Comparative Study of Electroless Copper Film on Different Self-Assembled Monolayers Modified ABS Substrate
Abstract
:1. Introduction
2. Results and Discussion
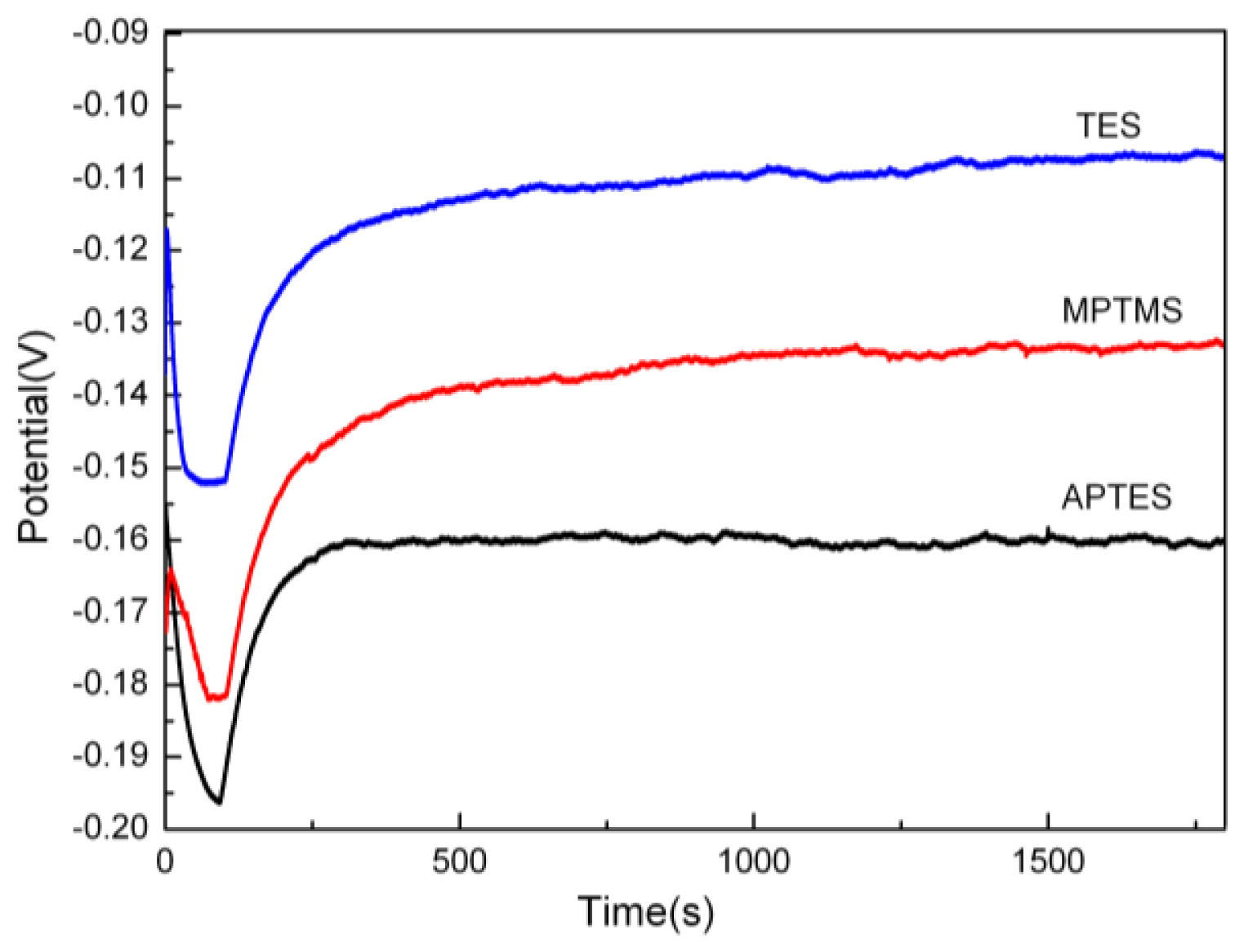
2.1. Deposition Rate and Contact Angle
2.2. Surface Morphology-SEM Studies for Copper Films
2.3. Adhesion Property of Copper Film
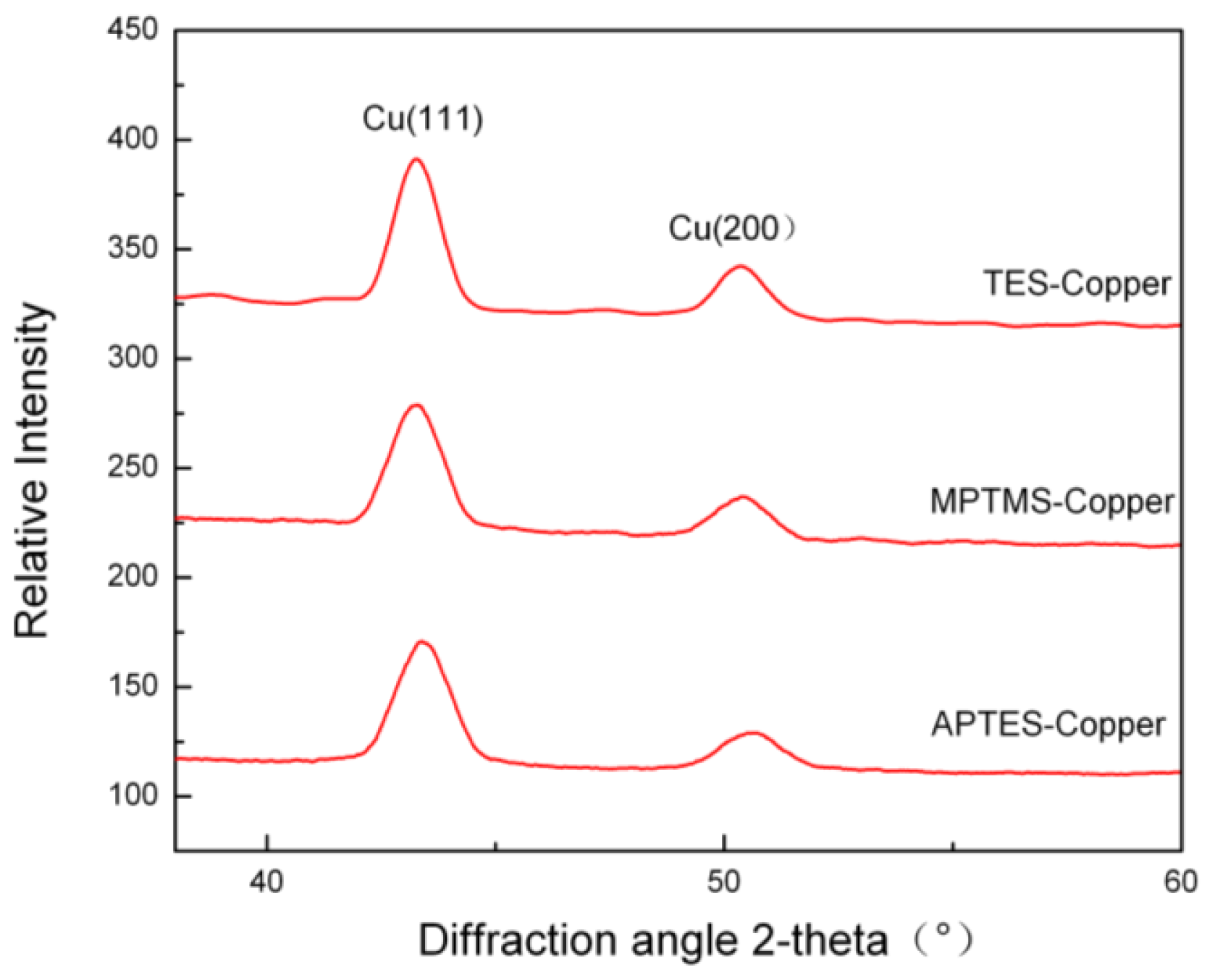
2.4. XRD Analysis of Copper Film
3. Experimental Section
3.1. Materials
3.2. Preparation of Different Self-Assembled Monolayers on ABS Surface
3.3. Electroless Copper Plating
3.4. Measurement and Characterization
3.4.1. Deposition Rate and Contact Angle
3.4.2. Adhesion Property Test
3.4.3. Scanning Electron Microscopy
3.4.4. X-ray Diffraction
4. Conclusions
Acknowledgments
Conflicts of Interest
Reference
- Tang, X.J.; Bi, C.L.; Han, C.X.; Zhang, B.G. A new palladium-free surface activation process for Ni electroless plating on ABS plastic. Mater. Lett 2009, 63, 840–842. [Google Scholar]
- Tang, X.J.; Cao, M.; Bi, C.L.; Yang, L.J.; Zhang, B.G. Research on a new surface activation process for electroless plating on ABS plastic. Mater. Lett 2008, 62, 1089–1091. [Google Scholar]
- Wu, P.K.; Lu, T.M. Metallized Plastics 7: Fundamental and Applied Aspects; VSP: Utrecht, The Netherlands, 2001; pp. 215–245. [Google Scholar]
- Pimanpang, S.; Wang, P.I.; Wang, G.C.; Lu, T.M. Self-assembled monolayer growth on chemically modified polymer surfaces. Appl. Surf. Sci 2006, 252, 3532–3540. [Google Scholar]
- Jay, J.; Yang, G.R.; Lu, T.M. Aqueous ammonium sulfide to modify the surface of low k dielectric thin films. Colloids Surf. A 2003, 214, 119–126. [Google Scholar]
- Friedrich, J.F.; Geng, S.; Unger, W.; Lippitz, A.; Erdmann, J.; Gorsler, H.V.; Wöll, C.; Schertel, A.; Bierbaum, K. Plasma functionalization and reorientation of macromolecules at polymer surfaces. Surf. Coat. Technol 1995, 74–75, 664–669. [Google Scholar]
- Wang, F.; Wang, J.; Zhang, H.N.; Wang, D.; Li, Y.N. Electroless copperplating on polypropylene modified by heterocyclic organosilane self-assembled nanofilm. Mod. Chem. Ind 2012, 6, 52–54. [Google Scholar]
- Garcia, A.; Berthelot, T.; Viel, P.; Polesel-Maris, J.; Palacin, S. Microscopic study of a ligand induced electroless plating process onto polymers. ACS Appl. Mater. Interfaces 2010, 11, 3043–3051. [Google Scholar]
- Wang, F.; Zhang, H.N.; Wang, J.; Fan, R.B.; Li, Y.N. Electroless copperplating on ABS resin modified by heterocyclic organosilane self-assembled film. J. Mater. Sci. Eng 2012, 5, 698–701. [Google Scholar]
- Nuzzo, R.G.; Dubios, L.H.; Allara, D.L. Fundamental studies of microscopic wetting on organic surfaces. 1. Formation and structural characterization of a self-consistent series of polyfunctional organic monolayers. J. Am. Chem. Soc 1990, 112, 558–569. [Google Scholar]
- Laibinis, P.E.; Whitesides, G.M. Self-assembled monolayers of n-alkanethiolates on copper are barrier films that protect the metal against oxidation by air. J. Am. Chem. Soc 1992, 114, 9022–9028. [Google Scholar]
- Kumar, A.; Biebuyck, H.A. The use of self-assembled monolayers and a selective etch to generate patterned gold features. J. Am. Chem. Soc 1992, 114, 9188–9189. [Google Scholar]
- Liu, Z.Z.; Wang, Q.; Liu, X.; Bao, J.Q. Effects of amino-terminated self-assembled monolayers on nucleation and growth of chemical vapor-deposited copper films. Thin Solid Films 2008, 517, 635–640. [Google Scholar]
- Venables, J.A.; Spiller; Hanbucken, M.; et al. Nucleation and growth of thin films. Rep. Prog. Phys 1984, 47, 399–459. [Google Scholar]
- Semaltianos, N.G.; Pastol, J.L.; Doppelt, P. Copper nucleation by chemical vapour depositionon organosilane treated SiO2 surfaces. Surf. Sci 2004, 562, 157–169. [Google Scholar]
- Morrison, I.D.; Ross, S. Colloidal Dispersions: Suspensions, Emulsion and Foams; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Vondrak, T.; Wang, H.; Winget, P.; Cramer, C.J.; Zhu, X.Y. Interfacial electronic structure in thiolate self-assembled monolayers: Implication for molecular electronics. J. Am. Chem. Soc 2000, 122, 4700–4707. [Google Scholar]
- Zhou, C.Z.; Nagy, G.; Walker, A.V. Toward molecular electronic circuitry: Selective deposition of metals on patterned self-assembled monolayer surfaces. J. Am. Chem. Soc 2005, 127, 12160–12161. [Google Scholar]
- Senkevich, J.; Yang, G.R.; Lu, T.M. Thermal stability of mercaptan terminated self-assembled multilayer films on SiO2 surfaces. Colloids Surf. A 2002, 207, 139–145. [Google Scholar]
- Cohen, S.L.; Liehr, M.; Kasi, S. Mechanisms of copper chemical vapor deposition. Appl. Phys. Lett 1992, 60, 50–52. [Google Scholar]
- Chen, H.; Wang, J.H.; Huo, Q. Self-assembled monolayer of 3-aminopropyltrimethoxysilane for improved adhesion between aluminum alloy substrate and polyurethane coating. Thin Solid Films 2007, 515, 7181–7189. [Google Scholar]
- Ryu, C.; Kwon, K.W.; Loke, A.L.S.; Lee, H.; Nogami, T.; Dubin, V.M. Microstructure and reliability of copper interconnects. Electron. Devices 1999, 46, 1113–1120. [Google Scholar]
- Wang, F.; Li, Y.N.; Wang, Y.B.; Cao, Z. Self-assembled monolayer of designed and synthesized triazinedithiolsilane molecule as interfacial adhesion enhancer for integrated circuit. Nanoscale Res. Lett 2011, 6, 483–487. [Google Scholar]





| SAMs type | Deposition rate (nm/min) | Contact angle (°) | Surface energy (J/m2) |
|---|---|---|---|
| TES-SAMs | 144 ± 10 | 71.9 ± 2 | 0.430 ± 0.01 |
| MPTMS-SAMs | 95 ± 10 | 78.9 ± 3 | 0.350 ± 0.02 |
| APTES-SAMs | 72 ± 10 | 87.8 ± 2 | 0.269 ± 0.01 |
| Copper plating | Contact angle (°) | Surface energy (J/m2) |
|---|---|---|
| blank copper | 94.3 ± 3 | 0.218 ± 0.02 |
| TES-copper | 99.3 ± 3 | 0.176 ± 0.02 |
| MPTMS-copper | 114 ± 3 | 0.092 ± 0.02 |
| APTES-copper | 122 ± 4 | 0.041 ± 0.01 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, J.; Fan, R.; Wang, J.; Jia, M.; Xiong, X.; Wang, F. Comparative Study of Electroless Copper Film on Different Self-Assembled Monolayers Modified ABS Substrate. Int. J. Mol. Sci. 2014, 15, 6412-6422. https://doi.org/10.3390/ijms15046412
Xu J, Fan R, Wang J, Jia M, Xiong X, Wang F. Comparative Study of Electroless Copper Film on Different Self-Assembled Monolayers Modified ABS Substrate. International Journal of Molecular Sciences. 2014; 15(4):6412-6422. https://doi.org/10.3390/ijms15046412
Chicago/Turabian StyleXu, Jiushuai, Ruibin Fan, Jiaolong Wang, Mengke Jia, Xuanrui Xiong, and Fang Wang. 2014. "Comparative Study of Electroless Copper Film on Different Self-Assembled Monolayers Modified ABS Substrate" International Journal of Molecular Sciences 15, no. 4: 6412-6422. https://doi.org/10.3390/ijms15046412





