Functional Diversity of Neurotrophin Actions on the Oculomotor System
Abstract
:1. Introduction
2. Extraocular Motoneurons as the Experimental Model
3. Extraocular Motoneurons Express the TrkA, TrkB and TrkC Receptors
4. The Expression of Trk Receptors Is Differentially Regulated after Axotomy
4.1. Changes in Expression of Trk Receptors after Axotomy
4.2. Correlations with Regenerative and Cholinergic Phenotypes
5. Neurotrophins Rescue Extraocular Motoneurons from Axotomy-Induced Cell Death during Postnatal Development
5.1. Survival of Extraocular Motoneurons after Postnatal Axotomy and Neurotrophin Administration
5.2. Maintenance of the Cholinergic Phenotype in Extraocular Motoneurons by Neurotrophins after Axotomy
5.3. Long-Lasting Effects of Neurotrophic Factors in Extraocular Motoneurons
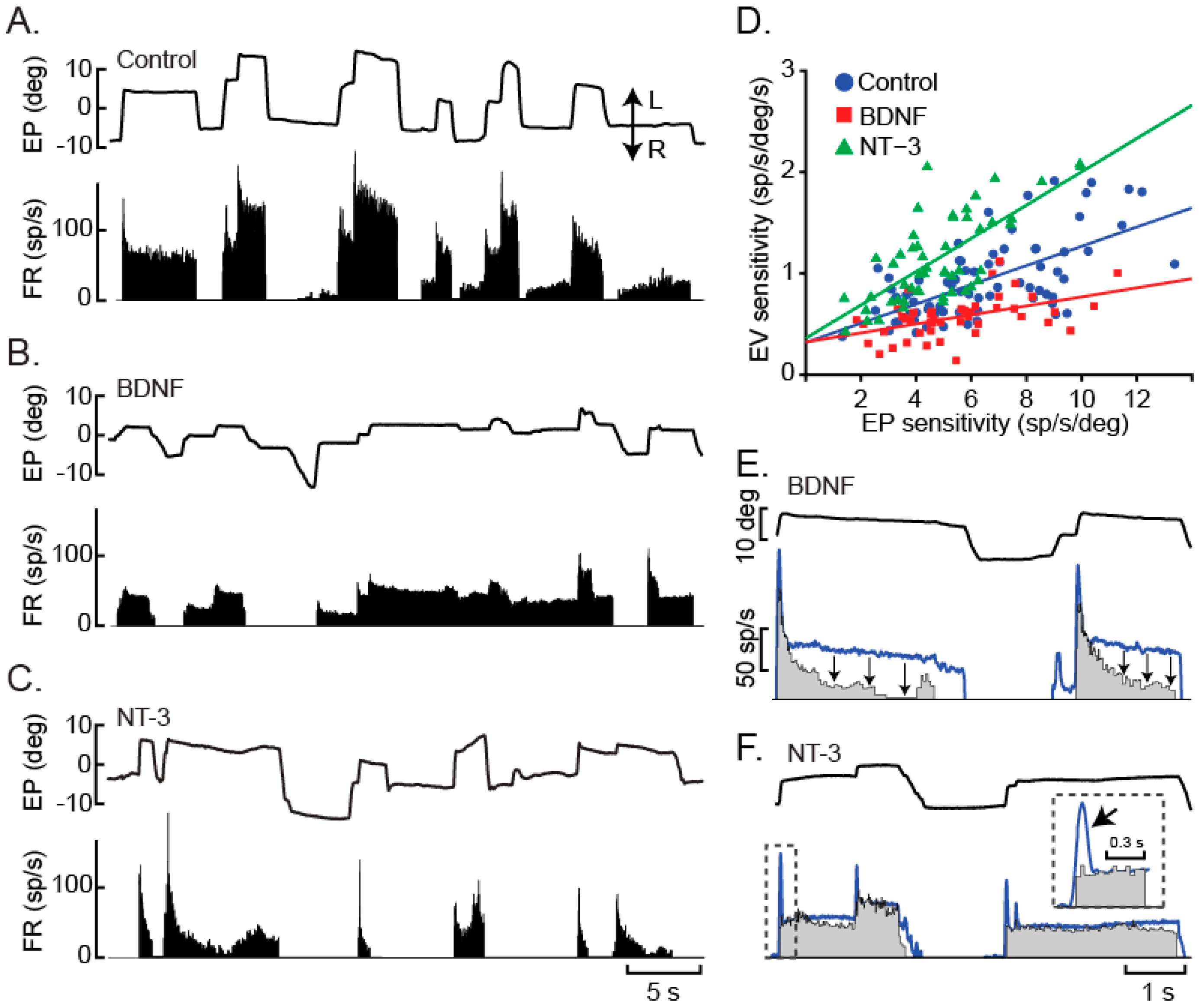
6. BDNF and NT-3 Exert Complementary Actions on the Discharge Activity of Extraocular Motoneurons
7. NGF Recovers above Control the Discharge Activity of Axotomized Motoneurons: Differential Role of TrkA and p75 Receptors
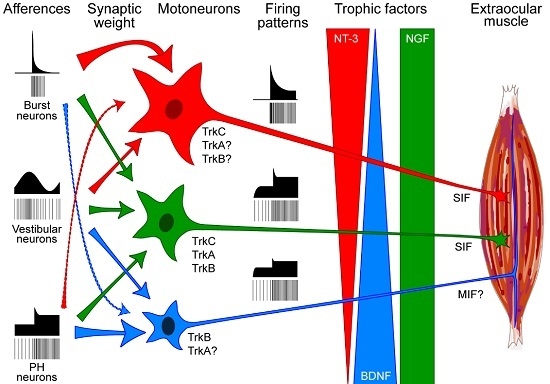
8. A Hypothesis Linking Neurotrophic Delivery with Motoneuronal Types and Their Afferences
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oppenheim, R.W.; Qin-Wei, Y.; Prevette, D.; Yan, Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature 1992, 360, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, D.; Hamnér, S.; Zirrgiebel, U. Neurotrophins and cerebellar development. Perspect. Dev. Neurobiol. 1997, 5, 83–94. [Google Scholar] [PubMed]
- Morrison, M.E.; Mason, C.A. Granule neuron regulation of Purkinje cell development: Striking a balance between neurotrophin and glutamate signaling. J. Neurosci. 1998, 18, 3563–3573. [Google Scholar] [PubMed]
- Mertz, K.; Koscheck, T.; Schilling, K. Brain-derived neurotrophic factor modulates dendritic morphology of cerebellar basket and stellate cells: An in vitro study. Neuroscience 2000, 97, 303–310. [Google Scholar] [CrossRef]
- Tanaka, S.; Sekino, Y.; Shirao, T. The effects of neurotrophin-3 and brain-derived neurotrophic factor on cerebellar granule cell movement and neurite extension in vitro. Neuroscience 2000, 97, 727–734. [Google Scholar] [CrossRef]
- Bennet, M.R.; Gibson, W.G.; Lemon, G. Neuronal cell death, nerve growth factor and neurotrophic models: 50 years on. Auton. Neurosci. 2002, 95, 1–23. [Google Scholar] [CrossRef]
- Gonzalez, M.; Collins, W.F. Modulation of motoneuron excitability by brain-derived neurotrophic factor. J. Neurophysiol. 1997, 77, 502–506. [Google Scholar] [PubMed]
- Desai, N.S.; Rutherford, L.C.; Turrigiano, G.G. BDNF regulates the intrinsic excitability of cortical neurons. Learn. Mem. 1999, 6, 284–291. [Google Scholar] [PubMed]
- Yamuy, J.; Pose, I.; Pedroarena, C.; Morales, F.R.; Chase, M.H. Neurotrophin-induced rapid enhancement of membrane potential oscillations in mesencephalic trigeminal neurons. Neuroscience 2000, 95, 1089–1100. [Google Scholar] [CrossRef]
- Causing, C.G.; Gloster, A.; Aloyz, R.; Bamji, S.X.; Chang, E.; Fawcett, J.; Kuchel, G.; Miller, F.D. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron 1997, 18, 257–267. [Google Scholar] [CrossRef]
- Novikov, L.N.; Novikova, L.N.; Holmberg, P.; Kellerth, J. Exogenous brain-derived neurotrophic factor regulates the synaptic composition of axonally lesioned and normal adult rat motoneurons. Neuroscience 2000, 100, 171–181. [Google Scholar] [CrossRef]
- Black, I.B. Trophic regulation of synaptic plasticity. J. Neurobiol. 1999, 41, 108–118. [Google Scholar] [CrossRef]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999, 22, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Schinder, A.F.; Poo, M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Cooper, J.D.; Svendsen, C.N.; Crossman, P.; Ip, N.Y.; Lindsay, R.M.; Zafra, F.; Lindholm, D. Atrophy but not death of adult septal cholinergic neurons after ablation of target capacity to produce mRNAs for NGF, BDNF, and NT3. J. Neurosci. 1993, 13, 5263–5276. [Google Scholar] [PubMed]
- Johnson, E.M.; Taniuchi, M.; DiStefano, P.S. Expression and possible function of nerve growth factor receptors on Schwann cells. Trends Neurosci. 1988, 11, 299–304. [Google Scholar] [CrossRef]
- Lindsay, R.M. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci. 1988, 8, 2394–2405. [Google Scholar] [PubMed]
- Grill, R.; Murai, K.; Blesch, A.; Gage, F.H.; Tuszynski, M.H. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 1997, 17, 5560–5572. [Google Scholar] [PubMed]
- Caleo, M.; Medini, P.; von Bartheld, C.S.; Maffei, L. Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J. Neurosci. 2003, 23, 287–296. [Google Scholar] [PubMed]
- De la Cruz, R.R.; Pastor, A.M.; Delgado-García, J.M. Effects of target depletion on adult mammalian central neurons: Functional correlates. Neuroscience 1994, 58, 81–97. [Google Scholar] [CrossRef]
- De la Cruz, R.R.; Pastor, A.M.; Delgado-García, J.M. Effects of target depletion on adult mammalian central neurons: Morphological correlates. Neuroscience 1994, 58, 59–79. [Google Scholar] [CrossRef]
- De la Cruz, R.R.; Delgado-García, J.M.; Pastor, A.M. Discharge characteristics of axotomized abducens internuclear neurons in the adult cat. J. Comp. Neurol. 2000, 427, 391–404. [Google Scholar] [CrossRef]
- Delgado-García, J.M.; del Pozo, F.; Spencer, R.F.; Baker, R. Behavior of neurons in the abducens nucleus of the alert cat—III. Axotomized motoneurons. Neuroscience 1988, 24, 143–160. [Google Scholar] [CrossRef]
- Pastor, A.M.; Delgado-García, J.M.; Martínez-Guijarro, F.J.; López-García, C.; de la Cruz, R.R. Response of abducens internuclear neurons to axotomy in the adult cat. J. Comp. Neurol. 2000, 427, 370–390. [Google Scholar] [CrossRef]
- Benítez-Temiño, B.; de la Cruz, R.R.; Pastor, A.M. Firing properties of axotomized central nervous system neurons recover after graft reinnervation. J. Comp. Neurol. 2002, 444, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Morado-Díaz, C.J.; Matarredona, E.R.; Morcuende, S.; Talaverón, R.; Davis-López de Carrizosa, M.A.; de la Cruz, R.R.; Pastor, A.M. Neural progenitor cell implants in the lesioned medial longitudinal fascicle of adult cats regulate synaptic composition and firing properties of abducens internuclear neurons. J. Neurosci. 2014, 34, 7007–7017. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Temiño, B.; de la Cruz, R.R.; Pastor, A.M. Grafting of a new target prevents synapse loss in abducens internuclear neurons induced by axotomy. Neuroscience 2003, 118, 611–626. [Google Scholar] [CrossRef]
- Büttner, U.; Büttner-Ennever, J.A. Present concepts of oculomotor organization. Prog. Brain Res. 2006, 151, 1–42. [Google Scholar] [PubMed]
- Delgado-García, J.M.; del Pozo, F.; Baker, R. Behavior of neurons in the abducens nucleus of the alert cat—II. Internuclear neurons. Neuroscience 1986, 17, 953–973. [Google Scholar] [CrossRef]
- Hikosaka, O.; Igusa, Y.; Nakao, S.; Shimazu, H. Direct inhibitory synaptic linkage of pontomedullary reticular burst neurons with abducens motoneurons in the cat. Exp. Brain Res. 1978, 33, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Grantyn, A.; Grantyn, R.; Gaunitz, U.; Robiné, K.P. Sources of direct excitatory and inhibitory inputs from the medial rhombencephalic tegmentum to lateral and medial rectus motoneurons in the cat. Exp. Brain Res. 1980, 39, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Igusa, Y.; Sasaki, S.; Shimazu, H. Excitatory premotor burst neurons in the cat pontine reticular formation related to the quick phase of vestibular nystagmus. Brain Res. 1980, 182, 451–456. [Google Scholar] [CrossRef]
- McCrea, R.A.; Yoshida, K.; Berthoz, A.; Baker, R. Eye movement related activity and morphology of second order vestibular neurons terminating in the cat abducens nucleus. Exp. Brain Res. 1980, 40, 468–473. [Google Scholar] [CrossRef] [PubMed]
- McCrea, R.A.; Baker, R. Anatomical connections of the nucleus prepositus of the cat. J. Comp. Neurol. 1985, 237, 377–407. [Google Scholar] [CrossRef] [PubMed]
- Escudero, M.; Delgado-García, J.M. Behavior of reticular, vestibular and prepositus neurons terminating in the abducens nucleus of the alert cat. Exp. Brain Res. 1988, 71, 218–222. [Google Scholar] [CrossRef] [PubMed]
- González-Forero, D.; de la Cruz, R.R.; Delgado-García, J.M.; Álvarez, F.J.; Pastor, A.M. Functional alterations of cat abducens neurons after peripheral tetanus neurotoxin injection. J. Neurophysiol. 2003, 89, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Delgado-García, J.M.; del Pozo, F.; Baker, R. Behavior of neurons in the abducens nucleus of the alert cat—I. Motoneurons. Neuroscience 1986, 17, 929–952. [Google Scholar] [CrossRef]
- Morcuende, S.; Benítez-Temiño, B.; Pecero, M.L.; Pastor, A.M.; de la Cruz, R.R. Abducens internuclear neurons depend on their target motoneurons for survival during early postnatal development. Exp. Neurol. 2005, 195, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.B.; Graeber, M.B. The facial nerve axotomy model. Brain Res. Rev. 2004, 44, 154–178. [Google Scholar] [CrossRef] [PubMed]
- Purves, D. Body and Brain: A Trophic Theory of Neural Connections; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Benítez-Temiño, B.; de la Cruz, R.R.; Tena, J.J.; Pastor, A.M. Cerebellar grafting in the oculomotor system as a model to study target influence on adult neurons. Brain Res. Rev. 2005, 49, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Barbacid, M. The Trk family of neurotrophin receptors. J. Neurobiol. 1994, 25, 1386–1403. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Jing, S.Q.; Nanduri, V.; O’Rourke, E.; Barbacid, M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 1991, 65, 189–197. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Martin-Zanca, D.; Parada, L.F. Tyrosine phosphorylation and tyrosine kinase activity of the Trk proto-oncogene product induced by NGF. Nature 1991, 350, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Nanduri, V.; Jing, S.A.; Lamballe, F.; Tapley, P.; Bryant, S.; Cordon-Cardo, C.; Jones, K.R.; Reichardt, L.F.; Barbacid, M. The TrkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 1991, 66, 395–403. [Google Scholar] [CrossRef]
- Ip, N.Y.; Ibáñez, C.F.; Nye, S.H.; McClain, J.; Jones, P.F.; Gies, D.R.; Belluscio, L.; Le Beau, M.M.; Espinosa, R.; Squinto, S.P. Mammalian neurotrophin-4: Structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl. Acad. Sci. USA 1992, 89, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Lamballe, F.; Klein, R.; Barbacid, M. trkC, a new member of the Trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 1991, 66, 967–979. [Google Scholar] [CrossRef]
- Snider, W.D. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell 1994, 77, 627–638. [Google Scholar] [CrossRef]
- Benítez-Temiño, B.; Morcuende, S.; Mentis, G.Z.; de la Cruz, R.R.; Pastor, A.M. Expression of Trk receptors in the oculomotor system of the adult cat. J. Comp. Neurol. 2004, 473, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Yamuy, J.; Sampogna, S.; Chase, M.H. Neurotrophin-receptor immunoreactive neurons in mesopontine regions involved in the control of behavioral states. Brain Res. 2000, 866, 1–14. [Google Scholar] [CrossRef]
- Steljes, T.P.; Kinoshita, Y.; Wheeler, E.F.; Oppenheim, R.W.; von Bartheld, C.S. Neurotrophic factor regulation of developing avian oculomotor neurons: Differential effects of BDNF and GDNF. J. Neurobiol. 1999, 41, 295–315. [Google Scholar] [CrossRef]
- Merlio, J.P.; Ernfors, P.; Jaber, M.; Persson, H. Molecular cloning of rat TrkC and distribution of cells expressing messenger RNAs for members of the Trk family in the rat central nervous system. Neuroscience 1992, 51, 513–532. [Google Scholar] [CrossRef]
- Moshnyakov, M.; Arumäe, U.; Saarma, M. mRNAs for one, two or three members of Trk receptor family are expressed in single rat trigeminal ganglion neurons. Brain Res. Mol. Brain Res. 1996, 43, 141–148. [Google Scholar] [CrossRef]
- Jacobs, J.S.; Miller, M.W. Expression of nerve growth factor, p75, and the high affinity neurotrophin receptors in the adult rat trigeminal system: Evidence for multiple trophic support systems. J. Neurocytol. 1999, 28, 571–595. [Google Scholar] [CrossRef] [PubMed]
- Burette, A.; Jalenques, I.; Romand, R. Neurotrophin receptor immunostaining in the rat ventral cochlear nucleus. Brain Res. 1997, 776, 10–23. [Google Scholar] [CrossRef]
- Gorba, T.; Wahle, P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur. J. Neurosci. 1999, 11, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Armanini, M.P.; Ling, L.H.; Phillips, H.S. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 1994, 12, 1161–1171. [Google Scholar] [CrossRef]
- Henderson, C.E.; Camu, W.; Mettling, C.; Gouin, A.; Poulsen, K.; Karihaloo, M.; Ruilamas, J.; Evans, T.; McMahon, S.B.; Armanini, M.P.; et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature 1993, 363, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, O.; Parsadanian, A.S.; Sendtner, M.; Thoenen, H. Expression of neurotrophins in skeletal muscle: Quantitative comparison and significance for motoneuron survival and maintenance of function. J. Neurosci. Res. 1995, 42, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.J.; Kobayashi, N.R.; Jasmin, B.J.; Tetzlaff, W. Acetylcholinesterase gene expression in axotomized rat facial motoneurons is differentially regulated by neurotrophins: Correlation with TrkB and TrkC mRNA levels and isoforms. J. Neurosci. 1998, 18, 9936–9947. [Google Scholar] [PubMed]
- Cooper, J.D.; Skepper, J.N.; Berzaghi, M.D.; Lindholm, D.; Sofroniew, M.V. Delayed death of septal cholinergic neurons after excitotoxic ablation of hippocampal neurons during early postnatal development in the rat. Exp. Neurol. 1996, 139, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Belluscio, L.; Friedman, B.; Alderson, R.F.; Wiegand, S.J.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron 1990, 5, 501–509. [Google Scholar] [CrossRef]
- Koliatsos, V.E.; Clatterbuck, R.E.; Winslow, J.W.; Cayouette, M.H.; Prices, D.L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 1993, 10, 359–367. [Google Scholar] [CrossRef]
- Ip, F.C.; Cheung, J.; Ip, N.Y. The expression profiles of neurotrophins and their receptors in rat and chicken tissues during development. Neurosci. Lett. 2001, 301, 107–110. [Google Scholar] [CrossRef]
- Harandi, V.M.; Lindquist, S.; Kolan, S.S.; Brännström, T.; Liu, J.X. Analysis of neurotrophic factors in limb and extraocular muscles of mouse model of amyotrophic lateral sclerosis. PLoS ONE 2014, 9, e109833. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [PubMed]
- Fawcett, J.P.; Bamji, S.X.; Causing, C.G.; Aloyz, R.; Ase, A.R.; Reader, T.A.; McLean, J.H.; Miller, F.D. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J. Neurosci. 1998, 18, 2808–2821. [Google Scholar] [PubMed]
- Korsching, S. The neurotrophic factor concept: A reexamination. J. Neurosci. 1993, 13, 2739–2748. [Google Scholar] [PubMed]
- Nawa, H.; Takei, N. BDNF as an anterophin; a novel neurotrophic relationship between brain neurons. Trends Neurosci. 2001, 24, 683–685. [Google Scholar] [CrossRef]
- Ridet, J.L.; Malhotra, S.K.; Privat, A.; Gage, F.H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Buj-Bello, A.; Davies, A.M. Paracrine interactions of BDNF involving NGF-dependent embryonic sensory neurons. Mol. Cell. Neurosci. 1996, 7, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, Y.; Nakamura, S.; Nakano, S.; Oka, N.; Akiguchi, I.; Kimura, J. Immunohistochemical localization of brain-derived neurotrophic factor in adult rat brain. Neuroscience 1996, 74, 1209–1226. [Google Scholar] [CrossRef]
- Furukawa, S.; Sugihara, Y.; Iwasaki, F.; Fukumitsu, H.; Nitta, A.; Nomoto, H.; Furukawa, Y. Brain-derived neurotrophic factor-like immunoreactivity in the adult rat central nervous system predominantly distributed in neurons with substantial amounts of brain-derived neurotrophic factor messenger RNA or responsiveness to brain-derived neurotrophi. Neuroscience 1997, 82, 653–670. [Google Scholar] [CrossRef]
- Talaverón, R.; Matarredona, E.R.; de la Cruz, R.R.; Pastor, A.M. Neural progenitor cell implants modulate vascular endothelial growth factor and brain-derived neurotrophic factor expression in rat axotomized neurons. PLoS ONE 2013, 8, e54519. [Google Scholar] [CrossRef] [PubMed]
- Koliatsos, V.E.; Crawford, T.O.; Price, D.L. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. Brain Res. 1991, 549, 297–304. [Google Scholar] [CrossRef]
- Piehl, F.; Frisén, J.; Risling, M.; Hökfelt, T.; Cullheim, S. Increased TrkB mRNA expression by axotomized motoneurones. Neuroreport 1994, 5, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Mosier, D.R.; Siklós, L.; Appel, S.H. Resistance of extraocular motoneuron terminals to effects of amyotrophic lateral sclerosis sera. Neurology 2000, 54, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Medina, L.; Figueredo-Cardenas, G.; Anfinson, S. Brainstem motoneuron pools that are selectively resistant in amyotrophic lateral sclerosis are preferentially enriched in parvalbumin: Evidence from monkey brainstem for a calcium-mediated mechanism in sporadic ALS. Exp. Neurol. 1995, 131, 239–250. [Google Scholar] [CrossRef]
- De la Cruz, R.R.; Pastor, A.M.; Martínez-Guijarro, F.J.; López-García, C.; Delgado-García, J.M. Localization of parvalbumin, calretinin, and calbindin D-28k in identified extraocular motoneurons and internuclear neurons of the cat. J. Comp. Neurol. 1998, 391, 377–391. [Google Scholar] [CrossRef]
- Yano, H.; Chao, M.V. Mechanisms of neurotrophin receptor vesicular transport. J. Neurobiol. 2004, 58, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Connor, B.; Young, D.; Lawlor, P.; Gai, W.; Waldvogel, H.; Faull, R.L.M.; Dragunow, M. Trk receptor alterations in Alzheimer’s disease. Mol. Brain Res. 1996, 42, 1–17. [Google Scholar] [CrossRef]
- Canals, J.M.; Checa, N.; Marco, S.; Michels, A.; Pérez-Navarro, E.; Alberch, J. The neurotrophin receptors TrkA, TrkB and TrkC are differentially regulated after excitotoxic lesion in rat striatum. Mol. Brain Res. 1999, 69, 242–248. [Google Scholar] [CrossRef]
- Duprey-Díaz, M.V.; Soto, I.; Blagburn, J.M.; Blanco, R.E. Changes in brain-derived neurotrophic factor and TrkB receptor in the adult Rana pipiens retina and optic tectum after optic nerve injury. J. Comp. Neurol. 2002, 454, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Morcuende, S.; Matarredona, E.R.; Benítez-Temiño, B.; Muñoz-Hernández, R.; Pastor, A.M.; de la Cruz, R.R. Differential regulation of the expression of neurotrophin receptors in rat extraocular motoneurons after lesion. J. Comp. Neurol. 2011, 519, 2335–2352. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.R.; Bedard, A.M.; Hincke, M.T.; Tetzlaff, W. Increased expression of BDNF and TrkB mRNA in rat facial motoneurons after axotomy. Eur. J. Neurosci. 1996, 8, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.P.; Gannon, S.M.; Hawk, K.; Walsh, B.F.; Isaacson, L.G. Transection of preganglionic axons leads to CNS neuronal plasticity followed by survival and target reinnervation. Auton. Neurosci. 2013, 179, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Hammarberg, H.; Piehl, F.; Risling, M.; Cullheim, S. Differential regulation of trophic factor receptor mRNAs in spinal motoneurons after sciatic nerve transection and ventral root avulsion in the rat. J. Comp. Neurol. 2000, 426, 587–601. [Google Scholar] [CrossRef]
- Boyce, V.S.; Park, J.; Gage, F.H.; Mendell, L.M. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur. J. Neurosci. 2012, 35, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.; Hökfelt, T.; Ulfhake, B. Expression of p75NTR, TrkB and TrkC in nonmanipulated and axotomized motoneurons of aged rats. Mol. Brain Res. 1999, 69, 21–34. [Google Scholar] [CrossRef]
- Sendtner, M.; Holtmann, B.; Hughes, R.A. The response of motoneurons to neurotrophins. Neurochem. Res. 1996, 21, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Mafong, E.; Meyer, S. Central infusions of brain-derived neurotrophic factor and neurotrophin-45, but not nerve growth factor and neurotrophin-3, prevent loss of the cholinergic phenotype in injured adult motor neurons. Neuroscience 1996, 71, 761–771. [Google Scholar] [CrossRef]
- Fukuoka, T.; Tokunaga, A.; Kondo, E.; Miki, K.; Tachibana, T.; Noguchi, K. Differential regulation of α- and β-CGRP mRNAs within oculomotor, trochlear, abducens, and trigeminal motoneurons in response to axotomy. Mol. Brain Res. 1999, 63, 304–315. [Google Scholar] [CrossRef]
- Sala, C.; Andreose, J.S.; Fumagalli, G.; Lømo, T. Calcitonin gene-related peptide: Possible role in formation and maintenance of neuromuscular junctions. J. Neurosci. 1995, 15, 520–528. [Google Scholar] [PubMed]
- Costigan, M.; Befort, K.; Karchewski, L.; Griffin, R.S.; D’Urso, D.; Allchorne, A.; Sitarski, J.; Mannion, J.W.; Pratt, R.E.; Woolf, C.J. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002, 3, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, T.; Yamashita, T.; Yamaguchi, A.; Hosokawa, K.; Tohyama, M. Analysis of genes induced in peripheral nerve after axotomy using cDNA microarrays. J. Neurochem. 2002, 82, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.B.; Breuer, S.; Liman, J.; Buss, A.; Schlangen, C.; Pech, K.; Hol, E.M.; Brook, G.A.; Noth, J.; Schwaiger, F.W. Identification of regeneration-associated genes after central and peripheral nerve injury in the adult rat. BMC Neurosci. 2003, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhang, F.X.; Huang, F.; Lu, Y.J.; Li, G.D.; Bao, L.; Xiao, H.S.; Zhang, X. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur. J. Neurosci. 2004, 19, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Wei, I.H.; Tseng, C.Y.; Lue, J.H.; Wen, C.Y.; Shieh, J.Y. Differential expression of calcitonin gene-related peptide (CGRP) and choline acetyltransferase (ChAT) in the axotomized motoneurons of normoxic and hypoxic rats. J. Chem. Neuroanat. 2004, 28, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Salvaterra, P.M.; Loera, S.; Chiu, A.Y. Brain-derived neurotrophic factor spares choline acetyltransferase mRNA following axotomy of motor neurons in vivo. J. Neurosci. Res. 1997, 47, 134–143. [Google Scholar] [CrossRef]
- Friedman, B.; Kleinfeld, D.; Ip, N.Y.; Verge, V.M.K.; Moulton, R.; Boland, P.; Zlotchenko, E.; Lindsay, R.M.; Liming, L. BDNF and NT-4/5 exert neurotrophic spinal motor neurons influences on injured adult. J. Neurosci. 1995, 15, 1044–1056. [Google Scholar] [PubMed]
- De la Cruz, R.R.; Pastor, A.M.; Delgado-García, J.M. Influence of the postsynaptic target on the functional properties of neurons in the adult mammalian central nervous system. Rev. Neurosci. 1996, 7, 115–149. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Vivó, M.; Valero-Cabré, A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007, 82, 163–201. [Google Scholar] [CrossRef] [PubMed]
- Titmus, M.J.; Faber, D.S. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog. Neurobiol. 1990, 35, 1–51. [Google Scholar] [CrossRef]
- Davis-López de Carrizosa, M.A.; Morado-Díaz, C.J.; Tena, J.J.; Benítez-Temiño, B.; Pecero, M.L.; Morcuende, S.R.; de la Cruz, R.R.; Pastor, A.M. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J. Neurosci. 2009, 29, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Davis-López de Carrizosa, M.A.; Morado-Díaz, C.J.; Morcuende, S.; de la Cruz, R.R.; Pastor, A.M. Nerve growth factor regulates the firing patterns and synaptic composition of motoneurons. J. Neurosci. 2010, 30, 8308–8319. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Sulaiman, O.; Boyd, J.G. Experimental strategies to promote functional recovery after peripheral nerve injuries. J. Peripher. Nerv. Syst. 2003, 8, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Terenghi, G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999, 194, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wu, W.; So, K.F.; Cheung, A.L.; Prevette, D.M.; Oppenheim, R.W. Effects of neurotrophic factors on motoneuron survival following axonal injury in newborn rats. Neuroreport 2000, 11, 2237–2241. [Google Scholar] [CrossRef] [PubMed]
- Clatterbuck, R.E.; Price, D.L.; Koliatsos, V.E. Further characterization of the effects of brain-derived neurotrophic factor and ciliary neurotrophic factor on axotomized neonatal and adult mammalian motor neurons. J. Comp. Neurol. 1994, 342, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Sendtner, M.; Holtmann, B.; Kolbeck, R.; Thoenen, H.; Barde, Y.A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 1992, 360, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Vejsada, R.; Sagot, Y.; Kato, A.C. Quantitative comparison of the transient rescue effects of neurotrophic factors on axotomized motoneurons in vivo. Eur. J. Neurosci. 1995, 7, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Elliott, J.; Snider, W.D. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature 1992, 360, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Morcuende, S.; Muñoz-Hernández, R.; Benítez-Temiño, B.; Pastor, A.M.; de la Cruz, R.R. Neuroprotective effects of NGF, BDNF, NT-3 and GDNF on axotomized extraocular motoneurons in neonatal rats. Neuroscience 2013, 250, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Houenou, L.J.; Li, L.; Lo, A.C.; Yan, Q.; Oppenheim, R.W. Naturally occurring and axotomy-induced motoneuron death and its prevention by neurotrophic agents: A comparison between chick and mouse. Prog. Brain Res. 1994, 102, 217–226. [Google Scholar] [PubMed]
- Haenggeli, C.; Kato, A.C. Differential vulnerability of cranial motoneurons in mouse models with motor neuron degeneration. Neurosci. Lett. 2002, 335, 39–43. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Young, W.G.; Yeung, G.; Shah, R.A.; Gordon, J.W.; Bloom, F.E.; Morrison, J.H.; Hof, P.R. Differential vulnerability of oculomotor, facial, and hypoglossal nuclei in G86R superoxide dismutase transgenic mice. J. Comp. Neurol. 2000, 416, 112–125. [Google Scholar] [CrossRef]
- Sharma, R.; Hicks, S.; Berna, C.M.; Kennard, C. Oculomotor dysfunction in amyotrophic lateral sclerosis. Arch. Neurol. 2011, 68, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Nishio, T.; Sunohara, N.; Furukawa, S. Neutrophin switching in spinal motoneurons of amyotrophic lateral sclerosis. Neuroreport 1998, 9, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Shiotani, A.; Watabe, K.; Takeda, Y.; Saito, K.; Mori, Y.; Ogawa, K. Adenoviral gene transfer of BDNF and GDNF synergistically prevent motoneuron loss in the nucleus ambiguus. Brain Res. 2006, 1076, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Kawazoe, Y.; Shen, J.S.; Takeda, Y.; Arakawa, Y.; Ogawa, J.; Oyanagi, K.; Ohashi, T.; Watanabe, K.; Inoue, K.; et al. Adenoviral gene transfer of GDNF, BDNF and TGF β 2, but not CNTF, cardiotrophin-1 or IGF1, protects injured adult motoneurons after facial nerve avulsion. J. Neurosci. Res. 2003, 72, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Elliott, J.L.; Matheson, C.; Sun, J.; Zhang, L.; Mu, X.; Rex, K.L.; Snider, W.D. Influences of neurotrophins on mammalian motoneurons in vivo. J. Neurobiol. 1993, 24, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.N.; Tetzlaff, W.; Mestres, P.; Giehl, K.M. BDNF, but not NT-3, promotes long-term survival of axotomized adult rat corticospinal neurons in vivo. Neuroreport 1999, 10, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- González-Forero, D.; Morcuende, S.; Álvarez, F.J.; de la Cruz, R.R.; Pastor, A.M. Transynaptic effects of tetanus neurotoxin in the oculomotor system. Brain 2005, 128, 2175–2188. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, J.; Ajiki, K.; Ichikawa, T.; Misawa, H. Changes of expression levels of choline acetyltransferase and vesicular acetylcholine transporter mRNAs after transection of the hypoglossal nerve in adult rats. Neurosci. Lett. 1997, 236, 95–98. [Google Scholar] [CrossRef]
- Yan, Q.; Matheson, C.; Lopez, O.T.; Miller, J.A. The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. J. Neurosci. 1994, 14, 5281–5291. [Google Scholar] [PubMed]
- Eriksson, N.P.; Lindsay, R.M.; Aldskogius, H. BDNF and NT-3 rescue sensory but not motoneurones following axotomy in the neonate. Neuroreport 1994, 5, 1445–1448. [Google Scholar] [PubMed]
- Vejsada, R.; Tseng, J.L.; Lindsay, R.M.; Acheson, A.; Aebischer, P.; Kato, A.C. Synergistic but transient rescue effects of BDNF and GDNF on axotomized neonatal motoneurons. Neuroscience 1998, 84, 129–139. [Google Scholar] [CrossRef]
- Sumner, B.E. A quantitative analysis of boutons with different types of synapse in normal and injured hypoglossal nuclei. Exp. Neurol. 1975, 49, 406–417. [Google Scholar] [CrossRef]
- Mendell, L.M. Modifiability of spinal synapses. Physiol. Rev. 1984, 64, 260–324. [Google Scholar] [PubMed]
- Brännström, T.; Kellerth, J.O. Recovery of synapses in axotomized adult cat spinal motoneurons after reinnervation into muscle. Exp. Brain Res. 1999, 125, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sumner, B.E. Quantitative ultrastructural observations on the inhibited recovery of the hypoglossal nucleus from the axotomy response when regeneration of the hypoglossal nerve is prevented. Exp. Brain Res. 1976, 26, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Vanden Noven, S.; Pinter, M.J. Effects of preventing reinnervation on axotomized spinal motoneurons in the cat. II. Changes in group Ia synaptic function. J. Neurophysiol. 1989, 62, 325–333. [Google Scholar] [PubMed]
- Pinter, M.J.; Vanden Noven, S. Effects of preventing reinnervation on axotomized spinal motoneurons in the cat. I. Motoneuron electrical properties. J. Neurophysiol. 1989, 62, 311–324. [Google Scholar] [PubMed]
- Davis-López de Carrizosa, M.A.; Tena, J.J.; Benítez-Temiño, B.; Morado-Díaz, C.J.; Pastor, A.M.; de la Cruz, R.R. A chronically implantable device for the controlled delivery of substances, and stimulation and recording of activity in severed nerves. J. Neurosci. Methods 2008, 167, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, L.C.; DeWan, A.; Lauer, H.M.; Turrigiano, G.G. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J. Neurosci. 1997, 17, 4527–4535. [Google Scholar] [PubMed]
- Rattiner, L.M.; Davis, M.; French, C.T.; Ressler, K.J. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J. Neurosci. 2004, 24, 4796–4806. [Google Scholar] [CrossRef] [PubMed]
- Bozdagi, O.; Rich, E.; Tronel, S.; Sadahiro, M.; Patterson, K.; Shapiro, M.L.; Alberini, C.M.; Huntley, G.W.; Salton, S.R.J. The neurotrophin-inducible gene VGF regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J. Neurosci. 2008, 28, 9857–9869. [Google Scholar] [CrossRef] [PubMed]
- Gajewska-Woźniak, O.; Skup, M.; Kasicki, S.; Ziemlińska, E.; Czarkowska-Bauch, J. Enhancing proprioceptive input to motoneurons differentially affects expression of neurotrophin 3 and brain-derived neurotrophic factor in rat hoffmann-reflex circuitry. PLoS ONE 2013, 8, e65937. [Google Scholar] [CrossRef] [PubMed]
- Kulakowski, S.A.; Parker, S.D.; Personius, K.E. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J. Appl. Physiol. 2011, 111, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, T.; Viegi, A.; Berardi, N.; Bozzi, Y.; Mascia, L.; Maffei, L. Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. J. Neurosci. 2003, 23, 3566–3571. [Google Scholar] [PubMed]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 1997, 18, 767–778. [Google Scholar] [CrossRef]
- Je, H.S.; Yang, F.; Zhou, J.; Lu, B. Neurotrophin 3 induces structural and functional modification of synapses through distinct molecular mechanisms. J. Cell Biol. 2006, 175, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Adamson, C.L.; Reid, M.A.; Davis, R.L. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J. Neurosci. 2002, 22, 1385–1396. [Google Scholar] [PubMed]
- Zhou, Z.; Liu, Q.; Davis, R.L. Complex regulation of spiral ganglion neuron firing patterns by neurotrophin-3. J. Neurosci. 2005, 25, 7558–7566. [Google Scholar] [CrossRef] [PubMed]
- Highstein, S.M.; Maekawa, K.; Steinacker, A.; Cohen, B. Synaptic input from the pontine reticular nuclei to abducens motoneurons and internuclear neurons in the cat. Brain Res. 1976, 112, 162–167. [Google Scholar] [CrossRef]
- Munson, J.B.; Shelton, D.L.; McMahon, S.B. Adult mammalian sensory and motor neurons: Roles of endogenous neurotrophins and rescue by exogenous neurotrophins after axotomy. J. Neurosci. 1997, 17, 470–476. [Google Scholar] [PubMed]
- Mendell, L.M.; Johnson, R.D.; Munson, J.B. Neurotrophin modulation of the monosynaptic reflex after peripheral nerve transection. J. Neurosci. 1999, 19, 3162–3170. [Google Scholar] [PubMed]
- Chen, H.-H.; Tourtellotte, W.G.; Frank, E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J. Neurosci. 2002, 22, 3512–3519. [Google Scholar] [PubMed]
- Arvanian, V.L.; Horner, P.J.; Gage, F.H.; Mendell, L.M. Chronic neurotrophin-3 strengthens synaptic connections to motoneurons in the neonatal rat. J. Neurosci. 2003, 23, 8706–8712. [Google Scholar] [PubMed]
- Aksay, E.; Gamkrelidze, G.; Seung, H.S.; Baker, R.; Tank, D.W. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat. Neurosci. 2001, 4, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Escudero, M.; de la Cruz, R.R.; Delgado-García, J.M. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J. Physiol. 1992, 458, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Yamada, M.K.; Nishiyama, N.; Matsuki, N.; Ikegaya, Y. BDNF boosts spike fidelity in chaotic neural oscillations. Biophys. J. 2004, 86, 1820–1828. [Google Scholar] [CrossRef]
- Youssoufian, M.; Walmsley, B. Brain-derived neurotrophic factor modulates cell excitability in the mouse medial nucleus of the trapezoid body. Eur. J. Neurosci. 2007, 25, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, N.; Mannen, H.; Sasaki, S.; Shimazu, H. Axonal branches and terminations in the cat abducens nucleus of secondary vestibular neurons in the horizontal canal system. Neurosci. Lett. 1980, 16, 143–148. [Google Scholar] [CrossRef]
- Ramachandran, R.; Lisberger, S.G. Transformation of vestibular signals into motor commands in the vestibuloocular reflex pathways of monkeys. J. Neurophysiol. 2006, 96, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Ebendal, T.; Larhammar, D.; Persson, H. Structure and expression of the chicken β nerve growth factor gene. EMBO J. 1986, 5, 1483–1487. [Google Scholar] [PubMed]
- Ernfors, P.; Henschen, A.; Olson, L.; Persson, H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron 1989, 2, 1605–1613. [Google Scholar] [CrossRef]
- Capsoni, S.; Ruberti, F.; di Daniel, E.; Cattaneo, A. Muscular dystrophy in adult and aged anti-NGF transgenic mice resembles an inclusion body myopathy. J. Neurosci. Res. 2000, 59, 553–560. [Google Scholar] [CrossRef]
- Lindsay, R.M. Trophic protection of motor neurons: Clinical potential in motor neuron diseases. J. Neurol. 1994, 242, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.C.; Ghasemlou, N.; Bisby, M.A.; Kawaja, M.D. Nerve growth factor alters p75 neurotrophin receptor-induced effects in mouse facial motoneurons following axotomy. Brain Res. 2002, 950, 180–185. [Google Scholar] [CrossRef]
- Weskamp, G.; Reichardt, L.F. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron 1991, 6, 649–663. [Google Scholar] [CrossRef]
- Frade, J.M.; Rodríguez-Tébar, A.; Barde, Y.A. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature 1996, 383, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Frade, J.M.; Barde, Y.A. Nerve growth factor: Two receptors, multiple functions. Bioessays 1998, 20, 137–145. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Signal transduction by the neurotrophin receptors. Curr. Opin. Cell Biol. 1997, 9, 213–221. [Google Scholar] [CrossRef]
- Barker, P.A. p75NTR: A study in contrasts. Cell Death Differ. 1998, 5, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Matusica, D.; Skeldal, S.; Sykes, A.M.; Palstra, N.; Sharma, A.; Coulson, E.J. An intracellular domain fragment of the p75 neurotrophin receptor (p75(NTR)) enhances tropomyosin receptor kinase A (TrkA) receptor function. J. Biol. Chem. 2013, 288, 11144–11154. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.M.R.; van Der Zee, C.E.E.M.; Hagg, T. p75 nerve growth factor receptor is important for retrograde transport of neurotrophins in adult cholinergic basal forebrain neurons. Neuroscience 1999, 94, 1163–1172. [Google Scholar] [CrossRef]
- Lee, F.S.; Kim, A.H.; Khursigara, G.; Chao, M.V. The uniqueness of being a neurotrophin receptor. Curr. Opin. Neurobiol. 2001, 11, 281–286. [Google Scholar] [CrossRef]
- Blum, R.; Konnerth, A. Neurotrophin-mediated rapid signaling in the central nervous system: Mechanisms and functions. Physiology 2005, 20, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hazari, M.S.; Pan, J.H.; Myers, A.C. Nerve growth factor acutely potentiates synaptic transmission in vitro and induces dendritic growth in vivo on adult neurons in airway parasympathetic ganglia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L992–L1001. [Google Scholar] [CrossRef] [PubMed]
- Hartikka, J.; Hefti, F. Development of septal cholinergic neurons in culture: Plating density and glial cells modulate effects of NGF on survival, fiber growth, and expression of transmitter-specific enzymes. J. Neurosci. 1988, 8, 2967–2985. [Google Scholar] [PubMed]
- Takei, N.; Kuramoto, H.; Endo, Y.; Hatanaka, H. NGF and BDNF increase the immunoreactivity of vesicular acetylcholine transporter in cultured neurons from the embryonic rat septum. Neurosci. Lett. 1997, 226, 207–209. [Google Scholar] [CrossRef]
- Huh, C.Y.L.; Danik, M.; Manseau, F.; Trudeau, L.E.; Williams, S. Chronic exposure to nerve growth factor increases acetylcholine and glutamate release from cholinergic neurons of the rat medial septum and diagonal band of Broca via mechanisms mediated by p75NTR. J. Neurosci. 2008, 28, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Yeh, H.H. Nerve growth factor rapidly increases muscarinic tone in mouse medial septum/diagonal band of Broca. J. Neurosci. 2005, 25, 4232–4242. [Google Scholar] [CrossRef] [PubMed]
- Auld, D.S.; Mennicken, F.; Quirion, R. Nerve growth factor rapidly induces prolonged acetylcholine release from cultured basal forebrain neurons: Differentiation between neuromodulatory and neurotrophic influences. J. Neurosci. 2001, 21, 3375–3382. [Google Scholar] [PubMed]
- Numakawa, T.; Nakayama, H.; Suzuki, S.; Kubo, T.; Nara, F.; Numakawa, Y.; Yokomaku, D.; Araki, T.; Ishimoto, T.; Ogura, A.; et al. Nerve growth factor-induced glutamate release is via p75 receptor, ceramide, and Ca2+ from ryanodine receptor in developing cerebellar neurons. J. Biol. Chem. 2003, 278, 41259–41269. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, A.; Sirrenberg, C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J. Biol. Chem. 1996, 271, 21100–21107. [Google Scholar] [PubMed]
- Heckman, C.J.; Binder, M.D. Computer simulations of the effects of different synaptic input systems on motor unit recruitment. J. Neurophysiol. 1993, 70, 1827–1840. [Google Scholar] [PubMed]
- Cope, T.C.; Sokoloff, A.J. Orderly recruitment among motoneurons supplying different muscles. J. Physiol. Paris 1999, 93, 81–85. [Google Scholar] [CrossRef]
- Burke, R.E. Motor units: Anatomy, physiology, and functional organization. In Handbook of Physiology—The Nervous System II. Volume 1, Part 1; Brooks, V.D., Ed.; American Physiological Society: Bethesda, MD, USA, 1981; pp. 345–422. [Google Scholar]
- González-Forero, D.; Álvarez, F.J.; de la Cruz, R.R.; Delgado-García, J.M.; Pastor, A.M. Influence of afferent synaptic innervation on the discharge variability of cat abducens motoneurones. J. Physiol. 2002, 541, 283–299. [Google Scholar] [CrossRef] [PubMed]
- González-Forero, D.; Pastor, A.M.; Delgado-García, J.M.; de la Cruz, R.R.; Álvarez, F.J. Synaptic structural modification following changes in activity induced by tetanus neurotoxin in cat abducens neurons. J. Comp. Neurol. 2004, 471, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Salama-Cohen, P.; Arévalo, M.Á.; Grantyn, R.; Rodríguez-Tébar, A. Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J. Neurochem. 2006, 97, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.B.; Turrigiano, G.G. Strength through diversity. Neuron 2008, 60, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Nja, A.; Purves, D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J. Physiol. 1978, 277, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Weigand, L.A.; Kwong, K.; Myers, A.C. The effects of nerve growth factor on nicotinic synaptic transmission in mouse airway parasympathetic neurons. Am. J. Respir. Cell Mol. Biol. 2015, 53, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Tep, C.; Benedick, A.; Saidi, N.; Ryu, J.C.; Kim, M.L.; Sadasivan, S.; Oberdick, J.; Smeyne, R.; Zhu, M.X.; et al. p75 Regulates Purkinje cell firing by modulating SK channel activity through Rac1. J. Biol. Chem. 2014, 289, 31458–31472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nicol, G. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci. Lett. 2004, 366, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.A.; Birren, S.J. p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents. J. Neurosci. 2009, 29, 5411–5424. [Google Scholar] [CrossRef] [PubMed]
- Nieto-González, J.L.; Carrascal, L.; Nuñez-Abades, P.; Torres, B. Muscarinic modulation of recruitment threshold and firing rate in rat oculomotor nucleus motoneurons. J. Neurophysiol. 2009, 101, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vasko, M.; Nicol, G. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory. J. Physiol. 2002, 544, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.; Koltzenburg, M.; Priestley, J.V.; Shelton, D.L.; McMahon, S.B. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur. J. Neurosci. 1998, 10, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Mendell, L.M. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci. Lett. 1999, 274, 159–162. [Google Scholar] [CrossRef]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Mantyh, P.W.; Koltzenburg, M.; Mendell, L.M.; Tive, L.; Shelton, D.L. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011, 115, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Ribrault, C.; Sekimoto, K.; Triller, A. From the stochasticity of molecular processes to the variability of synaptic transmission. Nat. Rev. Neurosci. 2011, 12, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Shadlen, M.N.; Newsome, W.T. The variable discharge of cortical neurons: Implications for connectivity, computation, and information coding. J. Neurosci. 1998, 18, 3870–3896. [Google Scholar] [PubMed]
- Lampl, I.; Reichova, I.; Ferster, D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron 1999, 22, 361–374. [Google Scholar] [CrossRef]
- Salinas, E.; Sejnowski, T.J. Impact of correlated synaptic input on output firing rate and variability in simple neuronal models. J. Neurosci. 2000, 20, 6193–6209. [Google Scholar] [PubMed]
- Mendonça, P.R.; Vargas-Caballero, M.; Erdélyi, F.; Szabó, G.; Paulsen, O.; Robinson, H.P. Stochastic and deterministic dynamics of intrinsically irregular firing in cortical inhibitory interneurons. Elife 2016, 5, e16475. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, K.M.; Englitz, B.; Sejnowski, T.J. Origin of intrinsic irregular firing in cortical interneurons. Proc. Natl. Acad. Sci. USA 2013, 110, 7886–7891. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E. Motor unit types: Functional specializations in motor control. Trends Neurosci. 1980, 3, 255–258. [Google Scholar] [CrossRef]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 1973, 234, 723–748. [Google Scholar] [CrossRef] [PubMed]
- Davis-López de Carrizosa, M.A.; Morado-Díaz, C.J.; Miller, J.M.; de la Cruz, R.R.; Pastor, A.M. Dual encoding of muscle tension and eye position by abducens motoneurons. J. Neurosci. 2011, 31, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Raikova, R.; Krutki, P.; Aladjov, H.; Celichowski, J. Variability of the twitch parameters of the rat medial gastrocnemius motor units-experimental and modeling study. Comput. Biol. Med. 2007, 37, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Büttner-Ennever, J.A.; Horn, A.K.; Scherberger, H.; D’Ascanio, P. Motoneurons of twitch and nontwitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J. Comp. Neurol. 2001, 438, 318–335. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, G.; Klam, F.; Doldan Dans, M.; Dubayle, D.; Brandi, A.M.; Büttner-Ennever, J.; Graf, W. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: Differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J. Comp. Neurol. 2006, 498, 762–785. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, C.S.; Jakobsen, J.; Flyvbjerg, A.; Andersen, H. Expression of neurotrophic factors in diabetic muscle--relation to neuropathy and muscle strength. Brain 2009, 132, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fukuda, K. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: The regulatory mechanisms of cardiac innervation and their critical roles in cardiac performance. J. Pharmacol. Sci. 2009, 109, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Soderstrom, K.E.; Kordower, J.H. Trophic factors therapy in Parkinson’s disease. Prog. Brain Res. 2009, 175, 201–216. [Google Scholar] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez-Temiño, B.; Davis-López de Carrizosa, M.A.; Morcuende, S.; Matarredona, E.R.; De la Cruz, R.R.; Pastor, A.M. Functional Diversity of Neurotrophin Actions on the Oculomotor System. Int. J. Mol. Sci. 2016, 17, 2016. https://doi.org/10.3390/ijms17122016
Benítez-Temiño B, Davis-López de Carrizosa MA, Morcuende S, Matarredona ER, De la Cruz RR, Pastor AM. Functional Diversity of Neurotrophin Actions on the Oculomotor System. International Journal of Molecular Sciences. 2016; 17(12):2016. https://doi.org/10.3390/ijms17122016
Chicago/Turabian StyleBenítez-Temiño, Beatriz, María A. Davis-López de Carrizosa, Sara Morcuende, Esperanza R. Matarredona, Rosa R. De la Cruz, and Angel M. Pastor. 2016. "Functional Diversity of Neurotrophin Actions on the Oculomotor System" International Journal of Molecular Sciences 17, no. 12: 2016. https://doi.org/10.3390/ijms17122016
APA StyleBenítez-Temiño, B., Davis-López de Carrizosa, M. A., Morcuende, S., Matarredona, E. R., De la Cruz, R. R., & Pastor, A. M. (2016). Functional Diversity of Neurotrophin Actions on the Oculomotor System. International Journal of Molecular Sciences, 17(12), 2016. https://doi.org/10.3390/ijms17122016