Cloning and Functional Characterization of c-Jun NH2-Terminal Kinase from the Mediterranean Species of the Whitefly Bemisia tabaci Complex
Abstract
:1. Introduction
2. Results
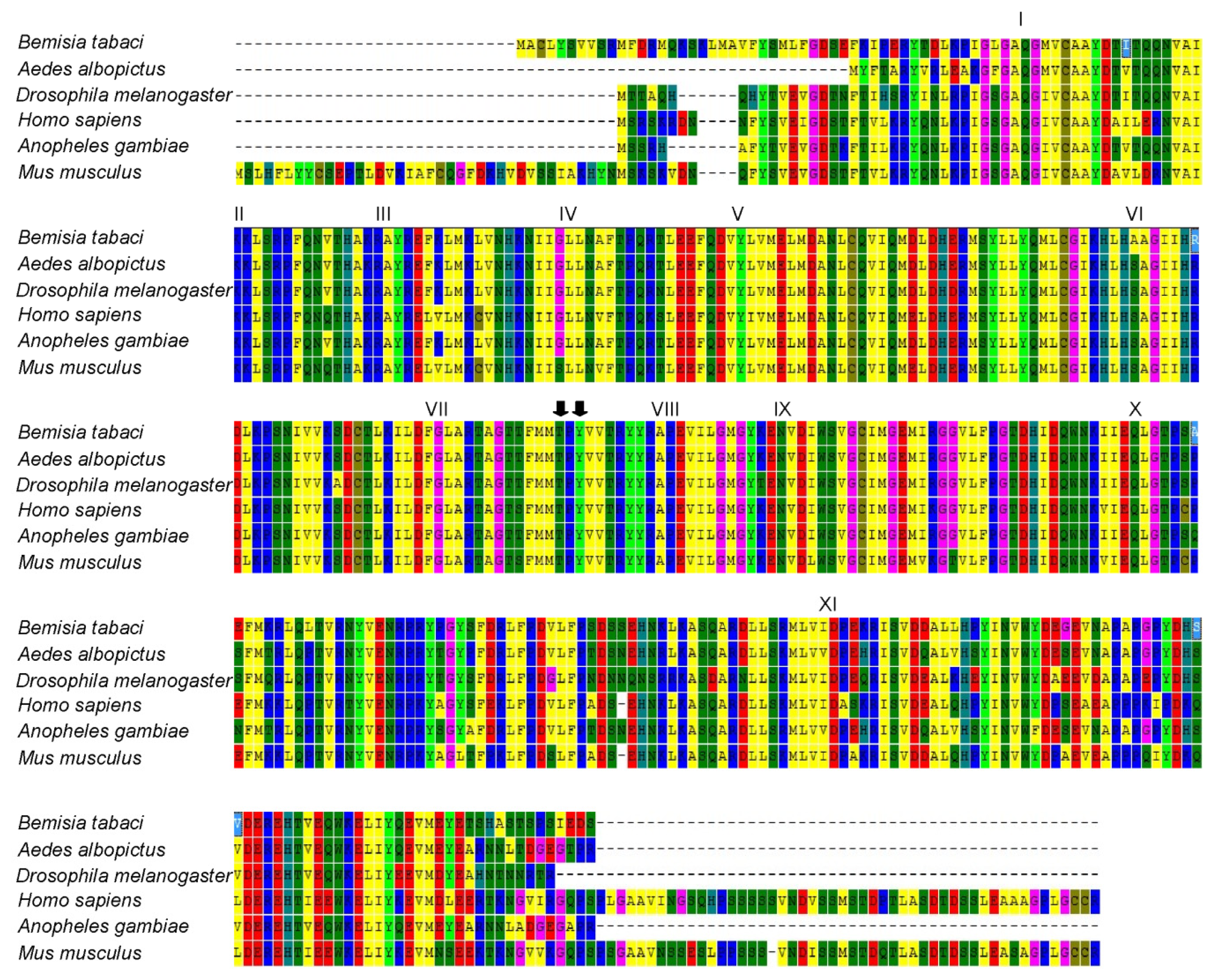
2.1. Cloning and Sequence Analyses of JNK
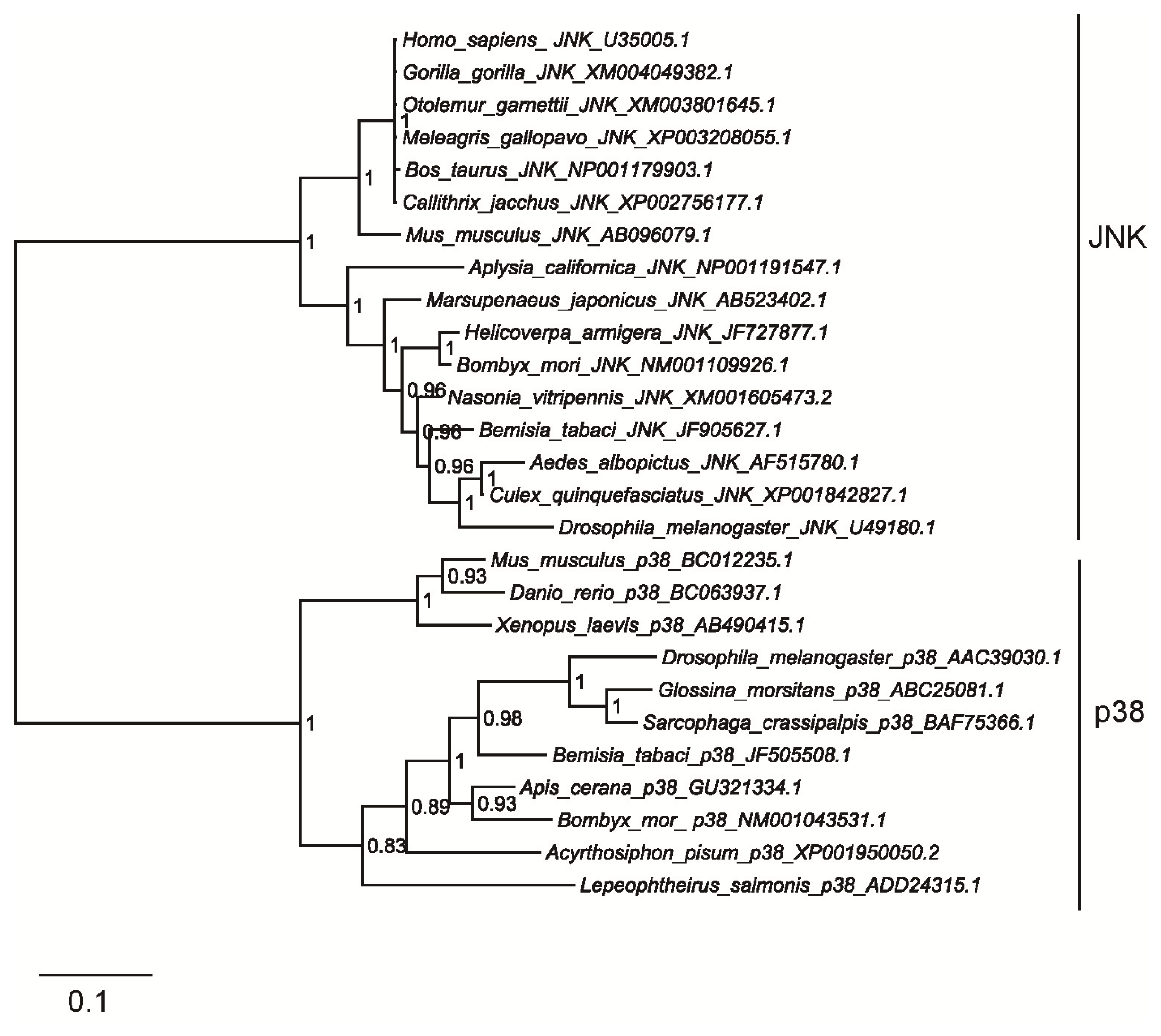
2.2. Homology and Phylogenetic Analyses of Bemisia tabaci JNK
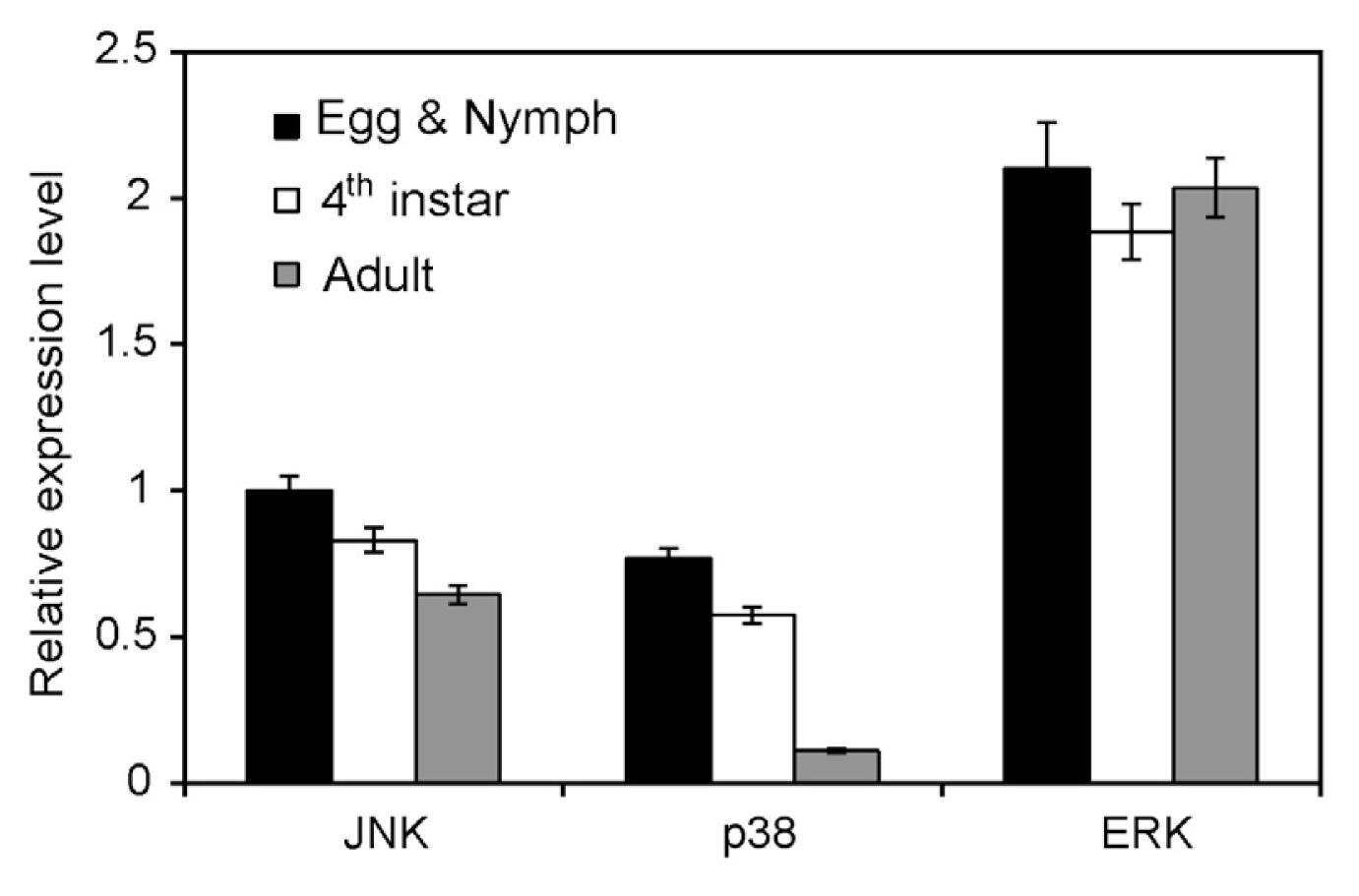
2.3. Expression in Different Developmental Stages
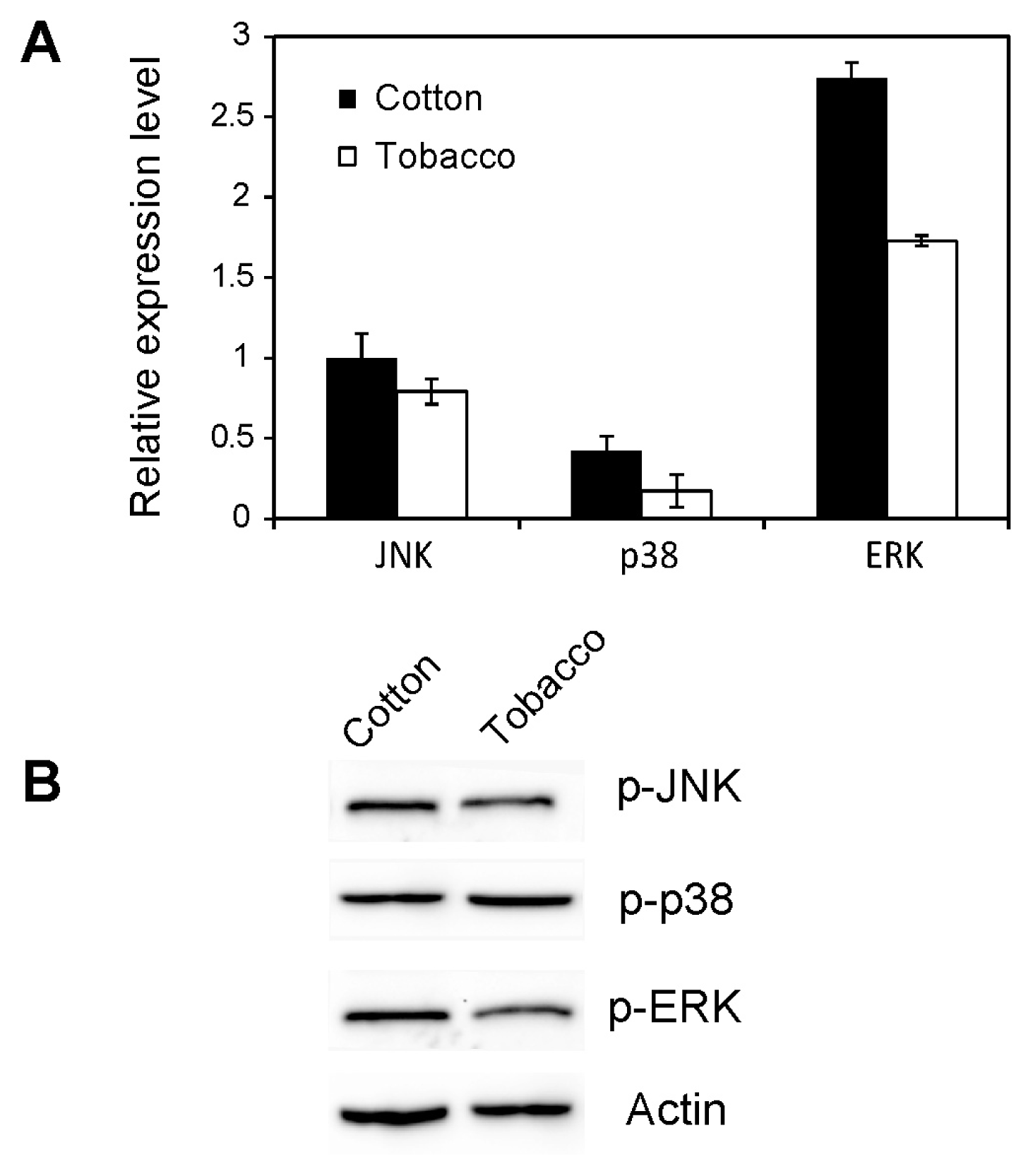
2.4. Effect of Host Shift on MAPK Activation
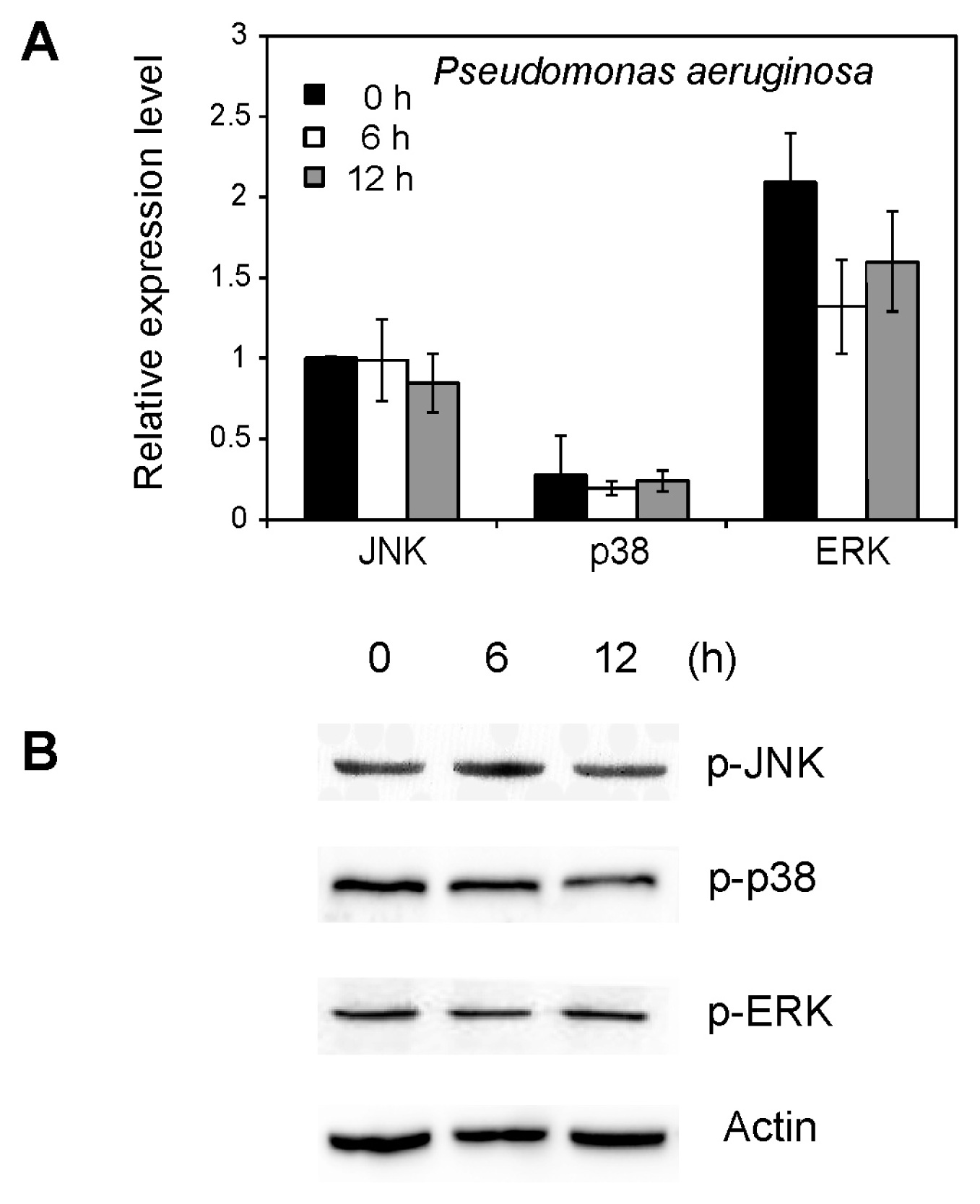
2.5. Effect of Pseudomonas aeruginosa on MAPK Activation
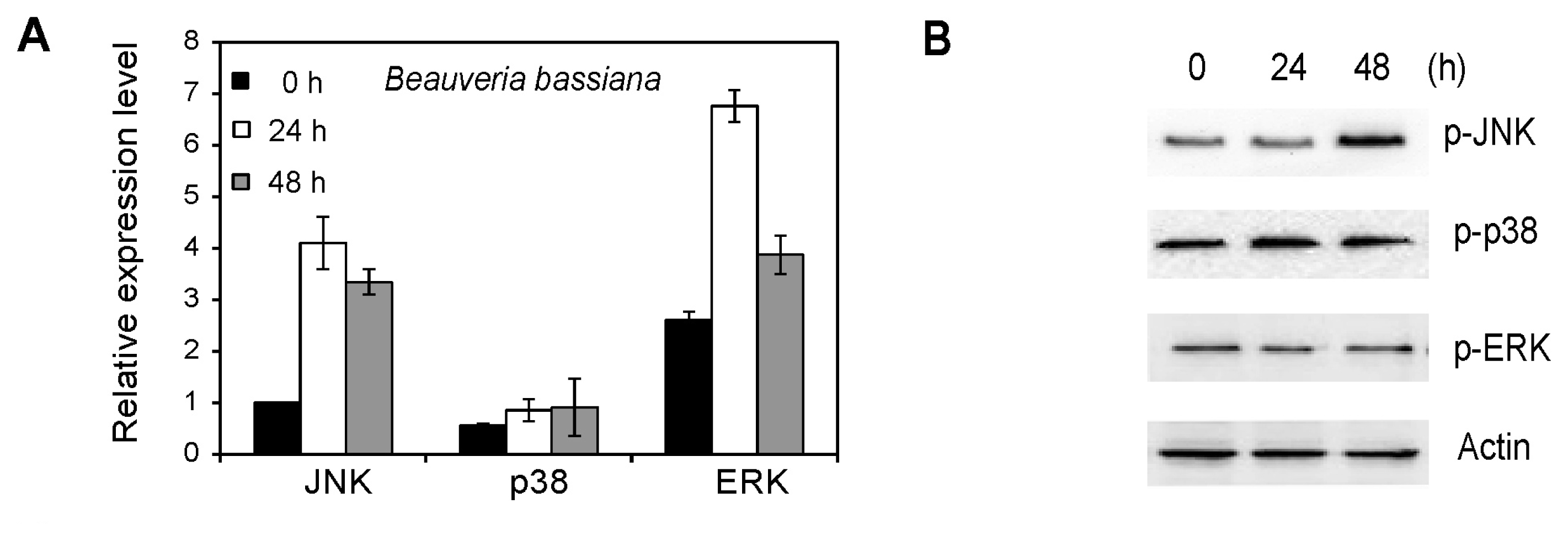
2.6. MAPK Activation in Whiteflies Treated with Beauveria bassiana
3. Discussion
4. Experimental Section
4.1. Source and Maintenance of Insects, Plants
4.2. Cloning of MED B. tabaci JNK
4.3. Sequence and Phylogenetic Analysis
4.4. RNA Isolation and Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
4.5. Western Blotting
4.6. Effect of Host Shift on MAPK Activation
4.7. Bacterial Treatment
4.8. Fungal Treatment
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Liu, S.S.; Colvin, J.; de Barro, P.J. Species concepts as applied to the whitefly Bemisia tabaci systematics: How many species are there? J. Integr. Agric 2012, 11, 176–186. [Google Scholar]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Ann. Rev. Entomol 2011, 56, 1–19. [Google Scholar]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; Barro, P.D. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am 2010, 103, 196–208. [Google Scholar]
- Tay, W.T.; Evans, G.A.; Boykin, L.M.; de Barro, P.J. Will the real Bemisia tabaci please stand up? PLoS One 2012, 7, e50550. [Google Scholar]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol 2003, 109, 195–219. [Google Scholar]
- Byrne, D.N.; Bellows, T.S., Jr. Whitefly biology. Ann. Rev. Entomol. 1991, 36, 431–457. [Google Scholar]
- Brown, J.K.; Frohlich, D.R.; Rosell, R.C. The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Ann. Rev. Entomol 1995, 40, 511–534. [Google Scholar]
- Liu, S.S.; de Barro, P.J.; Xu, J.; Luan, J.B.; Zang, L.S.; Ruan, Y.M.; Wan, F.H. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 2007, 318, 1769–1772. [Google Scholar]
- Broadbent, A.B.; Foottit, R.G.; Murphy, G.D. Sweetpotato whitefly Bemisia tabaci (Gennadius)(Homoptera: Aleyrodidae), a potential insect pest in Canada. Can. Entomol 1989, 121, 1027–1028. [Google Scholar]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 2001, 20, 709–723. [Google Scholar]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol 2005, 58, 216–225. [Google Scholar]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Ann. Rev. Immunol 2002, 20, 55–72. [Google Scholar]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar]
- Cowan, K.J.; Storey, K.B. Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol 2003, 206, 1107–1115. [Google Scholar]
- Rahaus, M.; Desloges, N.; Wolff, M.H. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J. Gen. Virol 2004, 85, 3529–3540. [Google Scholar]
- Katsuma, S.; Mita, K.; Shimada, T. ERK-and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J. Virol 2007, 81, 13700–13709. [Google Scholar]
- Ludwig, S.; Planz, O.; Pleschka, S.; Wolff, T. Influenza-virus-induced signaling cascades: Targets for antiviral therapy? Trends Mol. Med 2003, 9, 46–52. [Google Scholar]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar]
- Vogelweith, F.; Thiéry, D.; Quaglietti, B.; Moret, Y.; Moreau, J. Host plant variation plastically impacts different traits of the immune system of a phytophagous insect. Funct. Ecol 2011, 25, 1241–1247. [Google Scholar]
- Mizutani, T.; Kobayashi, M.; Eshita, Y.; Inanami, O.; Yamamori, T.; Goto, A.; Ako, Y.; Miyoshi, H.; Miyamoto, H.; Kariwa, H. Characterization of JNK-like protein derived from a mosquito cell line, C6/36. Insect Mol. Biol 2003, 12, 61–66. [Google Scholar]
- Mizutani, T.; Kobayashi, M.; Eshita, Y.; Shirato, K.; Kimura, T.; Ako, Y.; Miyoshi, H.; Takasaki, T.; Kurane, I.; Kariwa, H. Involvement of the JNK-like protein of the Aedes albopictus mosquito cell line, C6/36, in phagocytosis, endocytosis and infection of West Nile virus. Insect Mol. Biol 2003, 12, 491–499. [Google Scholar]
- Fujiwara, Y.; Denlinger, D.L. p38 MAPK is a likely component of the signal transduction pathway triggering rapid cold hardening in the flesh fly Sarcophaga crassipalpis. J. Exp. Biol 2007, 210, 3295–3300. [Google Scholar]
- Fujiwara, Y.; Denlinger, D.L. High temperature and hexane break pupal diapause in the flesh fly, Sarcophaga crassipalpis, by activating ERK/MAPK. J. Insect Physiol 2007, 53, 1276–1282. [Google Scholar]
- Yin, S.; Huo, Y.; Dong, Y.; Fan, L.; Yang, H.; Wang, L.; Ning, Y.; Hu, H. Activation of c-Jun NH (2)-terminal kinase is required for porcine reproductive and respiratory syndrome virus-induced apoptosis but not for virus replication. Virus Res 2012, 166, 103–108. [Google Scholar]
- Sluss, H.K.; Han, Z.; Barrett, T.; Davis, R.J.; Ip, Y.T. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Gene Dev 1996, 10, 2745–2758. [Google Scholar]
- Silverman, N.; Maniatis, T. NF-κB signaling pathways in mammalian and insect innate immunity. Gene Dev 2001, 15, 2321–2342. [Google Scholar]
- Chen, J.M.; Xie, C.C.; Tian, L.L.; Hong, L.X.; Wu, X.R.; Han, J.H. Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc. Natl. Acad. Sci. USA 2010, 107, 20774–20779. [Google Scholar]
- Li, F.F.; Xia, J.; Li, J.M.; Liu, S.S.; Wang, X.W. p38/MAPK is a component of the signal transduction pathway triggering cold stress response in the MED cryptic species of Bemisia tabaci. J. Integr. Agric 2012, 11, 303–311. [Google Scholar]
- Dérijard, B.; Hibi, M.; Wu, I.H.; Barrett, T.; Su, B.; Deng, T.; Karin, M.; Davis, R.J. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 1994, 76, 1025–1037. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar]
- Gorovits, R.; Czosnek, H. Expression of stress gene networks in tomato lines susceptible and resistant to Tomato yellow leaf curl virus in response to abiotic stresses. Plant Physiol. Biochem 2008, 46, 482–492. [Google Scholar]
- Moshe, A.; Pfannstiel, J.; Brotman, Y.; Kolot, M.; Sobol, I. Stress responses to Tomato Yellow Leaf Curl Virus (TYLCV) infection of resistant and susceptible tomato plants are different. Metabolomics 2012, S1, 006. [Google Scholar]
- Lee, Y.J.; Lee, C. Stress-activated protein kinases are involved in porcine reproductive and respiratory syndrome virus infection and modulate virus-induced cytokine production. Virology 2012, 427, 80–89. [Google Scholar]
- Davis, R.J. MAPKs: New JNK expands the group. Trends Biochem. Sci 1994, 19, 470–473. [Google Scholar]
- Shikano, I.; Ericsson, J.D.; Cory, J.S.; Myers, J.H. Indirect plant-mediated effects on insect immunity and disease resistance in a tritrophic system. Basic Appl. Ecol 2010, 11, 15–22. [Google Scholar]
- Zang, L.S.; Chen, W.Q.; Liu, S.S. Comparison of performance on different host plants between the B biotype and a non-B biotype of Bemisia tabaci from Zhejiang, China. Entomol. Exp. App 2006, 121, 221–227. [Google Scholar]
- Xu, J.; Lin, K.K.; Liu, S.S. Performance on different host plants of an alien and an indigenous Bemisia tabaci from China. J. Appl. Entomol 2011, 135, 771–779. [Google Scholar]
- Ferrandon, D.; Imler, J.L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol 2007, 7, 862–874. [Google Scholar]
- James, R.R. Effects of exogenous nutrients on conidial germination and virulence against the silverleaf whitefly for two hyphomycetes. J. Invertebr. Pathol 2001, 77, 99–107. [Google Scholar]
- Delaney, J.R.; Stöven, S.; Uvell, H.; Anderson, K.V.; Engström, Y.; Mlodzik, M. Cooperative control of Drosophila immune responses by the JNK and NF-κB signaling pathways. EMBO J 2006, 25, 3068–3077. [Google Scholar]
- Mendes-Giannini, M.J.S.; Soares, C.P.; Silva, J.L.M.; Andreotti, P.F. Interaction of pathogenic fungi with host cells: Molecular and cellular approaches. FEMS Immunol. Med. Microbiol 2005, 45, 383–394. [Google Scholar]
- Wang, X.W.; Luan, J.B.; Li, J.M.; Bao, Y.Y.; Zhang, C.X.; Liu, S.S. De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC Genomics 2010, 11, 400. [Google Scholar]
- DNAMAN. Available online: http://www.lynnon.com (on accessed 10 January 2013).
- Prot-Param Tools. Available online: http://kr.expasy.org/tools/protparam.html (on accessed 1 February 2013).
- BLAST Search Program. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (on accessed 1 January 2013).
- Clustal, W. Available online: http://www.ebi.ac.uk/Tools/msa/clustalw2 (on accessed 1 February 2013).
- MrBayes 3.1. Available online: http://mrbayes.net (on accessed 25 May 2013).
- Shi, W.B.; Feng, M.G. Effect of fungal infection on reproductive potential and survival time of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol 2009, 48, 229–237. [Google Scholar]

| Gene | Primer sequence |
|---|---|
| JNK | forward primer: 5′-TGTTGAGCAGTGGAAAGAGC-3′ |
| reverse primer: 5′-TTCGATAGACGGACTCGTTG-3′ | |
| p38 | forward primer: 5′-CATGGAAATTCTTGGAACCC-3′ |
| reverse primer: 5′-TTGGCTCCTTTGAACACTTG-3′ | |
| ERK | forward primer: 5′-TGGAATGGTCGTATCTGCAT-3′ |
| reverse primer: 5′-CATGCCTGAATCTTGTGAGG-3′ | |
| TAF | forward primer: 5′-TGTGGGACACCCATTATCAG-3′ |
| reverse primer: 5′-TGTGCAGCCAAGGAAATAAG-3′ | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, L.-L.; Huang, H.; Zhang, C.-R.; Xia, J.; Liu, S.-S.; Wang, X.-W. Cloning and Functional Characterization of c-Jun NH2-Terminal Kinase from the Mediterranean Species of the Whitefly Bemisia tabaci Complex. Int. J. Mol. Sci. 2013, 14, 13433-13446. https://doi.org/10.3390/ijms140713433
Wang L-L, Huang H, Zhang C-R, Xia J, Liu S-S, Wang X-W. Cloning and Functional Characterization of c-Jun NH2-Terminal Kinase from the Mediterranean Species of the Whitefly Bemisia tabaci Complex. International Journal of Molecular Sciences. 2013; 14(7):13433-13446. https://doi.org/10.3390/ijms140713433
Chicago/Turabian StyleWang, Lan-Lan, Huang Huang, Chang-Rong Zhang, Jun Xia, Shu-Sheng Liu, and Xiao-Wei Wang. 2013. "Cloning and Functional Characterization of c-Jun NH2-Terminal Kinase from the Mediterranean Species of the Whitefly Bemisia tabaci Complex" International Journal of Molecular Sciences 14, no. 7: 13433-13446. https://doi.org/10.3390/ijms140713433




