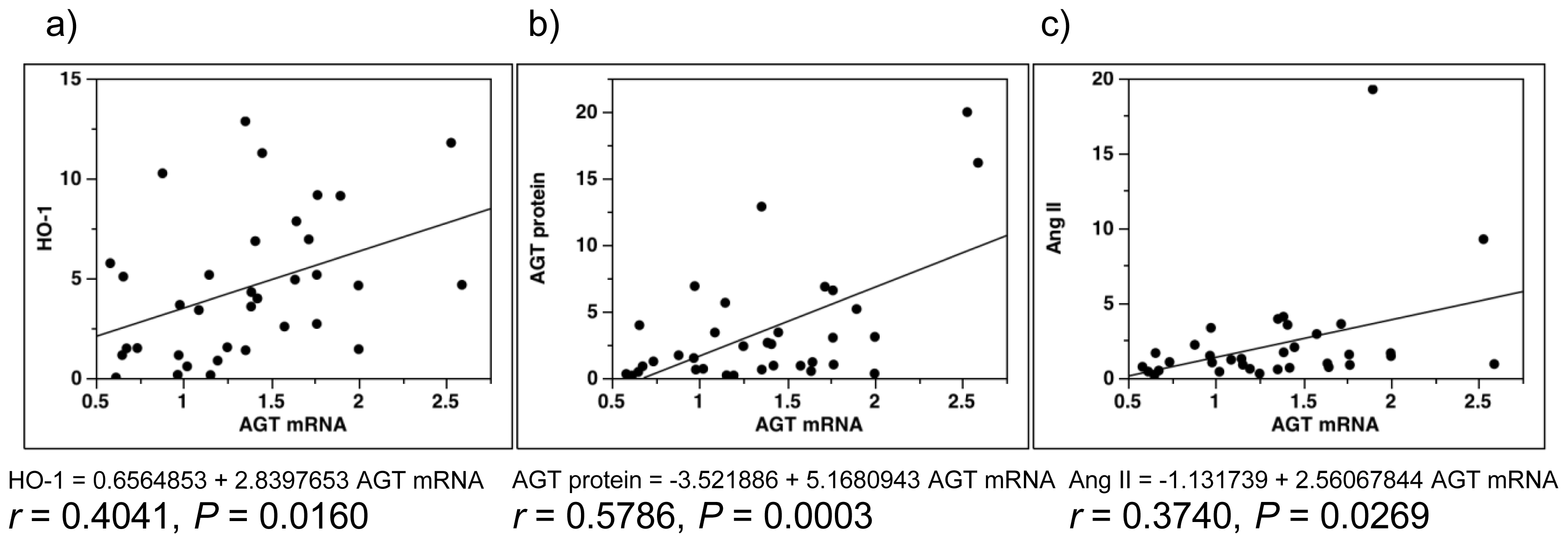
2.5. Single-Regression Analysis for AGT mRNA Levels with the Expression Level of ROS- and RAS-Related Factors in All Subjects
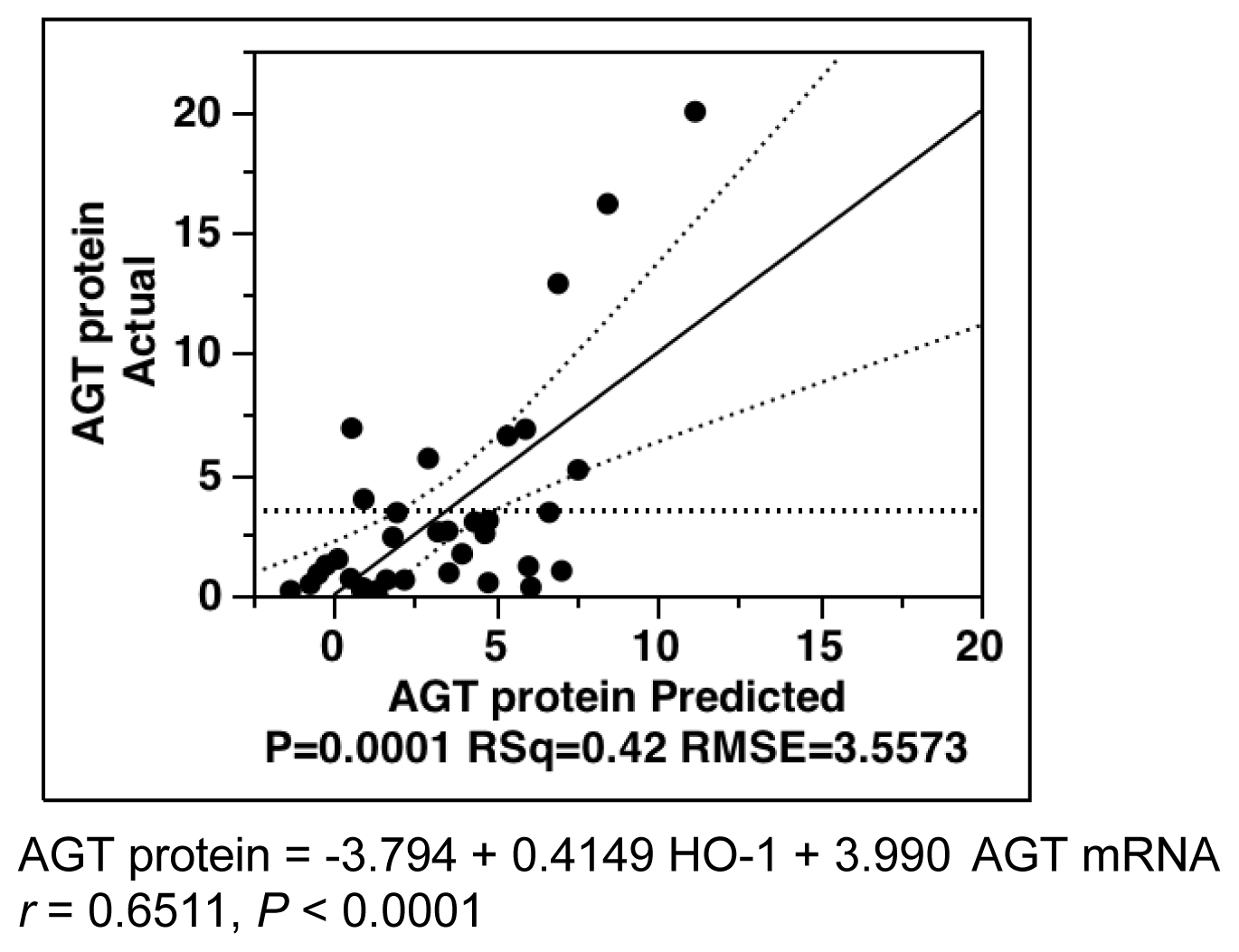
2.6. Multiple-Regression Analysis for AGT Protein Levels in All Subjects
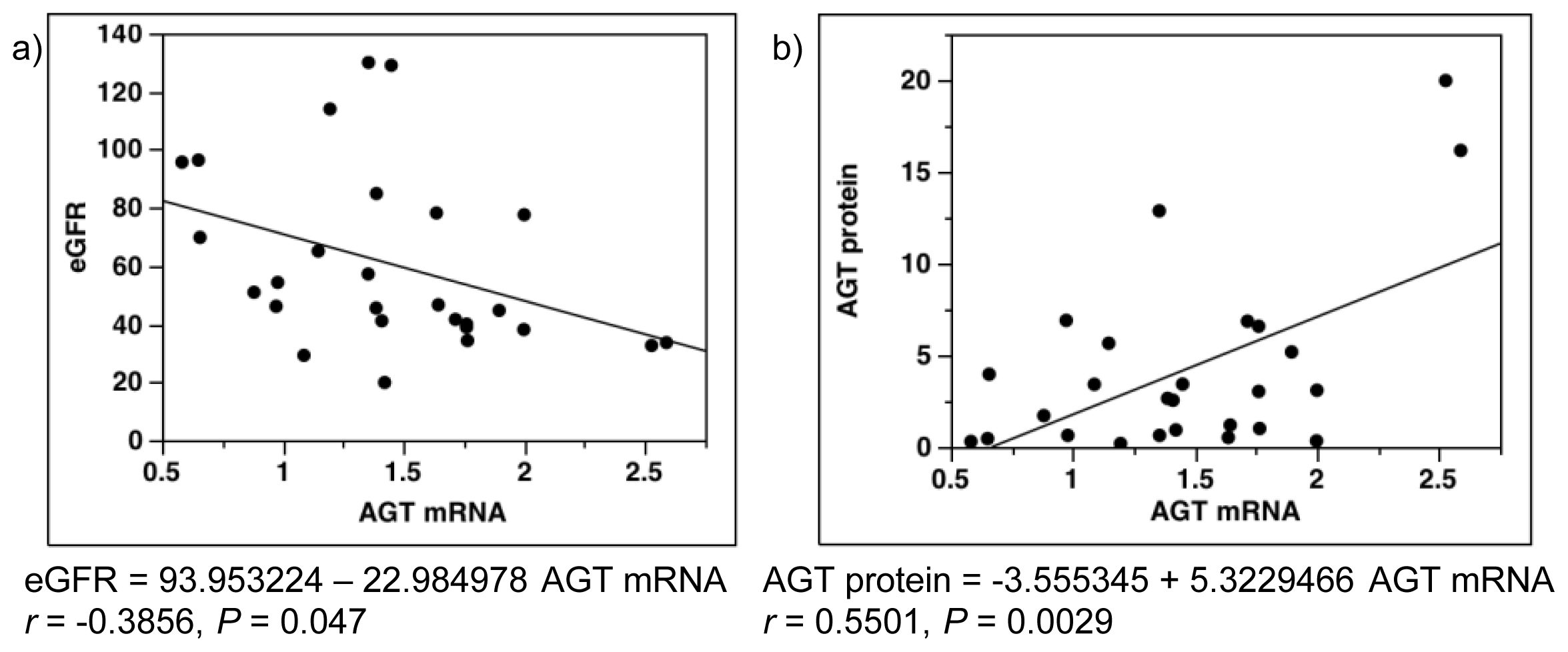
2.7. Single-Regression Analysis for AGT mRNA Levels with eGFR and AGT Protein in Patients with Diabetes
2.8. Comparison of AGT Expression in Patients with Diabetic Nephropathy Receiving Diabetes Medications (Insulin or Sulfonylurea (SU)) with Those Who Did Not Receive Such Medications
2.9. Discussion
3. Experimental Section
3.1. Study Design and Sample Collections
3.1.1. Control Subjects
3.1.2. Patients with Diabetic Nephropathy
3.2. Measurements
3.3. FISH
3.4. Immunohistochemistry (IHC)
3.5. Histological Analysis
3.6. Statistical Analysis
4. Conclusions
| Parameters | Control subjects (N = 8) | Patients with type 2 diabetes (N = 27) | p-Value | χ2 |
|---|---|---|---|---|
| Gender, W/M | 7/1 | 9/18* | 0.0069 | 7.296 |
| Age, y | 37.0 ± 3.3 | 54.6 ± 2.7* | 0.0020 | - |
| Height, cm | 161.1 ± 2.9 | 161.2 ± 1.8 | 0.9885 | - |
| BW, kg | 51.5 ± 5.0 | 65.4 ± 2.2* | 0.0071 | - |
| BMI | 19.6 ± 1.1 | 25.2 ± 0.8* | 0.0013 | - |
| SBP, mmHg | 116.8 ± 5.1 | 157.9 ± 6.0* | 0.0010 | - |
| DBP, mmHg | 69.3 ± 3.1 | 88.5 ± 2.7* | 0.0010 | - |
| Parameters | Control subjects (N = 8) | Patients with type 2 diabetes (N = 27) | p-Value |
|---|---|---|---|
| BG, mg/dL | 91.7 ± 6.2 | 168.1 ± 11.7 * | 0.0027 |
| HbA1c, % | (N/A) | 7.5 ± 0.4 | (N/A) |
| T-Cho, mg/dL | 195.0 ± 19.0 | 237.0 ± 12.9 | 0.1141 |
| TG, mg/dL | 136.8 ± 52.7 | 243.9 ± 27.4 | 0.0732 |
| HDL, mg/dL | 68.7 ± 6.9 | 51.4 ± 2.2 * | 0.0036 |
| LDL, mg/dL | 104.6 ± 14.3 | 135.4 ± 11.4 | 0.2014 |
| UNA, g/day | 2.3 ± 0.2 | 2.5 ± 0.2 | 0.4555 |
| UCl, g/day | 3.2 ± 0.4 | 3.3 ± 0.3 | 0.8884 |
| UNaCl, g/day | 5.5 ± 0.6 | 5.8 ± 0.5 | 0.6877 |
| UPro, mg/day | 105.6 ± 34.2 | 3727.1 ± 685.1 * | 0.0075 |
| Serum Cr, mg/dL | 0.6 ± 0.1 | 1.1 ± 0.1 * | 0.0095 |
| Ccr, mL/min/1.73 m2 | 136.7 ± 13.8 | 76.9 ± 7.9 * | 0.0009 |
| eGFR, mL/min/1.73 m2 | 102.3 ± 8.7 | 60.6 ± 5.9 * | 0.0012 |
| Parameters | Estimate | SE | T Ratio | p Value |
|---|---|---|---|---|
| Intercept | −3.7943 | 1.7293 | −2.19 | 0.0356 * |
| 4-HNE | −0.0519 | 0.0496 | −1.05 | 0.3029 |
| HO-1 | 0.4149 | 0.1865 | 2.23 | 0.0332 * |
| AGT mRNA | 3.9899 | 1.3102 | 3.05 | 0.0046 * |
Acknowledgments
Conflicts of Interest
References
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med 1993, 329, 977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853.
- Anderson, S.; Jung, F.F.; Ingelfinger, J.R. Renal renin-angiotensin system in diabetes: Functional, immunohistochemical, and molecular biological correlations. Am. J. Physiol 1993, 265, F477–F486. [Google Scholar]
- Yamamoto, T.; Nakamura, T.; Noble, N.A.; Ruoslahti, E.; Border, W.A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1993, 90, 1814–1818. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar]
- Kobori, H.; Harrison-Bernard, L.M.; Navar, L.G. Role of Activated Renin-Angiotensin System in the Pathogenesis of Diabetic Nephropathy. In Advances in Pathogenesis of Diabetic Nephropathy; Prabhakar, S.S., Ed.; Nova Science Publishers, Inc: New York, NY, USA, 2012; pp. 161–198. [Google Scholar]
- Nagai, Y.; Yao, L.; Kobori, H.; Miyata, K.; Ozawa, Y.; Miyatake, A.; Yukimura, T.; Shokoji, T.; Kimura, S.; Kiyomoto, H.; et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J. Am. Soc. Nephrol 2005, 16, 703–711. [Google Scholar]
- Kobori, H.; Kamiyama, M.; Harrison-Bernard, L.M.; Navar, L.G. Cardinal role of the intrarenal Renin-Angiotensin system in the pathogenesis of diabetic nephropathy. J. Investig. Med 2013, 61, 256–264. [Google Scholar]
- Taguma, Y.; Kitamoto, Y.; Futaki, G.; Ueda, H.; Monma, H.; Ishizaki, M.; Takahashi, H.; Sekino, H.; Sasaki, Y. Effect of captopril on heavy proteinuria in azotemic diabetics. N. Engl. J. Med 1985, 313, 1617–1620. [Google Scholar]
- Mann, J.F.; Schmieder, R.E.; McQueen, M.; Dyal, L.; Schumacher, H.; Pogue, J.; Wang, X.; Maggioni, A.; Budaj, A.; Chaithiraphan, S.; et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 2008, 372, 547–553. [Google Scholar]
- Haller, H.; Ito, S.; Izzo, J.L., Jr.; Januszewicz, A.; Katayama, S.; Menne, J.; Mimran, A.; Rabelink, T.J.; Ritz, E.; Ruilope, L.M.; et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med 2011, 364, 907–917. [Google Scholar]
- Kobori, H.; Mori, H.; Masaki, T.; Nishiyama, A. Angiotensin II blockade and renal protection. Curr. Pharm. Des 2013, 19, 3033–3042. [Google Scholar]
- Navar, L.G.; Harrison-Bernard, L.M.; Nishiyama, A.; Kobori, H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 2002, 39, 316–322. [Google Scholar]
- Gould, A.B.; Green, D. Kinetics of the human renin and human substrate reaction. Cardiovasc. Res 1971, 5, 86–89. [Google Scholar]
- Brasier, A.R.; Li, J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension 1996, 27, 465–475. [Google Scholar]
- Kobori, H.; Harrison-Bernard, L.M.; Navar, L.G. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J. Am. Soc. Nephrol 2001, 12, 431–439. [Google Scholar]
- Kamiyama, M.; Garner, M.K.; Farragut, K.M.; Kobori, H. The establishment of a primary culture system of proximal tubule segments using specific markers from normal mouse kidneys. Int. J. Mol. Sci 2012, 13, 5098–5111. [Google Scholar]
- Kamiyama, M.; Farragut, K.M.; Garner, M.K.; Navar, L.G.; Kobori, H. Divergent localization of angiotensinogen mRNA and protein in proximal tubule segments of normal rat kidney. J. Hypertens 2012, 30, 2365–2372. [Google Scholar]
- Ohashi, N.; Urushihara, M.; Satou, R.; Kobori, H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species—ERK/JNK pathways. Hypertens. Res 2010, 33, 1174–1181. [Google Scholar]
- Zhang, S.L.; Filep, J.G.; Hohman, T.C.; Tang, S.S.; Ingelfinger, J.R.; Chan, J.S. Molecular mechanisms of glucose action on angiotensinogen gene expression in rat proximal tubular cells. Kidney Int 1999, 55, 454–464. [Google Scholar]
- Vidotti, D.B.; Casarini, D.E.; Cristovam, P.C.; Leite, C.A.; Schor, N.; Boim, M.A. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am. J. Physiol. Ren. Physiol 2004, 286, F1039–F1045. [Google Scholar]
- Singh, R.; Singh, A.K.; Alavi, N.; Leehey, D.J. Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J. Am. Soc. Nephrol 2003, 14, 873–880. [Google Scholar]
- Kamiyama, M.; Zsombok, A.; Kobori, H. Urinary angiotensinogen as a novel early biomarker of intrarenal Renin-Angiotensin system activation in experimental type 1 diabetes. J. Pharmacol. Sci 2012, 119, 314–323. [Google Scholar]
- Miyata, K.; Ohashi, N.; Suzaki, Y.; Katsurada, A.; Kobori, H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol 2008, 35, 922–927. [Google Scholar]
- Suzaki, Y.; Ozawa, Y.; Kobori, H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int. J. Biol. Sci 2007, 3, 40–46. [Google Scholar]
- Lai, K.N.; Leung, J.C.; Lai, K.B.; To, W.Y.; Yeung, V.T.; Lai, F.M. Gene expression of the renin-angiotensin system in human kidney. J. Hypertens 1998, 16, 91–102. [Google Scholar]
- Kobori, H.; Ohashi, N.; Katsurada, A.; Miyata, K.; Satou, R.; Saito, T.; Yamamoto, T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J. Am. Soc. Hypertens 2008, 2, 349–354. [Google Scholar]
- Saito, T.; Urushihara, M.; Kotani, Y.; Kagami, S.; Kobori, H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am. J. Med. Sci 2009, 338, 478–480. [Google Scholar]
- Ogawa, S.; Kobori, H.; Ohashi, N.; Urushihara, M.; Nishiyama, A.; Mori, T.; Ishizuka, T.; Nako, K.; Ito, S. Angiotensin II type 1 receptor blockers reduce urinary angiotensinogen excretion and the levels of urinary markers of oxidative stress and inflammation in patients with type 2 diabetic nephropathy. Biomark Insights 2009, 4, 97–102. [Google Scholar]
- Park, S.; Bivona, B.J.; Kobori, H.; Seth, D.M.; Chappell, M.C.; Lazartigues, E.; Harrison-Bernard, L.M. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am. J. Physiol. Ren. Physiol 2010, 298, F37–F48. [Google Scholar]
- Kobori, H.; Katsurada, A.; Miyata, K.; Ohashi, N.; Satou, R.; Saito, T.; Hagiwara, Y.; Miyashita, K.; Navar, L.G. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am. J. Physiol. Ren. Physiol 2008, 294, F1257–F1263. [Google Scholar]
- Nakano, D.; Kobori, H.; Burford, J.L.; Gevorgyan, H.; Seidel, S.; Hitomi, H.; Nishiyama, A.; Peti-Peterdi, J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J. Am. Soc. Nephrol 2012, 23, 1847–1856. [Google Scholar]
- Hsieh, T.J.; Zhang, S.L.; Filep, J.G.; Tang, S.S.; Ingelfinger, J.R.; Chan, J.S. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology 2002, 143, 2975–2985. [Google Scholar]
- Brezniceanu, M.L.; Liu, F.; Wei, C.C.; Tran, S.; Sachetelli, S.; Zhang, S.L.; Guo, D.F.; Filep, J.G.; Ingelfinger, J.R.; Chan, J.S. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 2007, 71, 912–923. [Google Scholar]
- Konoshita, T.; Wakahara, S.; Mizuno, S.; Motomura, M.; Aoyama, C.; Makino, Y.; Kawai, Y.; Kato, N.; Koni, I.; Miyamori, I.; et al. Tissue gene expression of renin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care 2006, 29, 848–852. [Google Scholar]
- Min, T.Z.; Stephens, M.W.; Kumar, P.; Chudleigh, R.A. Renal complications of diabetes. Br. Med. Bull 2012, 104, 113–127. [Google Scholar]
- Joss, N.; Paterson, K.R.; Deighan, C.J.; Simpson, K.; Boulton-Jones, J.M. Diabetic nephropathy: How effective is treatment in clinical practice? QJM 2002, 95, 41–49. [Google Scholar]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar]
- Sofue, T.; Kiyomoto, H.; Kobori, H.; Urushihara, M.; Nishijima, Y.; Kaifu, K.; Hara, T.; Matsumoto, S.; Ichimura, A.; Ohsaki, H.; et al. Early treatment with olmesartan prevents juxtamedullary glomerular podocyte injury and the onset of microalbuminuria in type 2 diabetic rats. Am. J. Hypertens 2012, 25, 604–611. [Google Scholar]
- Yamamoto, T.; Nakagawa, T.; Suzuki, H.; Ohashi, N.; Fukasawa, H.; Fujigaki, Y.; Kato, A.; Nakamura, Y.; Suzuki, F.; Hishida, A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J. Am. Soc. Nephrol 2007, 18, 1558–1565. [Google Scholar]
- Sawaguchi, M.; Araki, S.I.; Kobori, H.; Urushihara, M.; Haneda, M.; Kashiwagi, A.; Uzu, T.; Maegawa, H. Association between urinary angiotensinogen levels and renal and cardiovascular prognoses in patients with type 2 diabetes mellitus. J. Diabetes Investig 2012, 3, 318–324. [Google Scholar]
- Burns, K.D.; Hiremath, S. Urinary angiotensinogen as a biomarker of chronic kidney disease: Ready for prime time? Nephrol. Dial. Transplant 2012, 27, 3010–3013. [Google Scholar]
- Hsieh, T.J.; Fustier, P.; Wei, C.C.; Zhang, S.L.; Filep, J.G.; Tang, S.S.; Ingelfinger, J.R.; Fantus, I.G.; Hamet, P.; Chan, J.S. Reactive oxygen species blockade and action of insulin on expression of angiotensinogen gene in proximal tubular cells. J. Endocrinol 2004, 183, 535–550. [Google Scholar]
- Chen, X.; Zhang, S.L.; Pang, L.; Filep, J.G.; Tang, S.S.; Ingelfinger, J.R.; Chan, J.S. Characterization of a putative insulin-responsive element and its binding protein(s) in rat angiotensinogen gene promoter: Regulation by glucose and insulin. Endocrinology 2001, 142, 2577–2585. [Google Scholar]
- Kobori, H.; Nishiyama, A.; Abe, Y.; Navar, L.G. Enhancement of intrarenal angiotensinogen in dahl salt-sensitive rats on high salt diet. Hypertension 2003, 41, 592–597. [Google Scholar]
- Kobori, H.; Nishiyama, A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem. Biophys. Res. Commun 2004, 315, 746–750. [Google Scholar]
- Brezniceanu, M.L.; Liu, F.; Wei, C.C.; Chenier, I.; Godin, N.; Zhang, S.L.; Filep, J.G.; Ingelfinger, J.R.; Chan, J.S. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 2008, 57, 451–459. [Google Scholar]
- Konishi, Y.; Nishiyama, A.; Morikawa, T.; Kitabayashi, C.; Shibata, M.; Hamada, M.; Kishida, M.; Hitomi, H.; Kiyomoto, H.; Miyashita, T.; et al. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension 2011, 58, 205–211. [Google Scholar]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis 2009, 53, 982–992. [Google Scholar]
- Robertson, K.L.; Thach, D.C. LNA flow-FISH: A flow cytometry-fluorescence in situ hybridization method to detect messenger RNA using locked nucleic acid probes. Anal. Biochem 2009, 390, 109–114. [Google Scholar]
- Thomsen, R.; Nielsen, P.S.; Jensen, T.H. Dramatically improved RNA in situ hybridization signals using LNA-modified probes. RNA 2005, 11, 1745–1748. [Google Scholar]
- Kobori, H.; Katsurada, A.; Ozawa, Y.; Satou, R.; Miyata, K.; Hase, N.; Suzaki, Y.; Shoji, T. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem. Biophys. Res. Commun 2007, 358, 156–163. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kamiyama, M.; Urushihara, M.; Morikawa, T.; Konishi, Y.; Imanishi, M.; Nishiyama, A.; Kobori, H. Oxidative Stress/Angiotensinogen/Renin-Angiotensin System Axis in Patients with Diabetic Nephropathy. Int. J. Mol. Sci. 2013, 14, 23045-23062. https://doi.org/10.3390/ijms141123045
Kamiyama M, Urushihara M, Morikawa T, Konishi Y, Imanishi M, Nishiyama A, Kobori H. Oxidative Stress/Angiotensinogen/Renin-Angiotensin System Axis in Patients with Diabetic Nephropathy. International Journal of Molecular Sciences. 2013; 14(11):23045-23062. https://doi.org/10.3390/ijms141123045
Chicago/Turabian StyleKamiyama, Masumi, Maki Urushihara, Takashi Morikawa, Yoshio Konishi, Masahito Imanishi, Akira Nishiyama, and Hiroyuki Kobori. 2013. "Oxidative Stress/Angiotensinogen/Renin-Angiotensin System Axis in Patients with Diabetic Nephropathy" International Journal of Molecular Sciences 14, no. 11: 23045-23062. https://doi.org/10.3390/ijms141123045
APA StyleKamiyama, M., Urushihara, M., Morikawa, T., Konishi, Y., Imanishi, M., Nishiyama, A., & Kobori, H. (2013). Oxidative Stress/Angiotensinogen/Renin-Angiotensin System Axis in Patients with Diabetic Nephropathy. International Journal of Molecular Sciences, 14(11), 23045-23062. https://doi.org/10.3390/ijms141123045




