Elevated O3 and TYLCV Infection Reduce the Suitability of Tomato as a Host for the Whitefly Bemisia tabaci
Abstract
:1. Introduction
2. Results
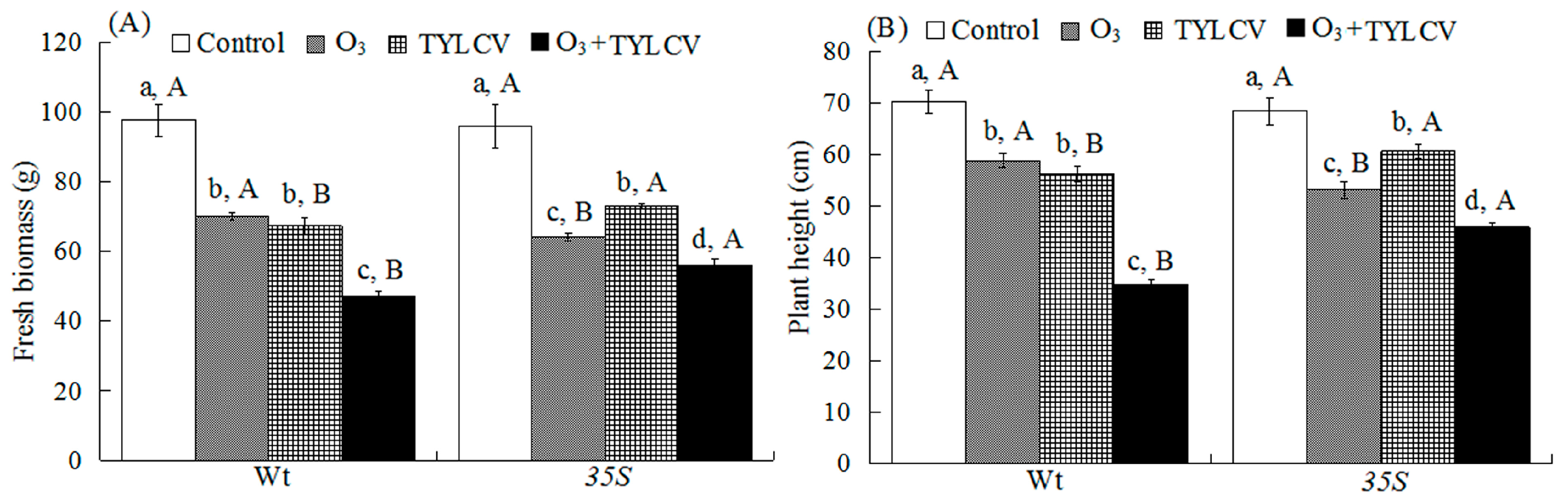
2.1. Tomato Growth Traits
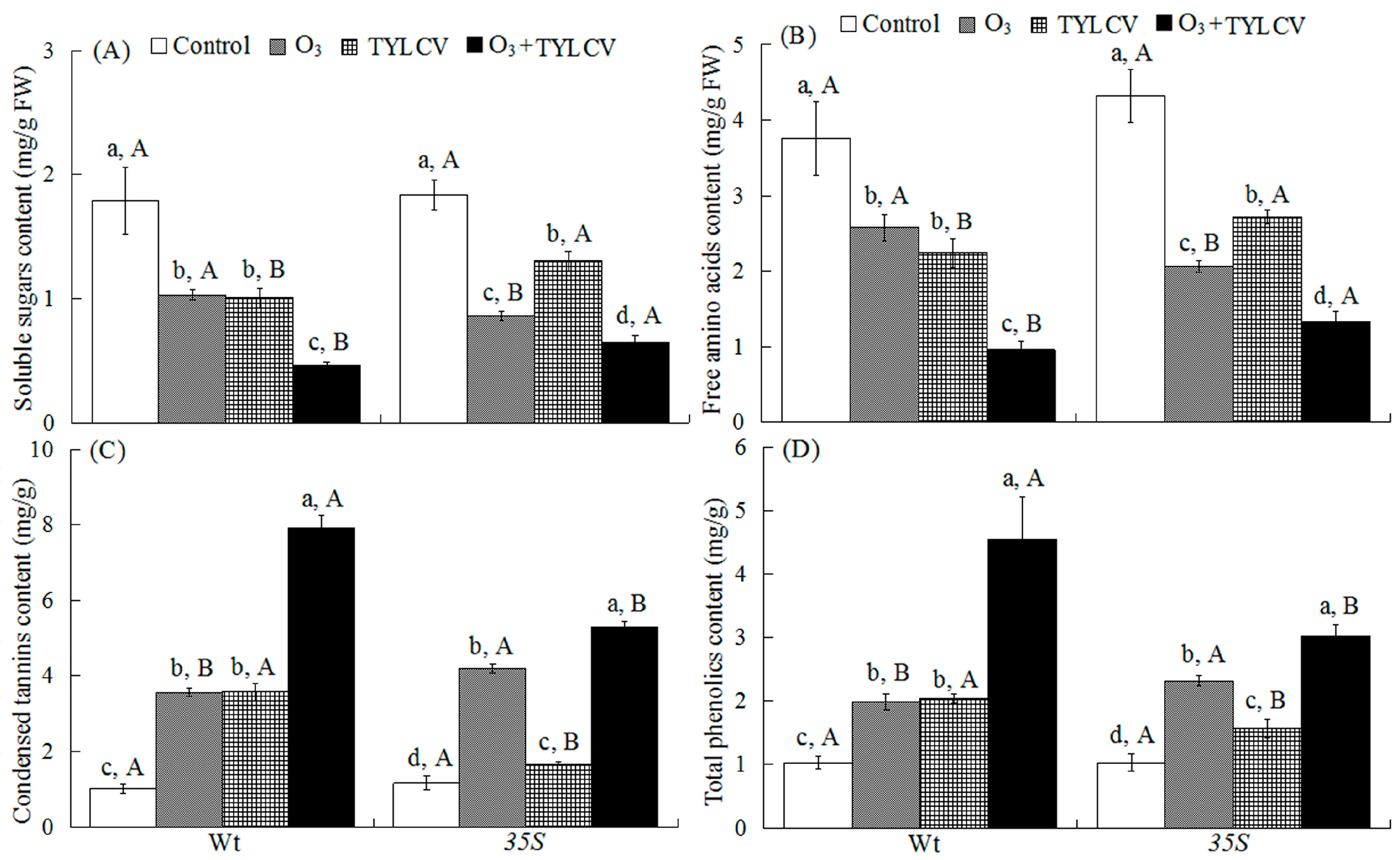
2.2. Foliar Soluble Sugar and Free Amino Acids of Tomato
2.3. Condensed Tannins and Total Phenolics in Tomato Leaves
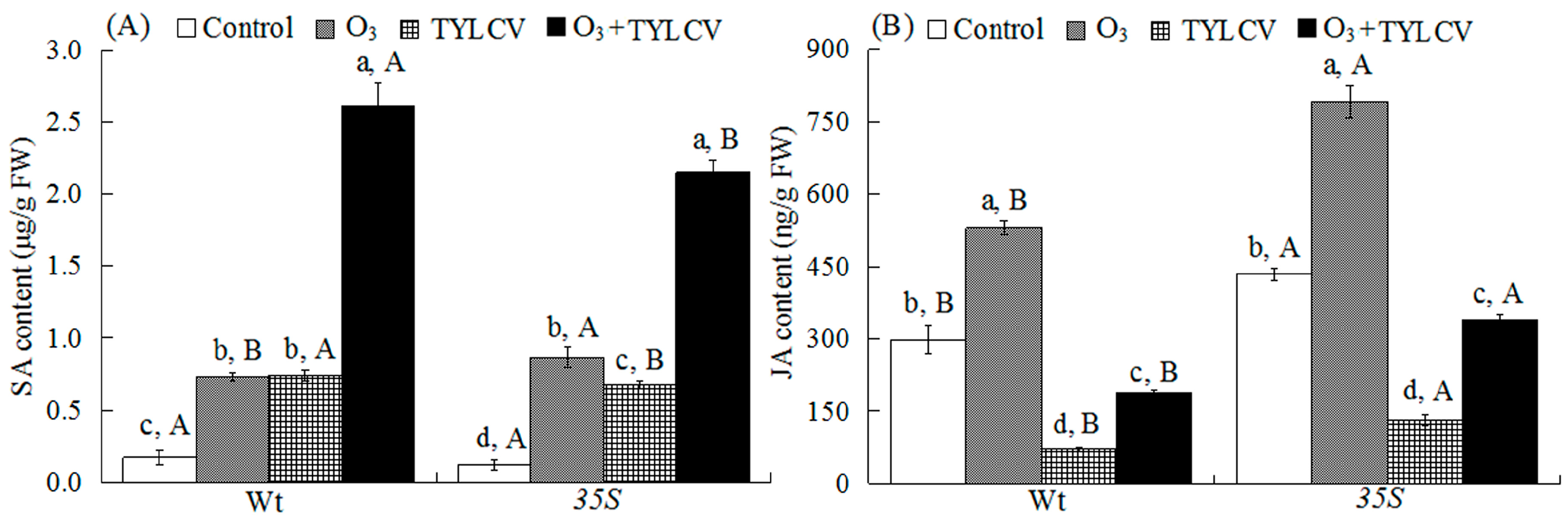
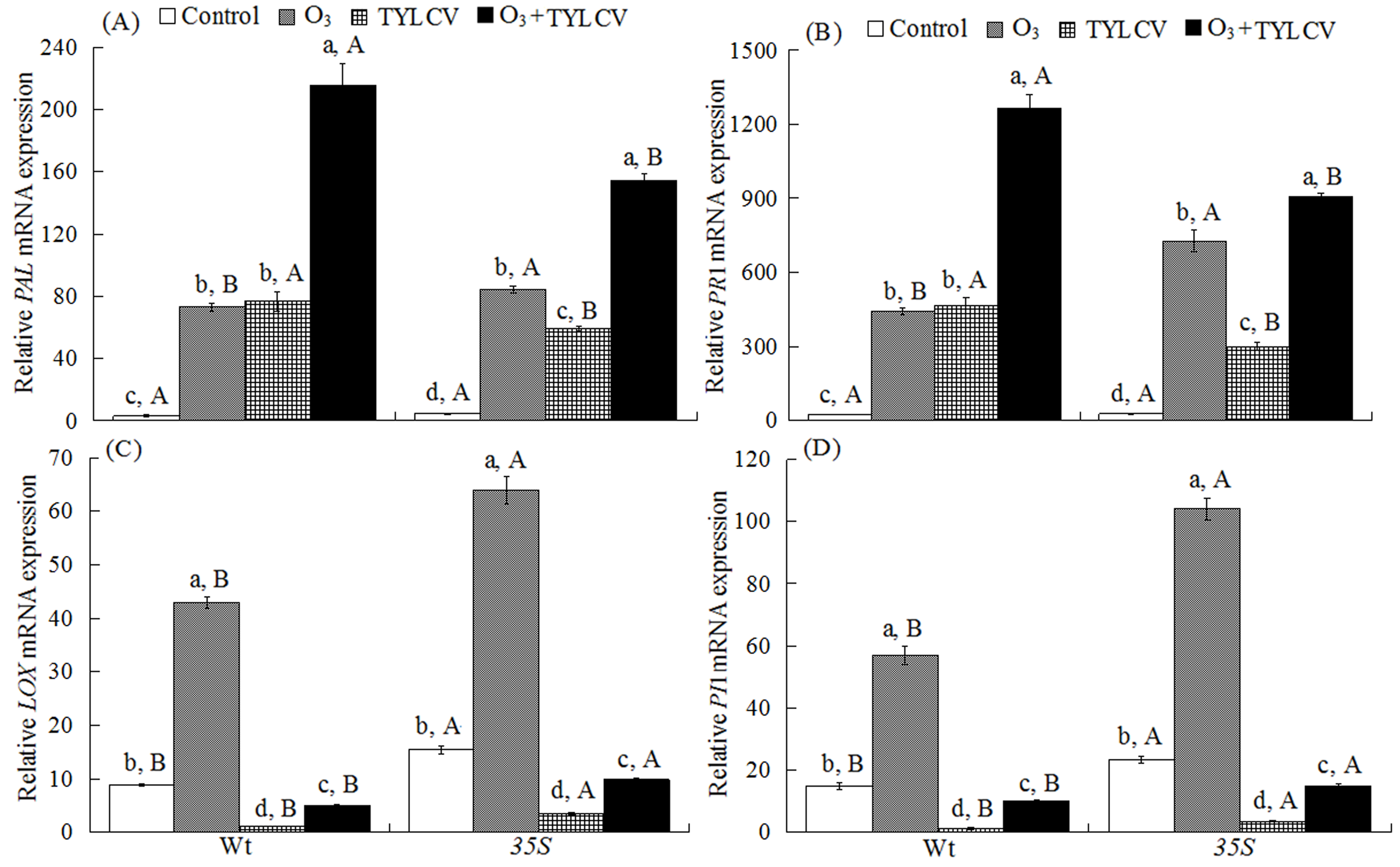
2.4. SA Content and Expression of Phenylalanine Ammonia Lyase Gene (PAL) and Pathogenesis-Related Protein Gene (PR1) in Tomato
2.5. JA Content and Expression of Lipoxygenase Gene (LOX) and Proteinase Inhibitor Gene (PI1) in Tomato
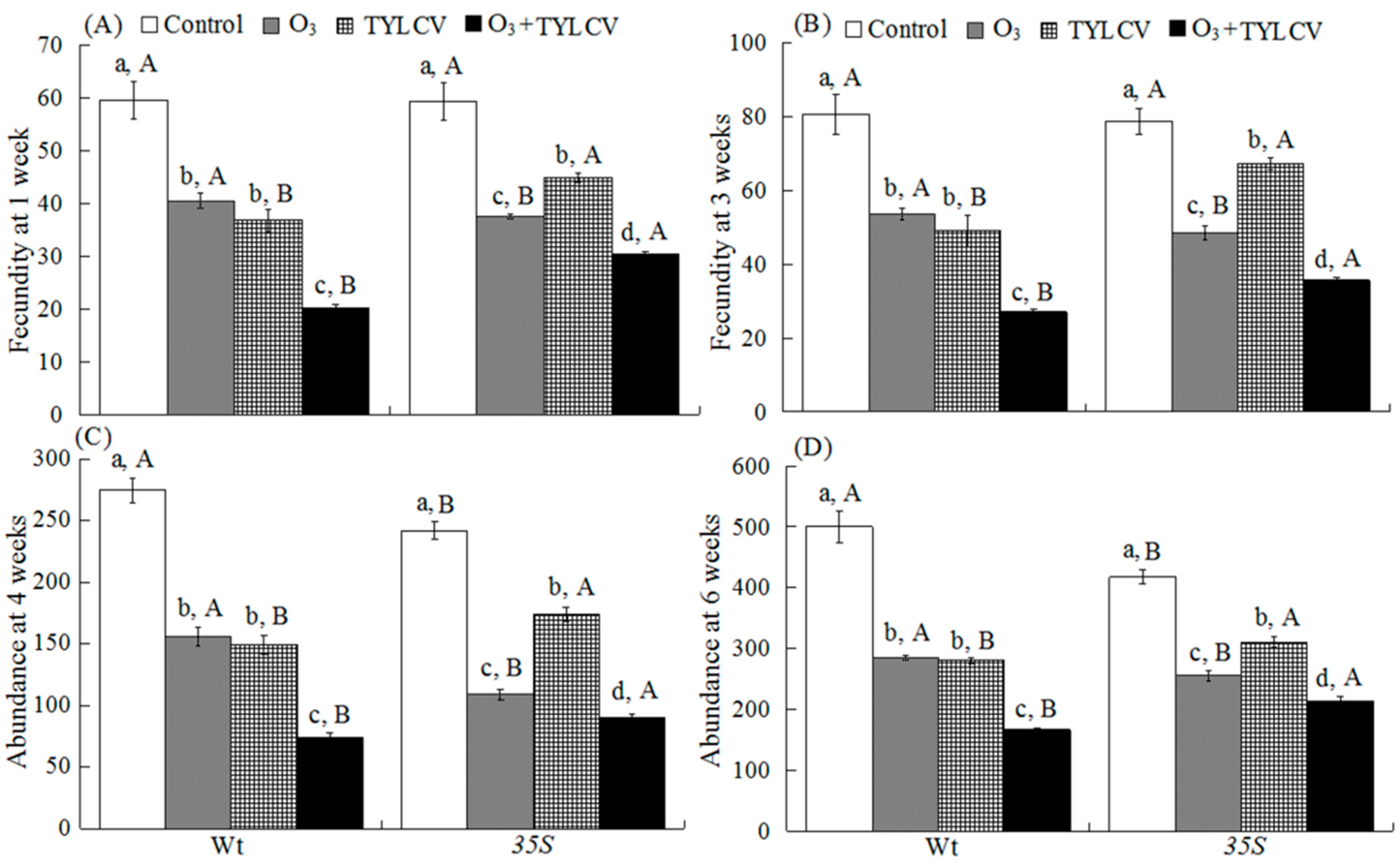
2.6. Fecundity and Abundance of B. tabaci
2.7. Pearson Correlations between B. tabaci Fecundity and Abundance and Biochemical Properties of Tomato Leaves
3. Discussion
4. Materials and Methods
4.1. Open-Top Chambers
4.2. Host Plants
4.3. Tomato yellow leaf curl virus Clone and Agroinoculation
4.4. Tomato Growth Traits
4.5. Tomato Foliar Chemistry
4.6. Fecundity and Abundance of B. tabaci
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TYLCV | Tomato yellow leaf curl virus |
| Wt | wild-type |
| JA | jasmonic acid |
| SA | salicylic acid |
| ppb | part per billion |
| ROS | reactive oxygen species |
| OTCs | open-top chambers |
| PR1 | pathogenesis-related protein |
| PAL | phenylalanine ammonia lyase |
| PI1 | proteinase inhibitor |
| LOX | lipoxygenase |
| rm | intrinsic rate of increase |
| TSWV | Tomato spotted wilt virus |
| AFLP | amplified fragment-length polymorphism |
References
- Van Dingenen, R.; Dentener, F.J.; Raes, F.; Krol, M.C.; Emberson, L.; Cofala, J. The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmos. Environ. 2009, 43, 604–618. [Google Scholar] [CrossRef]
- Blande, J.D.; Holopainen, J.K.; Li, T. Air pollution impedes plant-to-plant communication by volatiles. Ecol. Lett. 2010, 13, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- McGrath, J.M.; Betzelberger, A.M.; Wang, S.W.; Shook, E.; Zhu, X.G.; Long, S.P.; Ainsworth, E.A. An analysis of ozone damage to historical maize and soybean yields in the United States. Proc. Natl. Acad. Sci. USA 2015, 112, 14390–14395. [Google Scholar] [CrossRef] [PubMed]
- Langebartels, C.; Ernst, D.; Kangasjarvi, J.; Sandermann, H. Ozone effects on plant defense. Method Enzymol. 2000, 319, 520–535. [Google Scholar]
- Ye, L.F.; Fu, X.; Ge, F. Enhanced sensitivity to higher ozone in a pathogen-resistant tobacco cultivar. J. Exp. Bot. 2012, 63, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Cui, H.Y.; Sun, Y.C.; Su, J.W.; Ren, Q.; Li, C.Y.; Ge, F. Elevated O3 reduces the fitness of Bemisia tabaci via enhancement of the SA-dependent defense of the tomato plant. Arthropod Plant Interact. 2012, 6, 425–437. [Google Scholar] [CrossRef]
- Percy, K.E.; Awmack, C.S.; Lindroth, R.L.; Kubiske, M.E.; Kopper, B.J.; Isebrands, J.G.; Pregitzer, K.S.; Hendrey, G.R.; Dickson, R.E.; Zak, D.R.; et al. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature 2002, 420, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Agrell, J.; Kopper, B.; McDonald, E.P.; Lindroth, R.L. CO2 and O3 effects on host plant preferences of the forest tent caterpillar (Malacosoma disstria). Glob. Chang. Biol. 2005, 11, 588–599. [Google Scholar] [CrossRef]
- Pegadaraju, V.; Knepper, C.; Reese, J.; Shah, J. Premature leaf senescence modulated by the Arabidopsis Phytoalexin Deficient4 gene is associated with defence against the phloem-feeding green peach aphid. Plant Physiol. 2005, 139, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Eastburn, D.M.; DeGennaro, M.M.; DeLucia, E.H.; Dermody, O.; McElrone, A.J. Elevated atmospheric carbon dioxide and ozone alter soybean diseases at SoyFACE. Glob. Chang. Biol. 2010, 16, 320–330. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Whitham, S.A.; Yang, C.; Goodin, M.M. Global impact: Elucidating plant responses to viral infection. Mol. Plant Microbe Interact. 2006, 19, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Lewsey, M.G.; Murphy, A.M.; MacLean, D.; Dalchau, N.; Westwood, J.H.; Macaulay, K.; Bennett, M.H.; Moulin, M.; Hanke, D.E.; Powell, G.; et al. Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol. Plant Microbe Interact. 2010, 23, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Colvin, J.; Omongo, C.A.; Govindappa, M.R.; Stevenson, P.C.; Maruthi, M.N.; Gibson, G.; Seal, S.E.; Muniyappa, V. Host-plant viral infection effects on arthropod-vector population growth, development and behaviour: Management and epidemiological implications. Adv. Virus Res. 2006, 67, 419–452. [Google Scholar] [PubMed]
- Shi, X.B.; Pan, H.P.; Zhang, H.Y.; Jiao, X.G.; Xie, W.; Wu, Q.J.; Wang, S.L.; Fang, Y.; Chen, G.; Zhou, X.G.; et al. Bemisia tabaci Q carrying tomato yellow leaf curl virus strongly suppresses host plant defenses. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Kangasjarvi, J.; Jaspers, P.; Kollist, H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005, 28, 1021–1036. [Google Scholar] [CrossRef]
- Meur, G.; Budatha, M.; Srinivasan, T.; Kumar, K.R.R.; Gupta, A.D.; Kirti, P.B. Constitutive expression of Arabidopsis NPR1 confers enhanced resistance to the early instars of Spodoptera liturain transgenic tobacco. Physiol. Plant. 2008, 133, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Verhage, A.; van Wees, S.C.M.; Pieterse, C.M.J. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 2010, 154, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Tack, A.J.M.; Dicke, M. Plant pathogens structure arthropod communities across multiple spatial and temporal scales. Funct. Ecol. 2013, 27, 633–645. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Pieterse, C.M.J. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.A.; Agrawal, A.A.; Halitschke, R. Salicylate mediated interactions between pathogens and herbivores. Ecology 2010, 91, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Cardoza, V.; Mitchell, D.M.; Bright, L.; Oldroyd, G.; Harris, J.M. Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 2006, 46, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Doares, S.H.; Narvaezvasquez, J.; Conconi, A.; Ryan, C.A. Salicylic acid inhibits synthesis of proteinase-inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995, 108, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.; Enright, S.; Traw, M.B.; Bergelson, J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol. 2004, 13, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gong, H.; Zhou, X. Molecular characterization and pathogenicity of tomato yellow leaf curl virus in China. Virus Genes 2009, 39, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Bruessow, F.; Gouhier-Darimont, C.; Buchala, A.; Metraux, J.P.; Reymond, P. Insect eggs suppress plant defence against chewing herbivores. Plant J. 2010, 62, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Mescher, M.C.; Wang, S.L.; Chen, G.; Xie, W.; Wu, Q.J.; Wang, W.K.; Zhang, Y.J. Tomato yellow leaf curl virus differentially influences plant defense responses to a vector and a non-vector herbivore. Plant Cell Environ. 2016, 39, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Lee, H.I.; Creelman, R.A.; Mullet, J.E.; Davis, J.E. Jasmonic acid signalling modulates ozone-induced hypersensitive cell death. Plant Cell 2000, 12, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Kanna, M.; Tamaoki, M.; Kubo, A.; Nakajima, N.; Rakwal, R.; Agrawal, G.K.; Tamogami, S.; Loki, M.; Ogawa, D.; Saji, H.; et al. Isolation of an ozone-sensitive and jasmonate-semi-insensitive Arabidopsis mutant (oji1). Plant Cell Physiol. 2003, 44, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, D.D.; Aldea, M.; O’Neill, B.F.; Benitez, M.; Li, M.; Clough, S.J.; DeLucia, E.H. Elevated ozone alters soybean–virus interaction. Mol. Plant Microbe Interact. 2008, 21, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Ren, Q.; Sun, Y.C.; Ye, L.F.; Cao, H.F.; Ge, F. Lower incidence and severity of tomato virus in elevated CO2 is accompanied by modulated plant induced defence in tomato. Plant Biol. 2012, 14, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cai, X.; Zhou, X. Tomato leaf curl Guangxi virus is a distinct monopartite begomovirus species. Eur. J. Plant Pathol. 2007, 118, 287–294. [Google Scholar] [CrossRef]
- Wan, H.J.; Yuan, W.; Wang, R.Q.; Ye, Q.J.; Ruan, M.Y.; Li, Z.M.; Zhou, G.Z.; Yao, Z.P.; Yang, Y.J. Assessment of the genetic diversity of tomato yellow leaf curl virus. Genet. Mol. Res. 2015, 14, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Boykin, L.M.; Shatters, R.G.; Rosell, R.C.; McKenzie, C.L.; Bagnall, R.A.; Barro, P.D.; Frohlich, D.R. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007, 44, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.N.; Bellows, T.S. Whitefly biology. Annu. Rev. Entomol. 1991, 36, 431–457. [Google Scholar] [CrossRef]
- Bardin, M.; Fargues, J.; Nicot, P.C. Compatibility between biopesticides used to control grey mould, powdery mildew and whitefly on tomato. Biol. Control 2008, 46, 476–483. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schutz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Jiu, M.; Zhou, X.P.; Tong, L.; Xu, J.; Yang, X.; Wan, F.H.; Liu, S.S. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2007, 2, e182. [Google Scholar] [CrossRef] [PubMed]
- Oguntimehin, I.; Eissa, F.; Sakugawa, H. Simultaneous ozone fumigation and fluoranthene sprayed as mists negatively affected cherry tomato (Lycopersicon esculentum Mill). Ecotoxicol. Environ. Saf. 2010, 73, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- McConn, M.; Creelman, R.A.; Bell, E.; Mullet, J.E. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.D.; Thaler, J.S.; Granett, J.; Karban, R. Jasmonic acid induced resistance in grapevines to a root and leaf feeder. J. Econ. Entomol. 2000, 93, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, D.; Ray, J. Increase in surface ozone at rural sites in the western US. Atmos. Environ. 2007, 41, 5452–5463. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Kobayashi, K.; Ainsworth, E.A. Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): A meta-analysis. Glob. Chang. Biol. 2008, 14, 2696–2708. [Google Scholar]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Tsukahara, K.; Sawada, H.; Kohno, Y.; Matsuura, T.; Mori, I.C.; Terao, T.; Loki, M.; Tamaoki, M. Ozone-induced rice grain yield loss is triggered via a change in panicle morphology that is controlled by ABERRANT PANICLE ORGANIZATION 1 gene. PLoS ONE 2015, 10, e0123308. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ye, L.F.; Ge, F. Elevated CO2 shifts the focus of tobacco plant defences from cucumber mosaic virus to the green peach aphid. Plant Cell Environ. 2010, 33, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.J.; Taylor, R.; Karren, M.E. Divergent responses of two cereal aphids to previous infestation of their host plant. Entomol. Exp. Appl. 2002, 16, 43–50. [Google Scholar] [CrossRef]
- Cui, H.Y.; Sun, Y.C.; Su, J.W.; Li, C.Y.; Ge, F. Reduction in the fitness of Bemisia tabaci fed on three previously infested tomato genotypes differing in the jasmonic acid pathway. Environ. Entomol. 2012, 41, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; van Loon, L.C. NPR1: The spider in the web of induced resistance signaling pathways. Curr. Opin. Plant Biol. 2004, 7, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.F.; Chen, J.Y.; Kong, W.F.; Pan, Q.H.; Wan, S.B.; Huang, W.D. Salicylic acid induced the expression of phenylalanine ammonia lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- Liu, H.T.; Liu, Y.Y.; Pan, Q.H.; Yang, H.R.; Zhan, J.C.; Huang, W.D. Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J. Exp. Bot. 2006, 57, 3337–3347. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.R.; Jia, L.; Goggin, F.L. Acquired and R-gene-mediated resistance against the potato aphid in tomato. J. Chem. Ecol. 2004, 30, 2527–2542. [Google Scholar] [CrossRef] [PubMed]
- Chaman, M.E.; Copaja, S.V.; Argandona, V.H. Relationships between salicylic acid content, phenylalanine ammonia lyase (PAL) activity, and resistance of barley to aphid infestation. J. Agric. Food Chem. 2003, 51, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Ilarduya, O.; Xie, Q.G.; Kaloshian, I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant Microbe Interact. 2003, 16, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.H.; Zohdy, N.M.; El-Gengaihi, S.E.; Amr, A.E. The relationship between tannins concentration in some cotton varieties and susceptibility to piercing sucking insects. J. Appl. Entomol. 1997, 121, 321–325. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Jens, M.A.P.; Brotman, Y.; Mikhail, K.; Henryk, S.I.C.; Rena, G. Stress responses to Tomato yellow leaf curl virus (TYLCV) infection of resistant and susceptible tomato plants are different. Metab. Open Access 2012. [Google Scholar] [CrossRef]
- Abe, H.; Tomitaka, Y.; Shimoda, T.; Seo, S.; Sakurai, T.; Kugimiya, S.; Tsuda, S.; Kobayashi, M. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 2012, 53, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, M.; Lapidot, M.; Cohen, S.; Pilowsky, M. Novel source of resistance to Tomato yellow leaf curl virus exhibiting a symptomless reaction to viral infection. J. Am. Soc. Hortic. Sci. 1998, 123, 1004–1007. [Google Scholar]
- Lapidot, M.; Friedmann, M.; Pilowsky, M.; Ben-Joseph, R.; Cohen, S. Effect of host plant resistance to Tomato yellow leaf curl virus (TYLCV) on virus acquisition and transmission by its whitefly vector. Phytopathology 2001, 91, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Karafyllldis, I.; Turner, J.G. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant Microbe Interact. 2002, 15, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Kempema, L.A.; Cui, X.P.; Holzer, F.M.; Walling, L.L. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 2007, 143, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, G.; Xu, C.; Lee, G.; Bauer, P.; Ganal, M.; Ling, H.; Howe, G.A. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 2003, 15, 1646–1661. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.N.; Wang, L.; Zhao, J.; Li, C.; Ge, F.; Kang, L. Ecological trade-offs between jasmonic acid-dependent direct and indirect plant defences in tritrophic interactions. New Phytol. 2011, 189, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Yin, J.; Cao, H.F.; Li, C.Y.; Kang, L.; Ge, F. Elevated CO2 influences nematode-induced defense responses of tomato genotypes differing in the JA pathway. PLoS ONE 2011, 6, e19751. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Leon-Reyes, A.; Ritsema, T.; Verhage, A.; DenOtter, F.C.; van Loon, L.C.; Pieterse, C.M. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 2008, 147, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.J.; Kenton, P.; Atzorn, R.; Miersch, O.; Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006, 140, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Halim, V.A.; Altmann, S.; Ellinger, D.; Eschen-Lippold, L.; Miersch, O.; Scheel, D.; Rosahl, S. PAMP-induced defense responses in potato require both salicylic acid and jasmonic acid. Plant J. 2009, 57, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Su, J.W.; Wei, J.N.; Hu, Y.J.; Ge, F. Elevated O3 enhances the attraction of whitefly-infested tomato plants to Encarsia formosa. Sci. Rep. 2014, 4, 5350. [Google Scholar] [CrossRef] [PubMed]
- Al Abdallat, A.; Al Debei, H.; Asmar, H.; Misbeh, S.; Quraan, A.; Kvarnheden, A. An efficient in vitro-inoculation method for Tomato yellow leaf curl virus. Virol. J. 2010, 7, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Zhang, Y.J.; Zhang, W.J.; Wu, Q.J.; Xu, B.Y.; Chu, D. Analysis of genetic diversity among different geographical populations and determination of biotypes of Bemisia tabaci in China. J. Appl. Entomol. 2005, 129, 121–128. [Google Scholar] [CrossRef]
| Measured Indices | Value | Treatment(df) | ||||||
|---|---|---|---|---|---|---|---|---|
| O3 (1, 184) | TYLCV (1, 184) | Tomato Genotype (1, 184) | O3 × TYLCV (1, 184) | O3 × Genotype (1, 184) | TYLCV × Genotype (1, 184) | O3 × TYLCV × Genotype (1, 184) | ||
| Fresh biomass | F | 930.88 | 703.90 | 4.72 | 50.27 | 0.06 | 50.27 | 4.77 |
| p | 0.00 | 0.00 | 0.03 | 0.00 | 0.80 | 0.00 | 0.03 | |
| Plant height | F | 297.10 | 212.51 | 5.05 | 6.83 | 0.61 | 40.02 | 8.61 |
| p | 0.00 | 0.00 | 0.03 | 0.01 | 0.44 | 0.00 | 0.00 | |
| Fecundity at one week | F | 164.26 | 132.49 | 6.86 | 14.61 | 0.02 | 3.04 | 0.75 |
| p | 0.00 | 0.00 | 0.01 | 0.00 | 0.90 | 0.08 | 0.39 | |
| Fecundity at three weeks | F | 164.52 | 90.42 | 5.14 | 15.33 | 2.15 | 0.19 | 0.58 |
| p | 0.00 | 0.00 | 0.03 | 0.00 | 0.14 | 0.67 | 0.45 | |
| Abundance at four weeks | F | 1768.14 | 908.72 | 16.88 | 89.48 | 5.49 | 151.95 | 0.33 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.57 | |
| Abundance at six weeks | F | 1246.29 | 851.42 | 4.12 | 100.69 | 18.25 | 131.01 | 0.30 |
| p | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.58 | |
| Measured Indices | Value | Treatment(df) | ||||||
|---|---|---|---|---|---|---|---|---|
| O3 (1, 24) | TYLCV (1, 24) | Tomato Genotype (1, 24) | O3 × TYLCV (1, 24) | O3 × Genotype (1, 24) | TYLCV × Genotype (1, 24) | O3 × TYLCV × Genotype (1, 24) | ||
| Soluble sugars | F | 307.79 | 156.07 | 4.89 | 9.85 | 4.01 | 12.86 | 0.56 |
| p | 0.00 | 0.00 | 0.03 | 0.00 | 0.07 | 0.00 | 0.46 | |
| Free amino acids | F | 297.79 | 238.19 | 6.47 | 4.80 | 11.22 | 5.11 | 7.75 |
| p | 0.00 | 0.00 | 0.02 | 0.04 | 0.00 | 0.03 | 0.01 | |
| Condensed tannins | F | 681.27 | 269.61 | 52.13 | 21.25 | 0.18 | 104.37 | 5.37 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.68 | 0.00 | 0.03 | |
| Total phenolics | F | 159.07 | 96.41 | 11.39 | 12.30 | 2.04 | 22.38 | 7.74 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 | 0.01 | |
| SA a | F | 590.53 | 503.75 | 4.42 | 103.99 | 1.83 | 11.67 | 7.68 |
| p | 0.00 | 0.00 | 0.04 | 0.00 | 0.19 | 0.00 | 0.01 | |
| JA b | F | 304.52 | 641.86 | 136.01 | 26.12 | 17.05 | 12.89 | 0.48 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 | |
| PAL c | F | 515.41 | 406.44 | 15.52 | 24.91 | 4.15 | 29.07 | 9.94 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | |
| PR1 d | F | 926.49 | 428.75 | 8.23 | 12.23 | 1.15 | 97.03 | 33.08 |
| p | 0.00 | 0.00 | 0.01 | 0.00 | 0.29 | 0.00 | 0.00 | |
| LOX e | F | 984.33 | 1430.40 | 139.61 | 601.29 | 32.77 | 47.78 | 16.62 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| PI1 f | F | 829.22 | 1170.85 | 160.05 | 428.72 | 70.30 | 94.43 | 53.38 |
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Tomato Constituents | Fecundity at One Week | Fecundity at Three Weeks | Abundance at Four Weeks | Abundance at Six Weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | r | p | df | r | p | df | r | p | df | r | p | |
| Soluble sugars | 6 | 0.995 | 0.000 | 6 | 0.989 | 0.000 | 6 | 0.981 | 0.000 | 6 | 0.970 | 0.000 |
| Free amino acids | 6 | 0.984 | 0.000 | 6 | 0.973 | 0.000 | 6 | 0.955 | 0.000 | 6 | 0.928 | 0.001 |
| Condensed tannins | 6 | −0.952 | 0.000 | 6 | −0.966 | 0.000 | 6 | −0.901 | 0.002 | 6 | −0.827 | 0.011 |
| Total phenolics | 6 | −0.940 | 0.001 | 6 | −0.936 | 0.001 | 6 | −0.872 | 0.005 | 6 | −0.802 | 0.017 |
| SA a | 6 | −0.908 | 0.002 | 6 | −0.912 | 0.002 | 6 | −0.853 | 0.007 | 6 | −0.775 | 0.024 |
| PAL b | 6 | −0.963 | 0.000 | 6 | −0.955 | 0.000 | 6 | −0.909 | 0.002 | 6 | −0.853 | 0.007 |
| PR1 c | 6 | −0.966 | 0.000 | 6 | −0.970 | 0.000 | 6 | −0.943 | 0.000 | 6 | −0.869 | 0.005 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Sun, Y.; Chen, F.; Zhang, Y.; Ge, F. Elevated O3 and TYLCV Infection Reduce the Suitability of Tomato as a Host for the Whitefly Bemisia tabaci. Int. J. Mol. Sci. 2016, 17, 1964. https://doi.org/10.3390/ijms17121964
Cui H, Sun Y, Chen F, Zhang Y, Ge F. Elevated O3 and TYLCV Infection Reduce the Suitability of Tomato as a Host for the Whitefly Bemisia tabaci. International Journal of Molecular Sciences. 2016; 17(12):1964. https://doi.org/10.3390/ijms17121964
Chicago/Turabian StyleCui, Hongying, Yucheng Sun, Fajun Chen, Youjun Zhang, and Feng Ge. 2016. "Elevated O3 and TYLCV Infection Reduce the Suitability of Tomato as a Host for the Whitefly Bemisia tabaci" International Journal of Molecular Sciences 17, no. 12: 1964. https://doi.org/10.3390/ijms17121964
APA StyleCui, H., Sun, Y., Chen, F., Zhang, Y., & Ge, F. (2016). Elevated O3 and TYLCV Infection Reduce the Suitability of Tomato as a Host for the Whitefly Bemisia tabaci. International Journal of Molecular Sciences, 17(12), 1964. https://doi.org/10.3390/ijms17121964







