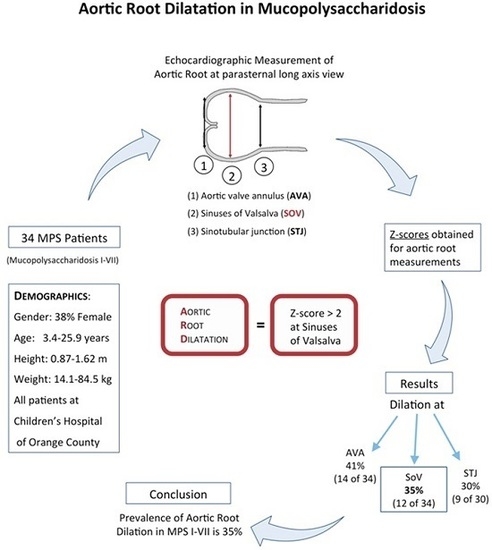
Aortic Root Dilatation in Mucopolysaccharidosis I–VII
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ARD | Aortic root dilatation |
| AVA | Aortic valve annulus |
| BSA | Body surface area |
| CHOC | Children’s Hospital of Orange County |
| CI | Confidence interval |
| ERT | Enzyme replacement therapy |
| GAGs | Glycosaminoglycans |
| HSCT | Hematopoietic stem cell transplantation |
| MPS | Mucopolysaccharidosis |
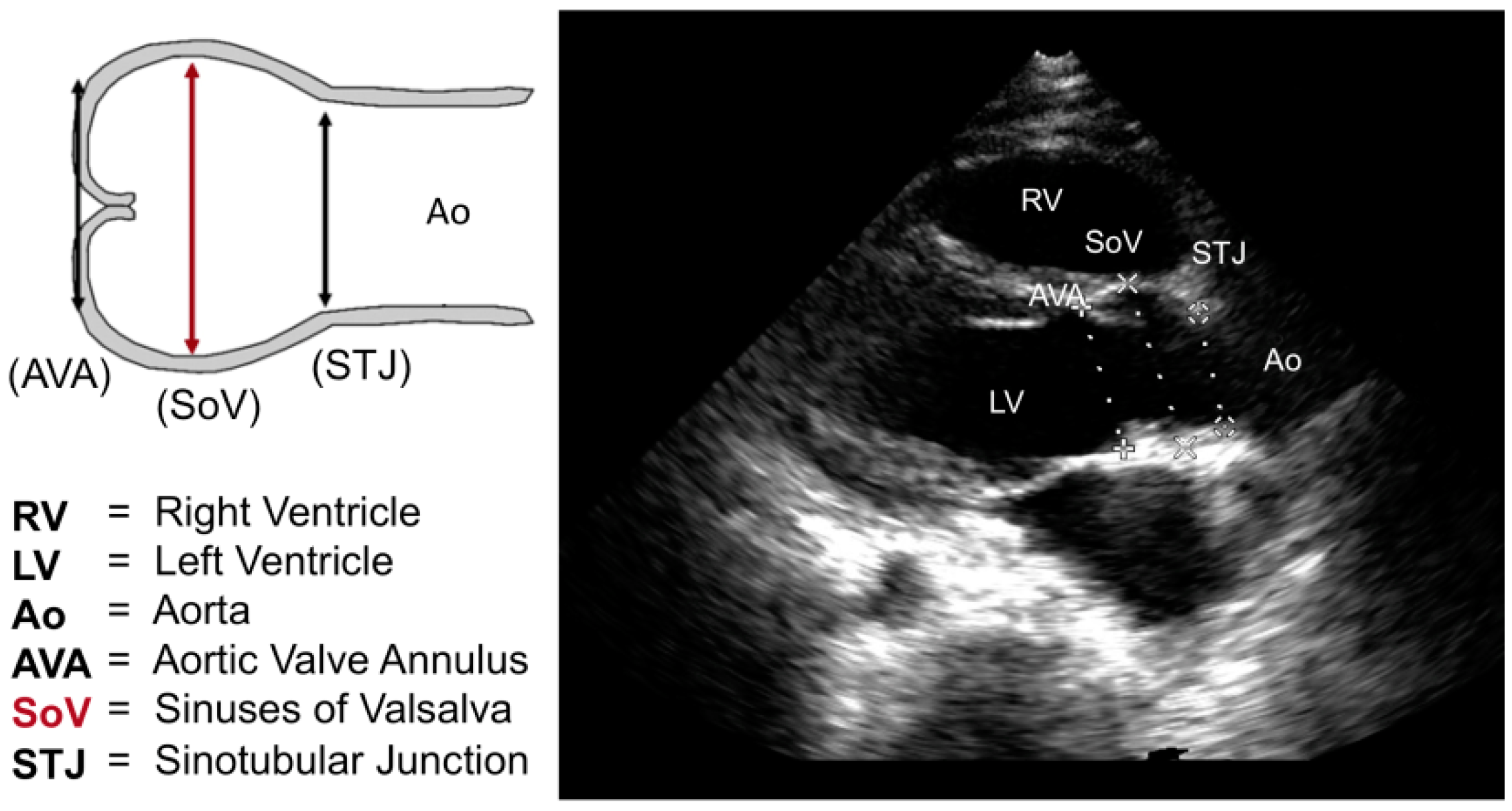
| SoV | Sinuses of valsalva |
| STJ | Sinotubular junction |
References
- Collins, J.A.; Munoz, J.V.; Patel, T.R.; Loukas, M.; Tubbs, R.S. The anatomy of the aging aorta. Clin. Anat. 2014, 27, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M.; Jing, H. Marfan’s syndrome: An overview. Sao Paulo Med. J. 2010, 128, 360–366. [Google Scholar] [PubMed]
- Fikar, C.R.; Fikar, R. Aortic dissection in childhood and adolescence: An analysis of occurrences over a 10-year interval in New York State. Clin. Cardiol. 2009, 32, E23–E26. [Google Scholar] [CrossRef] [PubMed]
- Shamszad, P.; Barnes, J.N.; Morris, S.A. Aortic dissection in hospitalized children and young adults: A multi-institutional study. Congenit. Heart Dis. 2014, 9, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.A.; Harmatz, P.R.; Scarpa, M.; Furlanetto, B.; Kampmann, C.; Loehr, J.P.; Ponder, K.P.; Roberts, W.C.; Rosenfeld, H.M.; Giugliani, R. Cardiac disease in patients with mucopolysaccharidosis: Presentation, diagnosis, and management. J. Inherit. Metab. Dis. 2011, 34, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.A.; Mackey-Bojack, S.; Panosckaltsis-Mortari, A.; Berry, J.M.; McElmurry, R.T.; Riddle, M.; Sun, L.Y.; Clarke, L.; Tolar, J. Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I: Implications for assessment of therapeutic interventions in hurler syndrome. Pediatr. Res. 2006, 59, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, M.M.; Fornasari, B.; Ellinwood, M.A.; Weil, J.; Melniczek, T.M.; O’Malley, C.D.; Sammarco, L.; Xu, K.P.; Ponder, K.P.; Haskins, M.E. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation 2004, 110, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, M.M.; Kusiak, C.M.; Shofer, F.S.; O’Donnell, P.; Bryan, C.; Ponder, K.P.; Haskins, M.E. Clinical characterization of cardiovascular abnormalities associated with feline mucopolysaccharidosis I and VI. J. Inherit. Metab. Dis. 2008, 31, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, L.; Orchard, P.; Whitley, C.B.; Berry, J.; Tolar, J.; Miller, W.; Braunlin, E.A. Cardiac ultrasound findings in infants with severe (Hurler phenotype) untreated mucopolysaccharidosis (MPS) Type I. J. Inherit. Metab. Dis. Rep. 2013, 10, 87–94. [Google Scholar]
- Yasuda, E.; Fushimi, K.; Suzuki, Y.; Shimizu, K.; Takami, T.; Zustin, J.; Patel, P.; Ruhnke, K.; Shimada, T.; Boyce, B.; et al. Pathogenesis of Morquio A syndrome: An autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013, 109, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.A.; Linders, B.; Wu, S.; Bigg, P.; O’Donnell, P.; Sleeper, M.M.; Whyte, M.P.; Haskins, M.; Ponder, K.P. Upregulation of elastase activity in aorta in mucopolysaccharidosis I and VII dogs may be due to increased cytokine expression. Mol. Genet. Metab. 2010, 99, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.A.; Orchard, P.J.; Whitley, C.B.; Schroeder, L.; Reed, R.C.; Manivel, J.C. Unexpected coronary artery findings in mucopolysaccharidosis—Report of four cases and literature review. Cardiovasc. Pathol. 2014, 23, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.A.; Berry, J.M.; Whitley, C.B. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am. J. Cardiol. 2006, 98, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Braunlin, E.A.; Rudser, K.D.; Dengel, D.R.; Metzig, A.M.; Covault, K.K.; Polgreen, L.E.; Shapiro, E.; Steinberger, J.; Kelly, A.S. Carotid intima–media thickness is increased in patients with treated mucopolysaccharidosis types I and II, and correlates with arterial stiffness. Mol. Genet. Metab. 2014, 111, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, C.; Bell, P.; Gurda, B.L.; Wang, Q.; Louboutin, J.P.; Zhu, Y.; Bagel, J.; O’Donnell, P.; Sikora, T.; Ruane, T.; et al. Liver-directed gene therapy corrects cardiovascular lesions in feline mucopolysaccharidosis type I. Proc. Natl. Acad. Sci. USA 2014, 111, 14894–14899. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, F.; Bigras, J.L.; Prsa, M.; Dahdah, N. Bias related to body mass index in pediatric echocardiographic z scores. Pediatr. Cardiol. 2015, 36, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kimmenade, R.R.V.; Kempers, M.; de Boer, M.J.; Loews, B.L.; Timmermans, J. A clinical appraisal of different z-score equations for aortic root assessment in the diagnostic evaluation of Marfan syndrome. Genet. Med. 2013, 15, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric method for measuring body surface area: A height-weight formula validated in infants, children and adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Colan, S.D.; McElhinney, D.B.; Crawford, E.C.; Keane, J.F.; Lock, J.E. Validation and re-evaluation of a discriminant model predicting anatomic suitability for biventricular repair in neonates with aortic stenosis. J. Am. Coll. Cardiol. 2006, 47, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Daubency, P.E.; Blacksone, E.H.; Weintraub, R.G.; Slavik, Z.; Scanion, J.; Webber, S.A. Relationship of the dimension of cardiac structures to body size: An echocardiographic study in normal infants and children. Cardiol. Young 1999, 9, 402–410. [Google Scholar]

| Demographics and Echocardiographic Parameters | MPS Type | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients | I | II | III | IVa | IVb | VI | VII | ||||||||||||||||
| n | Mean | Std | n | Mean | Std | n | Mean | Std | n | Mean | Std | n | Mean | Std | n | Mean | Std | n | Mean | n | Mean | Std | |
| Age (years) | 34 | 13.0 | 6.1 | 6 | 9.5 | 3.5 | 7 | 14.2 | 6.9 | 6 | 16.4 | 4.9 | 8 | 12.3 | 7.9 | 2 | 9.1 | 4.5 | 1 | 9.9 | 4 | 15.5 | 5.2 |
| Height (cm) | 34 | 123.9 | 21.4 | 6 | 115.9 | 28.2 | 7 | 131.2 | 16.6 | 6 | 136.2 | 14.9 | 8 | 110.2 | 18.8 | 2 | 119.5 | 19.1 | 1 | 95.0 | 4 | 142.0 | 8.2 |
| Weight (kg) | 34 | 34.4 | 18.0 | 6 | 28.8 | 14.7 | 7 | 41.7 | 23.7 | 6 | 36.8 | 17.3 | 8 | 26.0 | 13.7 | 2 | 24.6 | 7.9 | 1 | 17.1 | 4 | 52.8 | 11.7 |
| BSA (m2) | 34 | 1.1 | 0.4 | 6 | 1.0 | 0.3 | 7 | 1.2 | 0.4 | 6 | 1.2 | 0.3 | 8 | 0.9 | 0.3 | 2 | 0.9 | 0.2 | 1 | 0.7 | 4 | 1.5 | 0.2 |
| AVA (cm) | 34 | 1.8 | 0.4 | 6 | 1.7 | 0.2 | 7 | 1.7 | 0.3 | 6 | 1.8 | 0.4 | 8 | 1.9 | 0.5 | 2 | 1.7 | 0.4 | 1 | 1.5 | 4 | 2.2 | 0.5 |
| SoV (cm) | 34 | 2.3 | 0.5 | 6 | 2.1 | 0.2 | 7 | 2.3 | 0.4 | 6 | 2.5 | 0.6 | 8 | 2.4 | 0.7 | 2 | 2.1 | 0.6 | 1 | 1.7 | 4 | 2.5 | 0.3 |
| STJ (cm) | 32 | 1.9 | 0.5 | 5 | 1.5 | 0.7 | 7 | 1.9 | 0.4 | 5 | 2.3 | 0.3 | 8 | 2.0 | 0.5 | 2 | 1.7 | 0.4 | 1 | 1.4 | 4 | 2.1 | 0.3 |
| z-score (AVA) | 34 | 1.8 | 2.4 | 6 | 1.9 | 2.4 | 7 | 0.4 | 1.9 | 6 | 0.8 | 2.3 | 8 | 3.7 | 2.3 | 2 | 1.5 | 1.3 | 1 | 1.7 | 4 | 1.9 | 3.0 |
| z-score (SoV) | 34 | 1.2 | 1.8 | 6 | 0.8 | 1.6 | 7 | 0.6 | 1.4 | 6 | 1.6 | 2.7 | 8 | 2.5 | 1.7 | 2 | 0.8 | 1.7 | 1 | 0.0 | 4 | 0.3 | 0.6 |
| z-score (STJ) | 30 | 1.1 | 1.7 | 5 | 0.6 | 1.9 | 6 | 0.5 | 1.8 | 5 | 2.5 | 1.4 | 7 | 1.9 | 1.8 | 2 | 0.0 | 1.4 | 1 | 0.0 | 4 | 0.1 | 0.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolourchi, M.; Renella, P.; Wang, R.Y. Aortic Root Dilatation in Mucopolysaccharidosis I–VII. Int. J. Mol. Sci. 2016, 17, 2004. https://doi.org/10.3390/ijms17122004
Bolourchi M, Renella P, Wang RY. Aortic Root Dilatation in Mucopolysaccharidosis I–VII. International Journal of Molecular Sciences. 2016; 17(12):2004. https://doi.org/10.3390/ijms17122004
Chicago/Turabian StyleBolourchi, Meena, Pierangelo Renella, and Raymond Y. Wang. 2016. "Aortic Root Dilatation in Mucopolysaccharidosis I–VII" International Journal of Molecular Sciences 17, no. 12: 2004. https://doi.org/10.3390/ijms17122004