Transition Metal Intercalators as Anticancer Agents—Recent Advances
Abstract
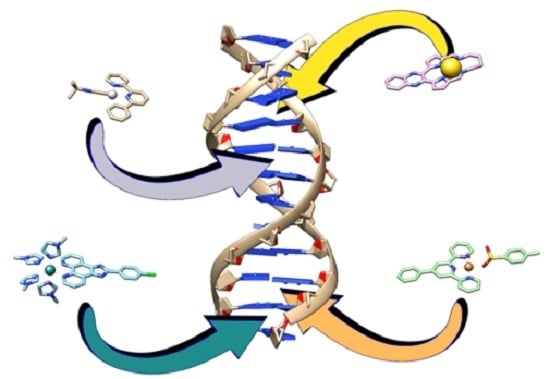
:1. Introduction
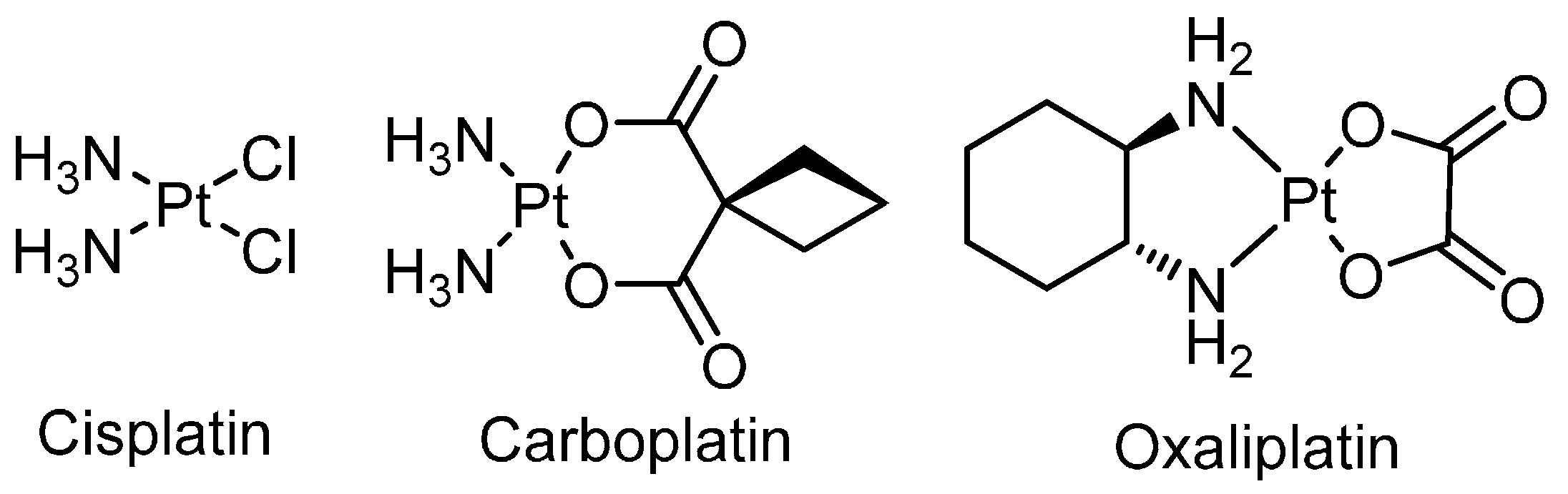
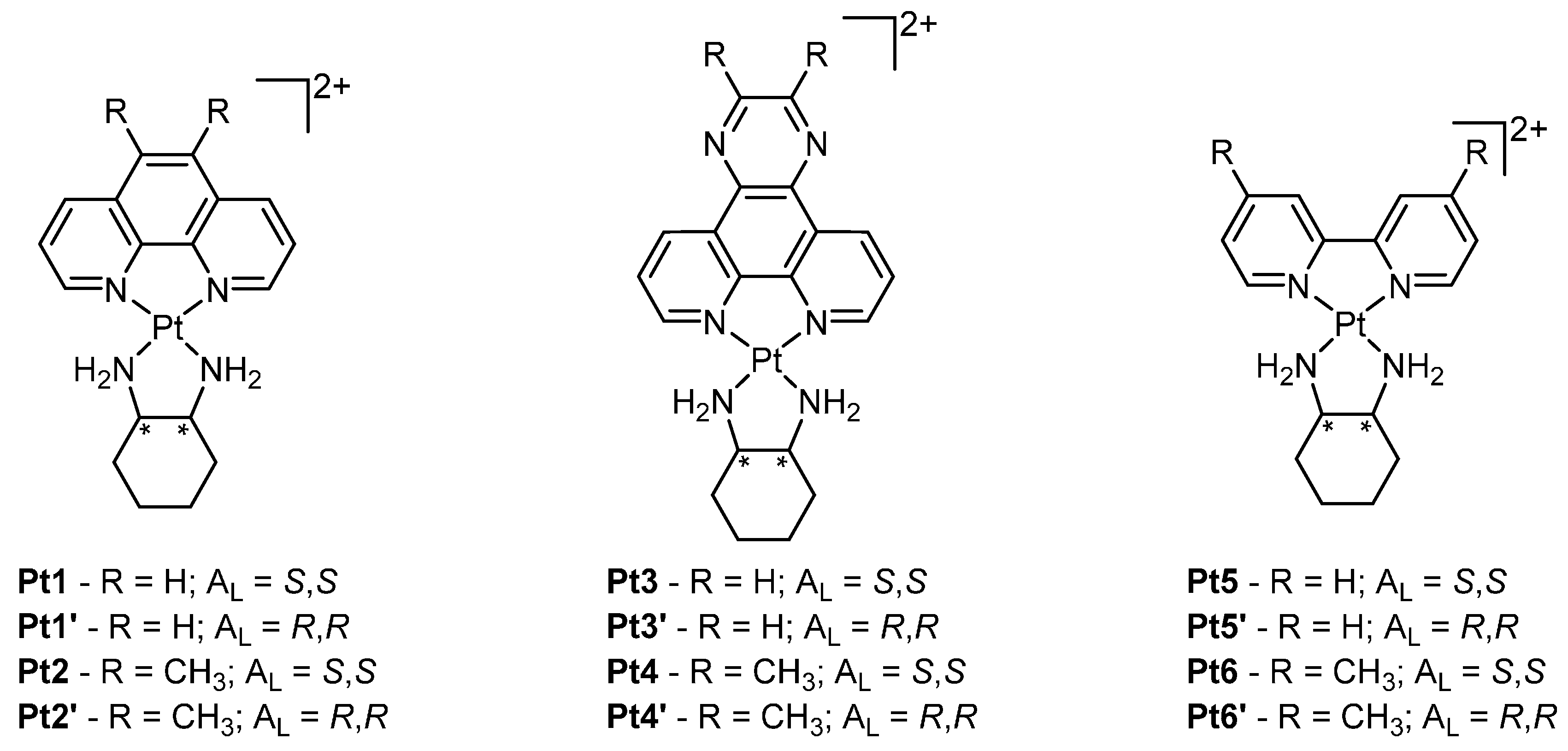
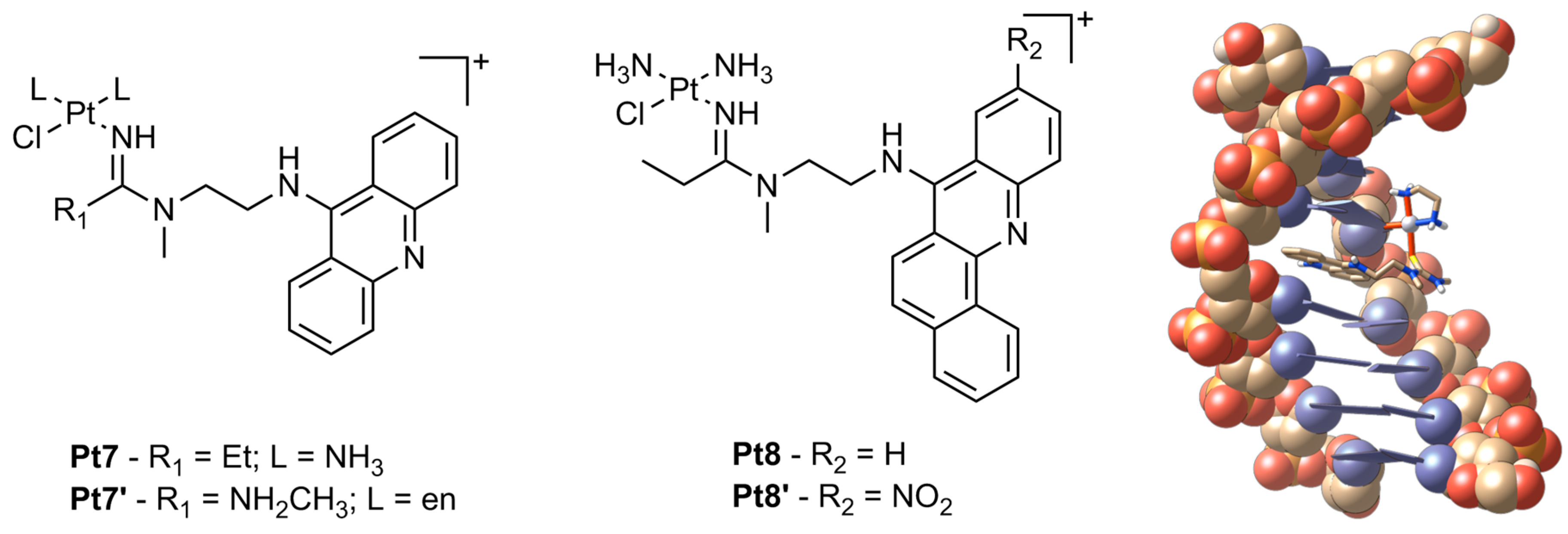
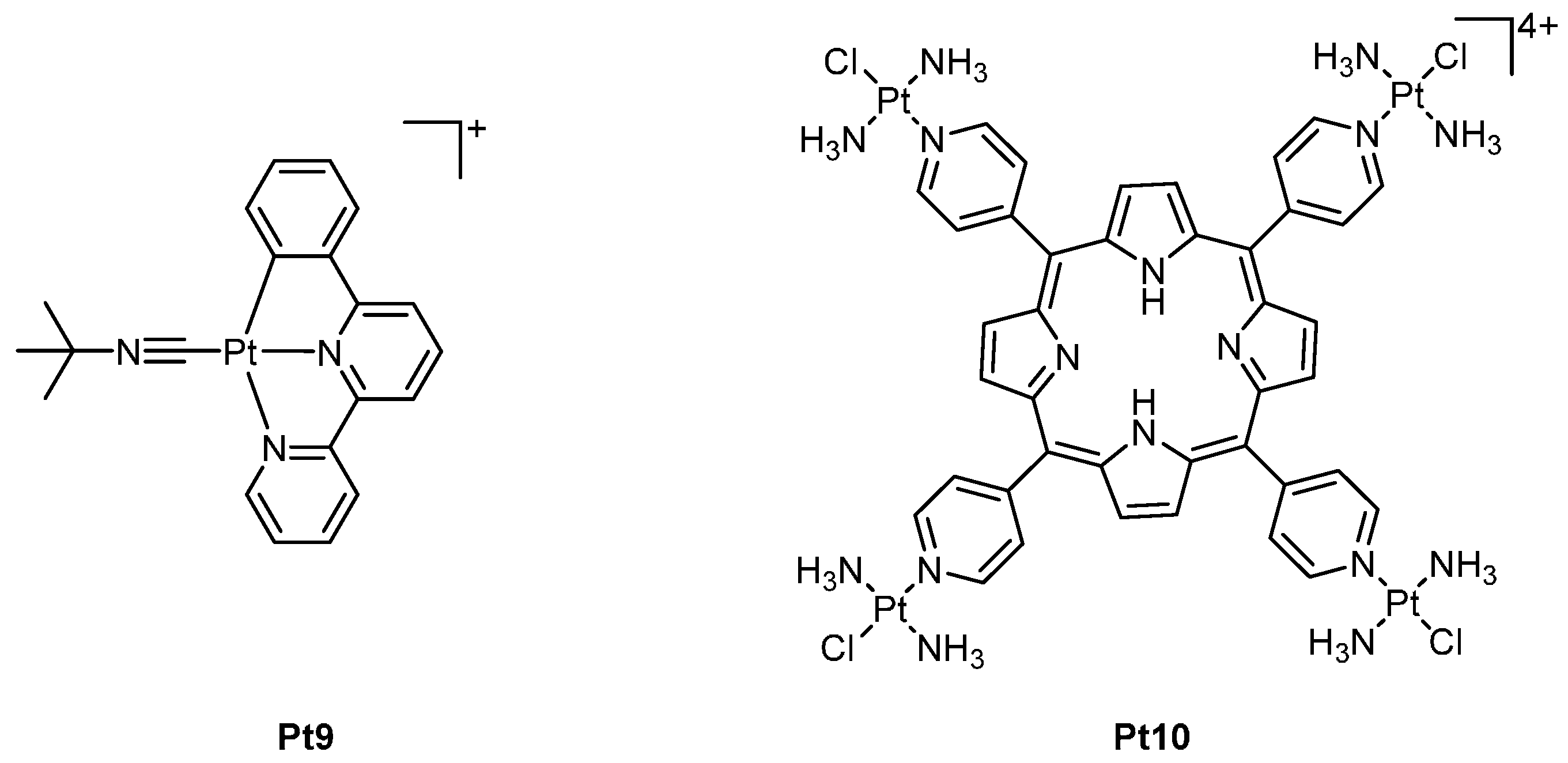
2. Platinum
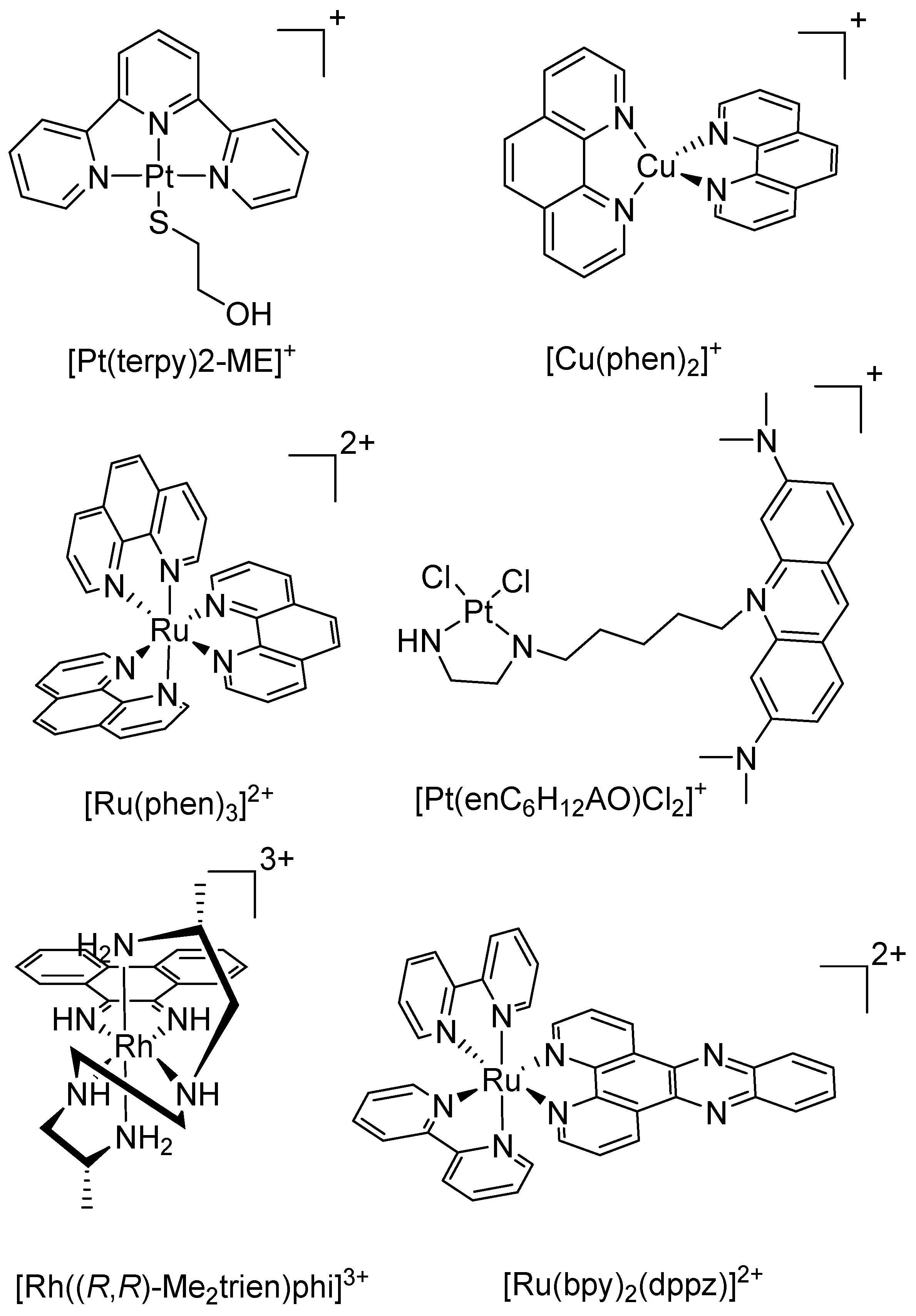
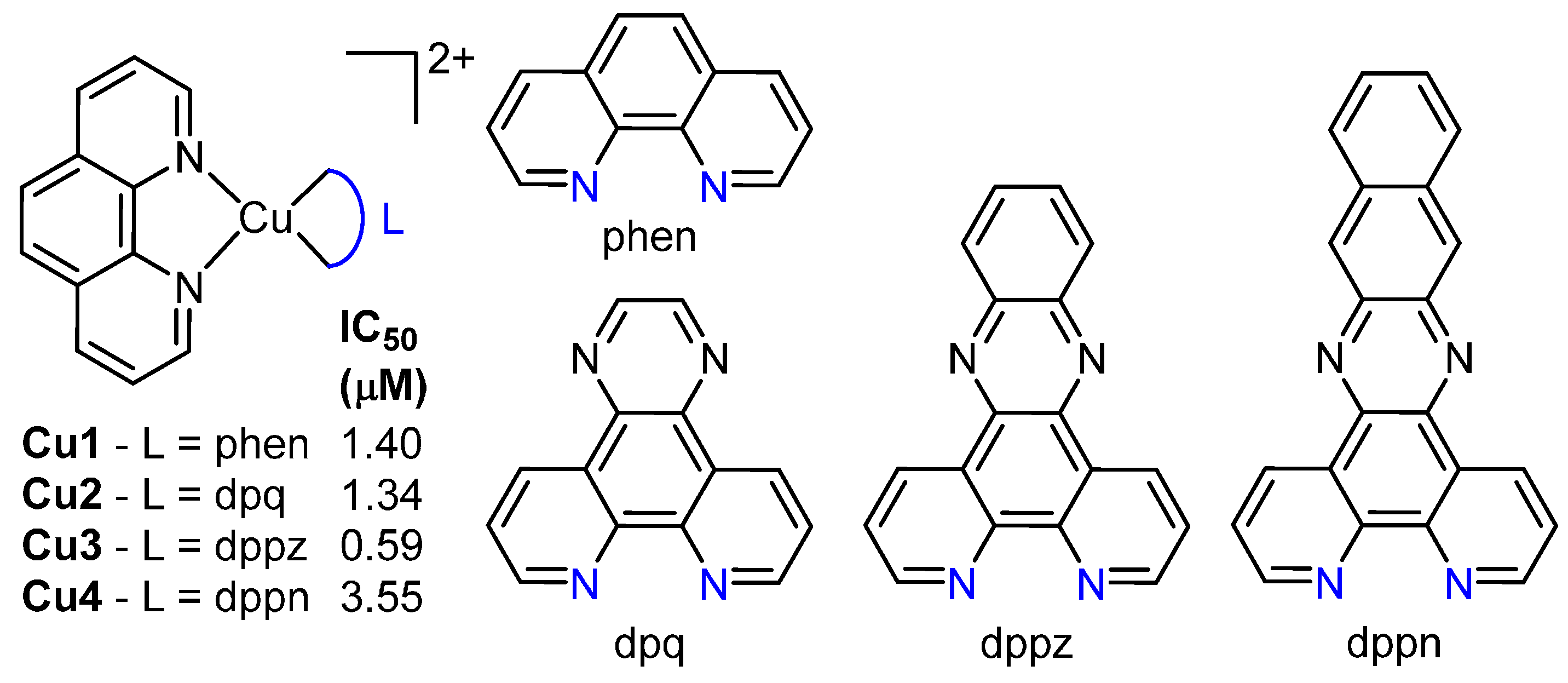
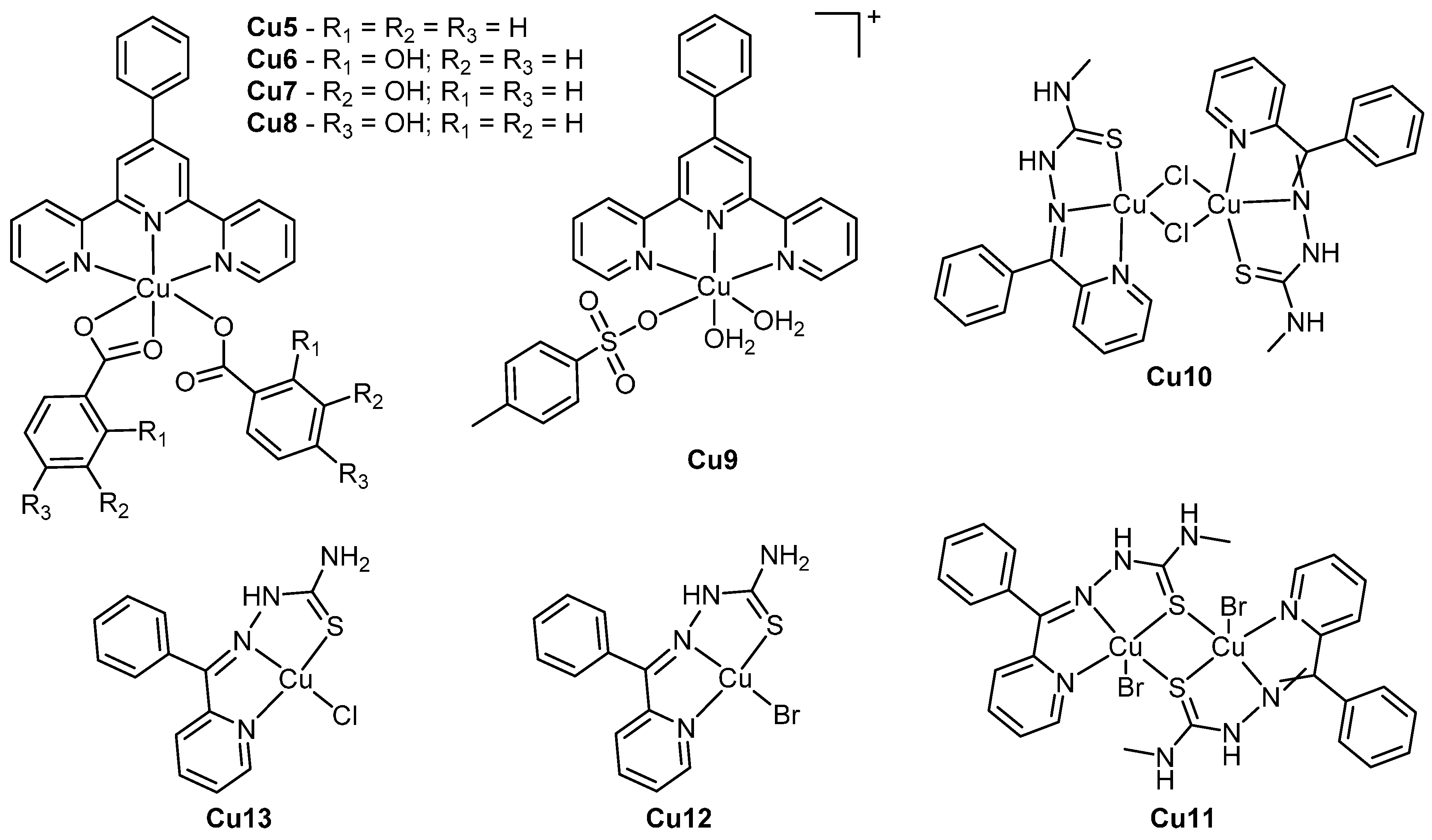
3. Copper
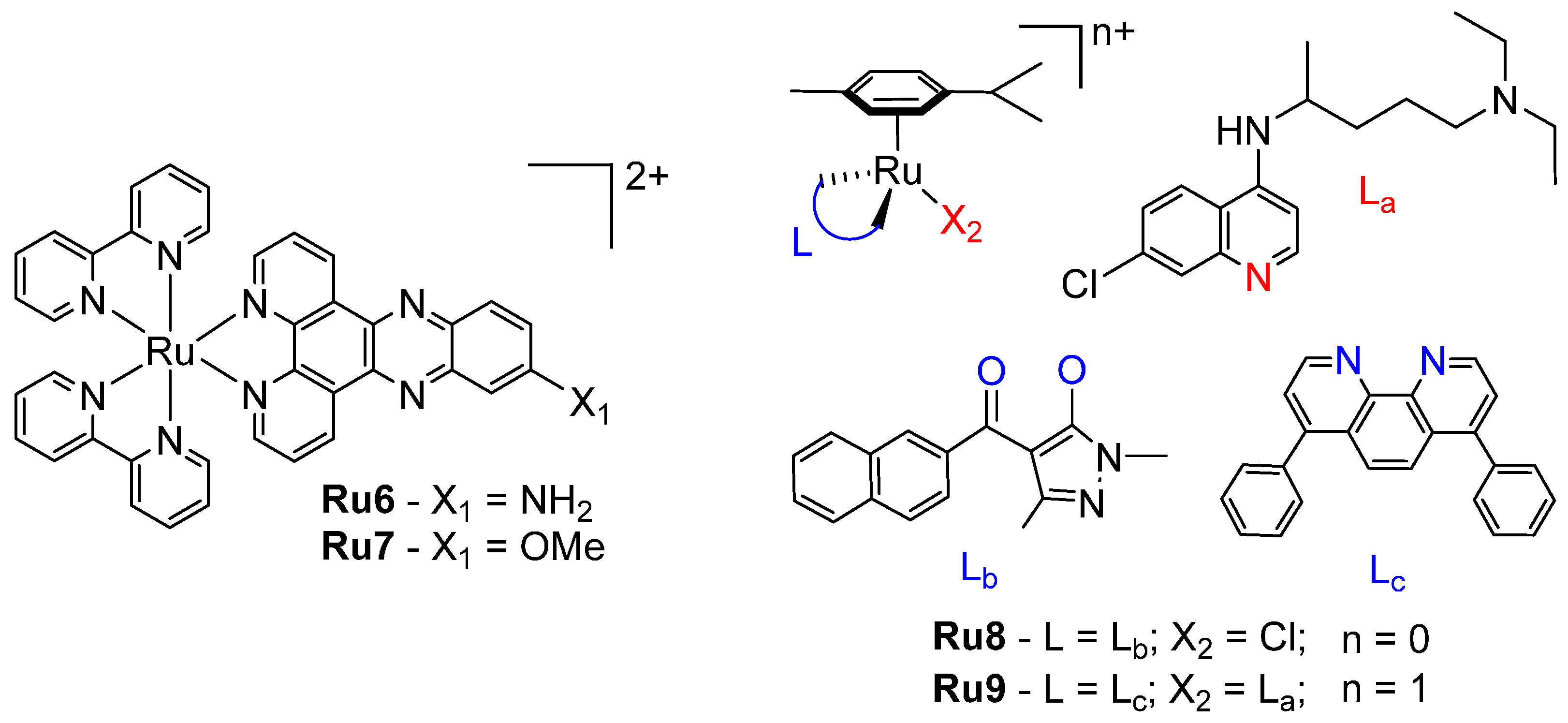
4. Ruthenium
5. Other Metals
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rosenberg, B.; van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J.; Einhorn, L.H. Drugs five years later. Cisplatin. Ann. Intern. Med. 1984, 100, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Provencher-Mandeville, J.; Debnath, C.; Mandal, S.K.; Leblanc, V.; Parent, S.; Asselin, É.; Bérubé, G. Design, synthesis and biological evaluation of estradiol-PEG-linked Platinum(II) hybrid molecules: Comparative molecular modeling study of three distinct families of hybrids. Steroids 2011, 76, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, V.; Fuertes, M.A.; Castilla, J.; Alonso, C.; Quevedo, C.; Pérez, J.M. Biochemical mechanisms of cisplatin cytotoxicity. Anti-Cancer Agents Med. Chem. 2007, 7, 3–18. [Google Scholar] [CrossRef]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Wilson, J.J.; Lippard, S.J. Monofunctional and higher-valent platinum anticancer agents. Inorg. Chem. 2013, 52, 12234–12249. [Google Scholar] [CrossRef] [PubMed]
- Cleare, M.J.; Hoeschele, J.D. Studies on the antitumor activity of group VIII transition metal complexes. Part I. Platinum (II) complexes. Bioinorg. Chem. 1973, 2, 187–210. [Google Scholar] [CrossRef]
- Lovejoy, K.S.; Lippard, S.J. Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting. Dalton Trans. 2009, 48, 10651–10659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Mathad, R.I.; Zhang, Z.; Sidell, N.; Yang, D. Solution structure of a 2:1 complex of anticancer drug XR5944 with TFF1 estrogen response element: Insights into DNA recognition by a bis-intercalator. Nucleic Acids Res. 2014, 42, 6012–6024. [Google Scholar] [CrossRef] [PubMed]
- Gelasco, A.; Lippard, S.J. NMR solution structure of a DNA dodecamer duplex containing a cis-Diammineplatinum(II) d(GpG) intrastrand cross-link, the major adduct of the anticancer drug cisplatin. Biochemistry 1998, 37, 9230–9239. [Google Scholar] [CrossRef] [PubMed]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Lerman, L.S. Structural considerations in the interaction of DNA and acridines. J. Mol. Biol. 1961, 3, 18–30. [Google Scholar] [CrossRef]
- Long, E.C.; Barton, J.K. On demonstrating DNA intercalation. Acc. Chem. Res. 1990, 23, 271–273. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Myers, S.; Harper, B.W.J.; Ng, N.S.; Dong, Q.; Xie, C.; Aldrich-Wright, J.R. The effects of 56MESS on mitochondrial and cytoskeletal proteins and the cell cycle in MDCK cells. Metallomics 2013, 5, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Jennette, K.W.; Lippard, S.J.; Vassiliades, G.A.; Bauer, W.R. Metallointercalation reagents. 2-hydroxyethanethiolato(2,2’,2’-terpyridine)-platinum(II) monocation binds strongly to DNA by intercalation. Proc. Natl. Acad. Sci. USA 1974, 71, 3839–3843. [Google Scholar] [CrossRef] [PubMed]
- Bowler, B.E.; Lippard, S.J. Modulation of platinum antitumor drug binding to DNA by linked and free intercalators. Biochemistry 1986, 25, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Sigman, D.S.; Graham, D.R.; D’Aurora, V.; Stern, A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline. Cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem. 1979, 254, 12269–12272. [Google Scholar] [PubMed]
- Kelly, J.M.; Tossi, A.B.; McConnell, D.J.; OhUigin, C. A Study of the interactions of some Polypyridylruthenium(II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucleic Acids Res. 1985, 13, 6017–6034. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.E.; Chambron, J.C.; Sauvage, J.P.; Turro, N.J.; Barton, J.K. A molecular light switch for DNA: Ru(bpy)2(dppz)2+. J. Am. Chem. Soc. 1990, 112, 4960–4962. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Erkkila, K.E.; Hudson, B.P.; Barton, J.K.; Rees, D.C. Structure of a photoactive rhodium complex intercalated into DNA. Nat. Struct. Mol. Biol. 2000, 7, 117–121. [Google Scholar]
- Yan, Y.K.; Melchart, M.; Habtemariam, A.; Sadler, P.J. Organometallic chemistry, biology and medicine: Ruthenium arene anticancer complexes. Chem. Commun. 2005, 38, 4764–4776. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Biol. 2007, 11, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Romero-Canelón, I.; Sadler, P.J. Next-generation metal anticancer complexes: Multitargeting via redox modulation. Inorg. Chem. 2013, 52, 12276–12291. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Safaei, E.; Ranjbar, B.; Hasani, L. Thermodynamic and spectroscopic study on the binding of cationic Zn(II) and Co(II) tetrapyridinoporphyrazines to calf thymus DNA: The role of the central metal in binding parameters. New J. Chem. 2004, 28, 1227–1234. [Google Scholar] [CrossRef]
- Pages, B.J.; Li, F.; Wormell, P.; Ang, D.L.; Clegg, J.K.; Kepert, C.J.; Spare, L.K.; Danchaiwijit, S.; Aldrich-Wright, J.R. Synthesis and analysis of the anticancer activity of Platinum(II) complexes incorporating dipyridoquinoxaline variants. Dalton Trans. 2014, 43, 15566–15575. [Google Scholar] [CrossRef] [PubMed]
- Pages, B.J.; Zhang, Y.; Li, F.; Sakoff, J.; Gilbert, J.; Aldrich-Wright, J.R. Cytotoxicity and structural analyses of 2,2′-Bipyridine-, 4,4′-Dimethyl-2,2′-bipyridine- and 2-(2′-Pyridyl)quinoxalineplatinum(II) complexes. Eur. J. Inorg. Chem. 2015, 2015, 4167–4175. [Google Scholar] [CrossRef]
- Pages, B.J.; Sakoff, J.; Gilbert, J.; Rodger, A.; Chmel, N.P.; Jones, N.C.; Kelly, S.M.; Ang, D.L.; Aldrich-Wright, J.R. Multifaceted studies of the DNA interactions and in vitro cytotoxicity of anticancer polyaromatic Platinum(II) complexes. Chem. Eur. J. 2016, 22, 8943–8954. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Higgins, V.; Aldrich-Wright, J.; Wu, M. Identification of the molecular mechanisms underlying the cytotoxic action of a potent platinum metallointercalator. J. Chem. Biol. 2012, 5, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, M.J.; Higgins, V.J.; Aldrich-Wright, J.R. Comparative analyses of cytotoxicity and molecular mechanisms between platinum metallointercalators and cisplatin. Metallomics 2012, 4, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Wheate, N.J.; Pisani, M.J.; Aldrich-Wright, J.R. Degradation of bidentate-coordinated platinum(II)-based DNA intercalators by reduced l-glutathione. J. Med. Chem. 2008, 51, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Taleb, R.I.; Krause-Heuer, A.M.; Cook, R.L.; Wang, S.; Higgins, V.J.; Aldrich-Wright, J.R. Novel Platinum(II)-based anticancer complexes and molecular hosts as their drug delivery vehicles. Dalton Trans. 2007, 43, 5055–5064. [Google Scholar] [CrossRef] [PubMed]
- Moretto, J.; Chauffert, B.; Ghiringhelli, F.; Aldrich-Wright, J.R.; Bouyer, F. Discrepancy between in vitro and in vivo antitumor effect of a new Platinum(II) metallointercalator. Investig. New Drug. 2011, 29, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.M.; Fenton, R.R.; Aldrich-Wright, J.R. In vivo studies of a Platinum(II) metallointercalator. Chem. Commun. 2008, 43, 5613–5615. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.J.; Liu, F.; Bartenstein, T.F.; Haines, L.G.; Levine, K.E.; Kucera, G.L.; Bierbach, U. Redesigning the DNA-targeted chromophore in platinum–acridine anticancer agents: A structure-activity relationship study. Chem. Eur. J. 2014, 20, 16174–16187. [Google Scholar] [CrossRef] [PubMed]
- Cheung-Ong, K.; Song, K.T.; Ma, Z.; Shabtai, D.; Lee, A.Y.; Gallo, D.; Heisler, L.E.; Brown, G.W.; Bierbach, U.; Giaever, G.; et al. Comparative chemogenomics to examine the mechanism of action of DNA-targeted platinum-acridine anticancer agents. ACS Chem. Biol. 2012, 7, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Kostrhunova, H.; Malina, J.; Pickard, A.J.; Stepankova, J.; Vojtiskova, M.; Kasparkova, J.; Muchova, T.; Rohlfing, M.L.; Bierbach, U.; Brabec, V. Replacement of a thiourea with an amidine group in a monofunctional platinum–acridine antitumor agent. Effect on DNA interactions, DNA adduct recognition and repair. Mol. Pharm. 2011, 8, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.T.; Baruah, H.; Kramarczyk, J.; Saluta, G.; Day, C.S.; Kucera, G.L.; Bierbach, U. Design, Synthesis, and biological activity of a novel non-cisplatin-type platinum−acridine pharmacophore. J. Med. Chem. 2001, 44, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Baruah, H.; Wright, M.W.; Bierbach, U. Solution structural study of a DNA duplex containing the Guanine-N7 adduct formed by a cytotoxic platinum−acridine hybrid agent. Biochemistry 2005, 44, 6059–6070. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Choudhury, J.R.; Wright, M.W.; Day, C.S.; Saluta, G.; Kucera, G.L.; Bierbach, U. A non-cross-linking platinum−acridine agent with potent activity in non-small-cell lung cancer. J. Med. Chem. 2008, 51, 7574–7580. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liu, J.; Lum, C.T.; Ma, C.; Chan, R.C.T.; Lok, C.N.; Kwok, W.M.; Che, C.M. Luminescent cyclometalated Platinum(II) complex forms emissive intercalating adducts with double-stranded DNA and RNA: Differential emissions and anticancer activities. Angew. Chem. Int. Ed. 2014, 53, 10119–10123. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Rubbiani, R.; Gasser, G.; Spingler, B. Visible-light-induced annihilation of tumor cells with platinum–porphyrin conjugates. Angew. Chem. 2014, 126, 7058–7061. [Google Scholar] [CrossRef]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Wende, C.; Lüdtke, C.; Kulak, N. Copper complexes of N-donor ligands as artificial nucleases. Eur. J. Inorg. Chem. 2014, 2014, 2597–2612. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Grover, D.K.; LeClaire, V.; Tseng, M.; Wicha, M.; Pienta, K.; Redman, B.G.; Jahan, T.; Sondak, V.K.; et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000, 6, 1–10. [Google Scholar] [PubMed]
- Pass, H.I.; Brewer, G.J.; Dick, R.; Carbone, M.; Merajver, S. A Phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: Final results. Ann. Thorac. Surg. 2008, 86, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, Q.; Li, J.L.; Jiang, L.; Gu, W.; Liu, X.; Tian, J.L.; Yan, S.P. Two water-soluble Copper(II) complexes: Synthesis, characterization, DNA cleavage, protein binding activities and in vitro anticancer activity studies. J. Inorg. Biochem. 2014, 137, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Li, A.; Shao, J.; Xie, C.Z.; Song, X.Q.; Bao, W.G.; Xu, J.Y. Four Cu(II) complexes based on antitumor chelators: Synthesis, structure, DNA binding/damage, HSA interaction and enhanced cytotoxicity. Dalton Trans. 2016, 45, 8036–8049. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, B.; Guedes da Silva, M.F.C.; Silva, J.; Mendo, A.S.; Baptista, P.V.; Fernandes, A.R.; Pombeiro, A.J.L. Synthesis, Characterization, thermal properties and antiproliferative potential of Copper(II) 4′-phenyl-terpyridine compounds. Dalton Trans. 2016, 45, 5339–5355. [Google Scholar] [CrossRef] [PubMed]
- Molphy, Z.; Prisecaru, A.; Slator, C.; Barron, N.; McCann, M.; Colleran, J.; Chandran, D.; Gathergood, N.; Kellett, A. Copper phenanthrene oxidative chemical nucleases. Inorg. Chem. 2014, 53, 5392–5404. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lutsenko, S. Human copper transporters: Mechanism, role in human diseases and therapeutic potential. Future Med. Chem. 2009, 1, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Palanimuthu, D.; Shinde, S.V.; Somasundaram, K.; Samuelson, A.G. In vitro and in vivo anticancer activity of copper bis(thiosemicarbazone) complexes. J. Med. Chem. 2013, 56, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Li, Y.; Zhang, D.Y.; Nie, Y.; Li, Z.J.; Gu, W.; Liu, X.; Tian, J.L.; Yan, S.P. Copper complexes based on chiral schiff-base ligands: DNA/BSA binding ability, DNA cleavage activity, cytotoxicity and mechanism of apoptosis. Eur. J. Med. Chem. 2016, 114, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.J.; Wang, X.T.; Xie, C.Z.; Tian, H.; Song, X.Q.; Pan, H.T.; Qiao, X.; Xu, J.Y. Mixed-ligand Copper(II) schiff base complexes: The role of the co-ligand in DNA binding, DNA cleavage, protein binding and cytotoxicity. Dalton Trans. 2016, 45, 9073–9087. [Google Scholar] [CrossRef] [PubMed]
- Meenongwa, A.; Brissos, R.F.; Soikum, C.; Chaveerach, P.; Gamez, P.; Trongpanich, Y.; Chaveerach, U. Effects of N,N-heterocyclic ligands on the in vitro cytotoxicity and DNA interactions of Copper(II) chloride complexes from amidino-O-methylurea ligands. New J. Chem. 2016, 40, 5861–5876. [Google Scholar] [CrossRef]
- Puckett, C.A.; Barton, J.K. Methods to explore cellular uptake of ruthenium complexes. J. Am. Chem. Soc. 2007, 129, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Howerton, B.S.; Heidary, D.K.; Glazer, E.C. Strained ruthenium complexes are potent light-activated anticancer agents. J. Am. Chem. Soc. 2012, 134, 8324–8327. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, a new redox-active anticancer agent—Preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Leijen, S.; Burgers, S.A.; Baas, P.; Pluim, D.; Tibben, M.; van Werkhoven, E.; Alessio, E.; Sava, G.; Beijnen, J.H.; Schellens, J.H.M. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs 2015, 33, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.P.; Cook, D.; Morte, S.R.; McIntyre, P.; Buchner, K.; Beer, H.; Cardin, D.J.; Brazier, J.A.; Winter, G.; Kelly, J.M.; et al. X-ray crystal structure of rac-[Ru(phen)2dppz]2+ with d(ATGCAT)2 shows enantiomer orientations and water ordering. J. Am. Chem. Soc. 2013, 135, 12652–12659. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.; Revankar, V.K.; Khan, A.; Butcher, R.J.; Thatipamula, K. Supramolecular architecture and photophysical and biological properties of Ruthenium(II) polypyridyl complexes. New J. Chem. 2015, 39, 3646–3657. [Google Scholar] [CrossRef]
- Chen, L.; Peng, F.; Li, G.; Jie, X.; Cai, K.R.; Cai, C.; Zhong, Y.; Zeng, H.; Li, W.; Zhang, Z.; et al. The studies on the cytotoxicity in vitro, cellular uptake, cell cycle arrest and apoptosis-inducing properties of ruthenium methylimidazole complex [Ru(MeIm)4(p-cpip)]2+. J. Inorg. Biochem. 2016, 156, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Pierroz, V.; Rubbiani, R.; Patra, M.; Hess, J.; Spingler, B.; Oehninger, L.; Schur, J.; Ott, I.; Salassa, L.; et al. DNA intercalating RuII polypyridyl complexes as effective photosensitizers in photodynamic therapy. Chem. Eur. J. 2014, 20, 14421–14436. [Google Scholar] [CrossRef] [PubMed]
- Kaspler, P.; Lazic, S.; Forward, S.; Arenas, Y.; Mandel, A.; Lilge, L. A Ruthenium(II) based photosensitizer and transferrin complexes enhance photo-physical properties, cell uptake, and photodynamic therapy safety and efficacy. Photochem. Photobiol. Sci. 2016, 15, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Kasimova, K.; Arenas, Y.; Kaspler, P.; Lazic, S.; Mandel, A.; Lilge, L. A novel class of ruthenium-based photosensitizers effectively kills in vitro cancer cells and in vivo tumors. Photochem. Photobiol. Sci. 2015, 14, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.; Rodriguez-Corrales, J.A.; Donohoe, L.E.; Winkel, B.S.J.; Brewer, K.J. A new class of Ru(II) polyazine agents with potential for photodynamic therapy. Chem. Commun. 2016, 52, 2705–2708. [Google Scholar] [CrossRef] [PubMed]
- Padilla, R.; Maza, W.A.; Dominijanni, A.J.; Winkel, B.S.J.; Morris, A.J.; Brewer, K.J. Pushing the limits of structurally-diverse light-harvesting Ru(II) metal-organic chromophores for photodynamic therapy. J. Photochem. Photobiol. A 2016, 322, 67–75. [Google Scholar] [CrossRef]
- Caruso, F.; Monti, E.; Matthews, J.; Rossi, M.; Gariboldi, M.B.; Pettinari, C.; Pettinari, R.; Marchetti, F. Synthesis, characterization, and antitumor activity of water-soluble (arene)ruthenium(II) derivatives of 1,3-Dimethyl-4-acylpyrazolon-5-ato ligands. First example of Ru(arene)(ligand) antitumor species involving simultaneous Ru–N7(guanine) bonding and ligand intercalation to DNA. Inorg. Chem. 2014, 53, 3668–3677. [Google Scholar] [PubMed]
- Colina-Vegas, L.; Villarreal, W.; Navarro, M.; de Oliveira, C.R.; Graminha, A.E.; Maia, P.I.D.S.; Deflon, V.M.; Ferreira, A.G.; Cominetti, M.R.; Batista, A.A. Cytotoxicity of Ru(II) piano–stool complexes with chloroquine and chelating ligands against breast and lung tumor cells: Interactions with DNA and BSA. J. Inorg. Biochem. 2015, 153, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, X.; Huang, H.; Zhao, C.; Liao, S.; Yang, C.; Liu, S.; Song, W.; Lu, X.; Lan, X.; et al. Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget 2014, 5, 5453–5471. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lum, C.T.; Lok, C.N.; Zhang, J.J.; Che, C.M. Chemical biology of anticancer Gold(III) and Gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- Nardon, C.; Fregona, D. Gold(III) complexes in the oncological preclinical arena: From aminoderivatives to peptidomimetics. Curr. Top. Med. Chem. 2016, 16, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Rubbiani, R.; Salassa, L.; de Almeida, A.; Casini, A.; Ott, I. Cytotoxic Gold(I) N-heterocyclic carbene complexes with phosphane ligands as potent enzyme inhibitors. ChemMedChem 2014, 9, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Holenya, P.; Can, S.; Rubbiani, R.; Alborzinia, H.; Junger, A.; Cheng, X.; Ott, I.; Wolfl, S. Detailed analysis of pro-apoptotic signaling and metabolic adaptation ttriggered by a N-heterocyclic carbene-gold(I) complex. Metallomics 2014, 6, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Nardon, C.; Schmitt, S.M.; Yang, H.; Zuo, J.; Fregona, D.; Dou, Q.P. Gold(III)-dithiocarbamato peptidomimetics in the forefront of the targeted anticancer therapy: Preclinical studies against human breast neoplasia. PLoS ONE 2014, 9, e84248. [Google Scholar] [CrossRef] [PubMed]
- Akerman, K.J.; Fagenson, A.M.; Cyril, V.; Taylor, M.; Muller, M.T.; Akerman, M.P.; Munro, O.Q. Gold(III) macrocycles: Nucleotide-specific unconventional catalytic inhibitors of human topoisomerase I. J. Am. Chem. Soc. 2014, 136, 5670–5682. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Oehninger, L.; Geldmacher, Y.; Alborzinia, H.; Wölfl, S.; Sheldrick, W.S.; Ott, I. Gold(I) N-heterocyclic carbene complexes with naphthalimide ligands as combined thioredoxin reductase inhibitors and DNA intercalators. ChemMedChem 2014, 9, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, W.; Yu, L.; Zheng, W.; Li, L.; Fan, C.; Chen, T. Zinc(II) complexes containing bis-benzimidazole derivatives as a new class of apoptosis inducers that trigger DNA damage-mediated p53 phosphorylation in cancer cells. Dalton Trans. 2013, 42, 5932–5940. [Google Scholar] [CrossRef] [PubMed]
- Haribabu, J.; Jeyalakshmi, K.; Arun, Y.; Bhuvanesh, N.S.P.; Perumal, P.T.; Karvembu, R. Synthesis, DNA/protein binding, molecular docking, DNA cleavage and in vitro anticancer activity of Nickel(II) bis(thiosemicarbazone) complexes. RSC Adv. 2015, 5, 46031–46049. [Google Scholar] [CrossRef]
- Zhang, H.R.; Liu, Y.C.; Meng, T.; Qin, Q.P.; Tang, S.F.; Chen, Z.F.; Zou, B.Q.; Liu, Y.N.; Liang, H. Cytotoxicity, DNA binding and cell apoptosis induction of a Zinc(II) complex of HBrQ. Med. Chem. Commun. 2015, 6, 2224–2231. [Google Scholar] [CrossRef]
- Tabrizi, L.; McArdle, P.; Erxleben, A.; Chiniforoshan, H. Nickel(II) and Cobalt(II) complexes of lidocaine: Synthesis, structure and comparative in vitro evaluations of biological perspectives. Eur. J. Med. Chem. 2015, 103, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Ting Hun, L. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Yu, Y.; Gutierrez, E.; Kovacevic, Z.; Saletta, F.; Obeidy, P.; Rahmanto, Y.S.; Richardson, D.R. Iron chelators for the treatment of cancer. Curr. Med. Chem. 2012, 19, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Del Oliveira, A.C.; dalSilva, E.G.; Rocha, D.D.; Hillard, E.A.; Pigeon, P.; Jaouen, G.; Rodrigues, F.A.R.; de Abreu, F.C.; da Rocha Ferreira, F.; Goulart, M.O.F.; et al. Molecular mechanism of action of 2-Ferrocenyl-1,1-diphenylbut-1-ene on HL-60 leukemia cells. ChemMedChem 2014, 9, 2580–2586. [Google Scholar] [CrossRef] [PubMed]


| Complex | IC50 (µM) | ||
|---|---|---|---|
| L1210 | Du145 | A2780 | |
| Pt1 | 0.10 ± 0.01 | 0.08 ± 0.05 | 0.27 ± 0.03 |
| Pt1’ | 1.5 ± 0.1 | 0.79 ± 0.08 | 2.7 ± 0.07 |
| Pt2 | 0.009 ± 0.002 | 0.007 ± 0.002 | 0.030 ± 0.004 |
| Pt2’ | 0.46 ± 0.01 | 0.41 ± 0.04 | 1.1 ± 0.1 |
| Pt3 | 0.19 ± 0.01 | 0.44 ± 0.06 | 2.0 ± 0.1 |
| Pt3′ | 0.8 ± 0.2 | 2.7 ± 0.2 | 6.5 ± 0.0 |
| Pt4 | 1.3 ± 0.4 | 2.2 ± 0.1 | 3.7 ± 0.4 |
| Pt4′ | 6 ± 2 | 3 ± 1 | 2.0 ± 0.1 |
| Pt5 | 0.6 ± 0.2 | 1.3 ± 0.4 | 2.6 ± 0.2 |
| Pt5′ | 5.5 ± 0.1 | n.d. | n.d |
| Pt6 | 0.36 ± 0.02 | 0.12 ± 0.03 | 1.1 ± 0.3 |
| Pt6’ | 1.8 ± 0.0 | 1.5 ± 0.03 | 5.6 ± 0.5 |
| Cisplatin | 0.35–1 [a] | 1.2 ± 0.1 | 1.0 ± 0.1 |
| Carboplatin | n.d. | 2.9 ± 0.4 | 0.16 ± 0.0 |
| Oxaliplatin | n.d. | 15 ± 1 | 9 ± 3 |
| Complex | IC50 (µM) | ||||
|---|---|---|---|---|---|
| Cell Line | |||||
| NCI-H460 | NCI-H520 | NCI-H522 | A549 | HL-60 | |
| Pt7 | 0.0052 ± 0.0001 | 0.043 ± 0.004 | 0.010 ± 0.001 | 0.0065 ± 0.0002 | – |
| Pt7’ | – | – | – | – | 0.13 |
| Pt8 | 0.24 ± 0.01 | 0.52 ± 0.01 | 0.12 ± 0.02 | 0.32 ± 0.06 | – |
| Pt8’ | 2.4 ± 0.5 | 2.2 ± 0.1 | 3.62 ± 0.08 | 12.4 ± 0.9 | – |
| Complex | IC50 (μM) | Reference | ||
|---|---|---|---|---|
| HCT116 | HepG-2 | NHF [a] | ||
| Cu5 | 0.31 ± 0.03 | 14.0 ± 0.5 | >20 | [48] |
| Cu6 | 0.468 ± 0.006 | 13.6 ± 0.5 | >20 | |
| Cu7 | 0.44 ± 0.09 | 0.54 ± 0.03 | >5 | |
| Cu8 | 1.5 ± 0.2 | 0.7 ± 0.1 | >5 | |
| Cu9 | 0.07 ± 0.05 | 0.24 ± 0.02 | 5.483 ± 0.003 | |
| Complex | HeLa | HepG-2 | NCI-H460 | Reference |
| Cu10 | 0.16 ± 0.05 | 0.10 ± 0.04 | 0.08 ± 0.01 | [47] |
| Cu11 | 0.59 ± 0.02 | 0.20 ± 0.01 | 0.16 ± 0.01 | |
| Cu12 | 1.4 ± 0.6 | 1.1 ± 0.4 | 2.0 ± 0.3 | |
| Cu13 | 1.3 ± 0.2 | 0.8 ± 0.2 | 1.5 ± 0.7 | |
| Complex | IC50 (µM) | Reference | Complex | IC50 (µM) | Reference |
|---|---|---|---|---|---|
| Ru1 | 28.0 ± 0.1 | [60] | Ru6 [a] | 2.0 ± 0.9 | [62] |
| Ru2 | 21.00 ± 0.08 | Ru7 [a] | 5.5 ± 0.7 | ||
| Ru3 | 19.00 ± 0.08 | – | – | – | |
| Ru4 | 27 ± 2 | [61] | Cisplatin | 15 ± 2 | [61] |
| Ru5 | 25 ± 2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deo, K.M.; Pages, B.J.; Ang, D.L.; Gordon, C.P.; Aldrich-Wright, J.R. Transition Metal Intercalators as Anticancer Agents—Recent Advances. Int. J. Mol. Sci. 2016, 17, 1818. https://doi.org/10.3390/ijms17111818
Deo KM, Pages BJ, Ang DL, Gordon CP, Aldrich-Wright JR. Transition Metal Intercalators as Anticancer Agents—Recent Advances. International Journal of Molecular Sciences. 2016; 17(11):1818. https://doi.org/10.3390/ijms17111818
Chicago/Turabian StyleDeo, Krishant M., Benjamin J. Pages, Dale L. Ang, Christopher P. Gordon, and Janice R. Aldrich-Wright. 2016. "Transition Metal Intercalators as Anticancer Agents—Recent Advances" International Journal of Molecular Sciences 17, no. 11: 1818. https://doi.org/10.3390/ijms17111818
APA StyleDeo, K. M., Pages, B. J., Ang, D. L., Gordon, C. P., & Aldrich-Wright, J. R. (2016). Transition Metal Intercalators as Anticancer Agents—Recent Advances. International Journal of Molecular Sciences, 17(11), 1818. https://doi.org/10.3390/ijms17111818