To our knowledge, this is the first study that has used transcriptomics methods to analyse the gene expression changes associated with OLE consumption in humans. It is important to acknowledge that this was a preliminary study to identify where changes in gene expression are occurring. The sample size was small. Thus, the results are indicative rather than conclusive but will help guide the direction of future research. The results suggest that anti-inflammatory and cancer-related gene expression changes are associated with the consumption of OLE and could explain the health benefits claimed with traditional use. Changes in gene expression indicate that the anti-inflammatory profile involves phospholipase and inflammatory pathways. There was very little evidence that changes in gene expression relating to the phase II enzymes and the Nrf2 canonical pathway were altered by OLE consumption, although inhibition of this pathway by olive oil phenolics has been suggested as instrumental in oxidative and inflammatory protection [
6,
7]. Many of the downregulated genes are pivotal in inflammation and disease (
OSM,
COX-2 and
IL-8). Metabolic processes were also emphasized and an important lipolysis-related gene (
G0S2) was altered by OLE consumption.
3.1. Gene Expression in PBMCs
PBMCs were chosen for analysis because they are easily accessible and express many genes believed to be restricted to non-blood tissues that respond to micro and macro alterations to organs, and will reflect effects of diet and nutrients [
23,
24]. Although mRNA expression profiles do not allow direct determination of protein function (especially those involved in phosphorylation signalling cascades), they do allow investigation of novel signalling pathways that OLE polyphenols may act through in human PBMCs. In a sample of healthy people taking a nutraceutical, changes are likely to be numerous but subtle [
25], and thus it was not unexpected that the FC and significance of gene expression was not very strong. It is acknowledged that in this study, using an adjusted
p-value cut off at 0.05, no changes in gene expressions would be deemed statistically significant. Therefore, an unadjusted
p-value cut off at 0.05 was used. Three genes differentially expressed in response to supplementation were selected for validation with real-time PCR. One gene was significantly downregulated (
EGR1) and one gene was significantly upregulated (
ID3) when assessed by microarray. The third gene (
COX-2) was selected because it plays an important role in inflammatory and cancer pathways. The expression levels between microarrays and real-time PCR for all three genes were similar, giving credibility to the use of unadjusted
p-values for microarray analysis. All three genes used for validation play a role in cancer.
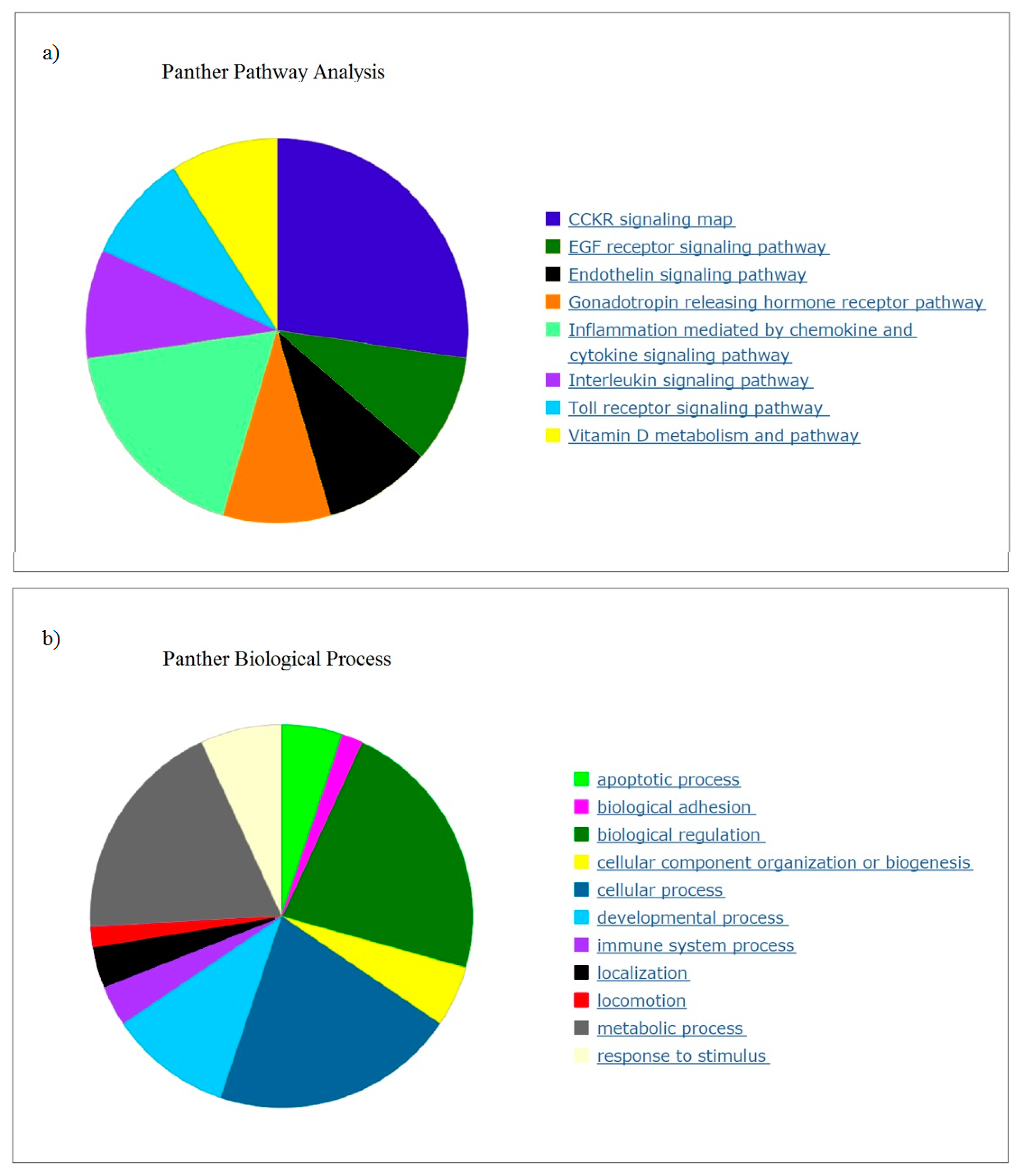
Many of the top genes downregulated in response to OLE were relevant to chronic and acute inflammation. Pathway analysis correlated the expression to inflammation and metabolic processes and identified cancer, arthritis, and adipogenesis as relevant. A recent study identified potential target genes for therapy for inflammation (
COX-2), immune imbalance (
IL-8), and active atherosclerosis (
OSM) [
26]. These genes were all downregulated with OLE.
3.2. COX-2 Expression in PBMCs
Research around the inflammatory enzyme COX-2 is extensive. This gene encoding this enzyme (COX-2) was downregulated in response to OLE consumption. At the time this study was completed, there were no existing studies that had demonstrated that olive polyphenols were able to regulate COX-2 beyond post-prandial consumption in healthy humans.
Interest in the anti-inflammatory properties of oleocanthal (an olive phenolic) was motivated by a study that demonstrated its ability to decrease the COX-2 enzyme levels in vitro [
27]. Expression of the gene was not investigated. It was hypothesized that oleocanthal was acting in a similar manner to ibuprofen (IB) (inhibiting the enzyme) but to gain the same effects that the recommended dose of IB has on COX-2, it has been estimated that approximately 500 g of extra virgin OO (EVOO) would need to be consumed each day [
27]. This is clearly not a realistic daily dose.
COX-2 inhibition has been definitively demonstrated in cell models [
28,
29] and mice [
30,
31] treated with olive polyphenols. Cell models used polyphenol extract while the animal models have distributed it in their food with OO or refined oil, respectively.
3.3. IL-8 Expression in PBMCs
In this study,
IL-8 was downregulated with OLE. This corresponds to the most recent in vivo work with the same OLE supplement in humans in which reduced levels of
IL-8 were observed in blood taken from participants after a six-week intervention [
9]. Ex vivo work by this group has also shown that OLE is able to downregulate
IL-8 [
32]. Another study looking at overweight males found no changes to
IL-8 with OLE
IL-8 is a pro-inflammatory chemokine involved in cellular response to inflammation, tumour proliferation, and has been acknowledged to play an important role in cancer [
33].
IL-8 is a target gene of NF-κB [
34], and, as mentioned above, has been identified as a potential target therapy for immune imbalance.
3.4. Other Genes with Inflammatory Relevance
This study measured a downregulation of transcription factor jun-B (
JUNB) with OLE.
JUNB is an AP1 transcription factor correlated to immune function, and recent experimental work has demonstrated its pivotal role in macrophage activation [
22,
35].
JUNB can be directly activated by NF-κB in dendritic cells with LPS stimulation [
36] and is required for the full activation of IL1β and tumor necrosis factor (TNF) in macrophages stimulated by LPS [
22].
Heparin binding EGF-like growth factor (
HBEGF) was also downregulated with OLE. This growth factor is activated by the transcription factors AP1 and NF-κB and plays a role in cancer and other chronic diseases [
37]. Upregulation of
HBEGF has been observed in several cancer cells including breast (MDA-MB-231) and prostate (PC3), and it has been suggested as a potential target for treatment [
38]. Its upregulation has also been correlated to NF-κB activation of inflammatory cytokines [
39].
OSM was downregulated with OLE consumption in this study. This gene encodes the pro-inflammatory cytokine OSM, expression of which has been correlated with metastasis of cancer tumours [
40,
41]. In endometrial cancer, OSM was shown to activate STAT3, enhance cell migration and invasion in vitro and promote cell proliferation, growth and angiogenesis in vivo [
42].
OSM receptor knock-out (KO) mice with cardiac disease have demonstrated improved survival and cardiac performance [
43]; therefore,
OSM could be a target for protection from cardiovascular complications.
3.5. NF-κB Inflammatory Pathway
The transcription factor NF-κB activates >150 genes in response to stress [
44]. The NF-κB complex resides inactively within the cytoplasm due to the presence of the inhibitor molecule IκB [
45]. Measuring changes in the gene expression of NF-κB is largely ineffective because it is already present and therefore does not require further protein synthesis, merely activation. The kinases inhibitor of nuclear factor κB kinase subunit (IKK)α and IKKβ phosphorylate IκB proteins, allowing NF-κB to enter the nucleus, bind to promoter regions of target genes and enhance transcription of genes [
46]. The intervention work revealed no measurable change in expression of
IκB or
NF-κB, although there was a modest decrease in the expression of
IKKβ. This would result in decreased phosphorylation of IκB and therefore decreased release of NF-κB. IKK can be triggered by pro-inflammatory cytokines [
47]. MyD88 was also downregulated and plays an important role in the activation of NF-κB triggered by TLRs. Lastly,
NKAPL was downregulated with OLE. An in vivo model has demonstrated that its suppression leads to downregulation of
NF-κB via inhibiting
TNF and
IL1β [
48].
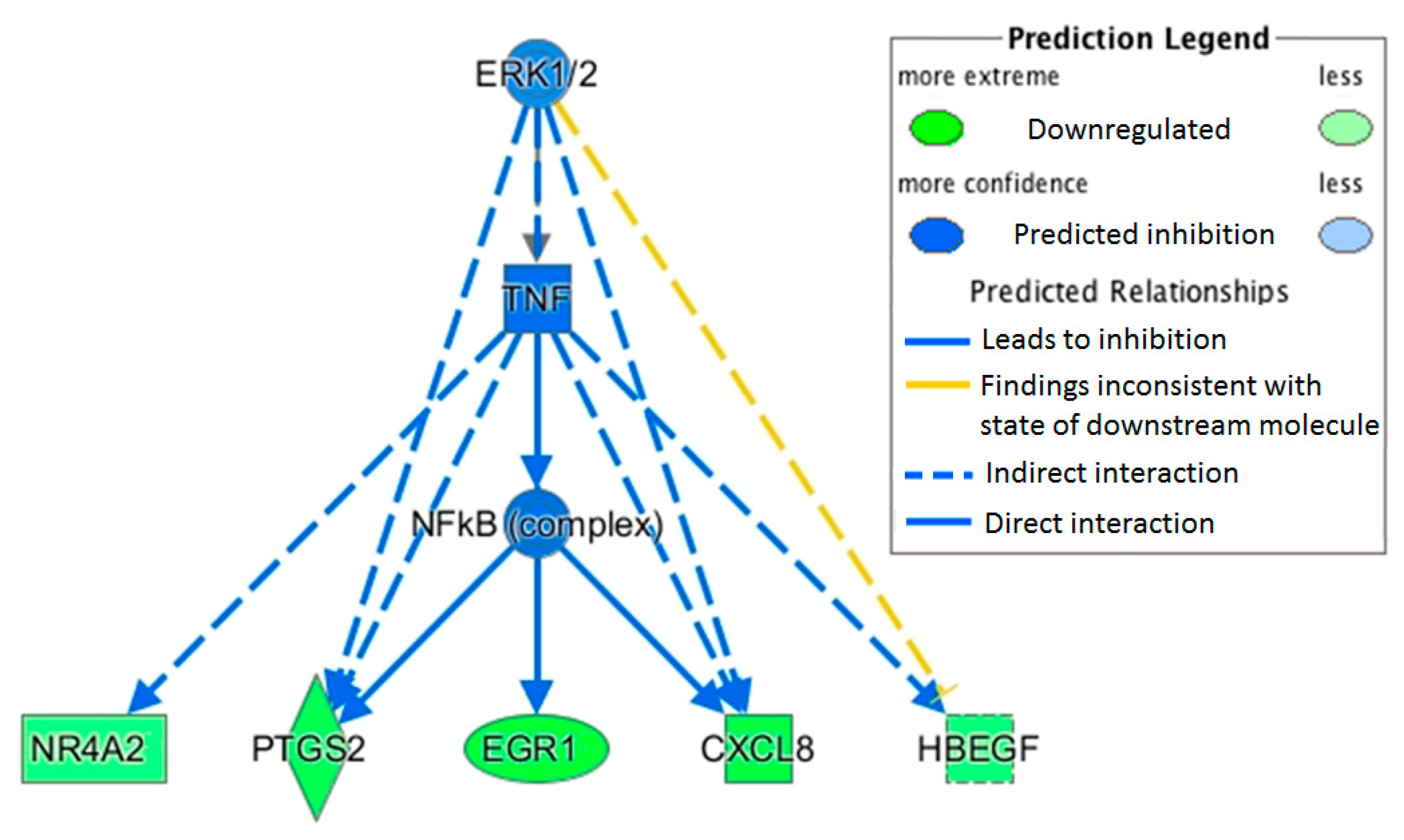
MAPK/AP1 may also play a role in OLE inflammatory changes. In this study,
JUNB expression was downregulated, and this plays a role in the MAPK pathway (
Figure 3).
3.6. Lipid Metabolism and Anti-Inflammatory Gene Expression
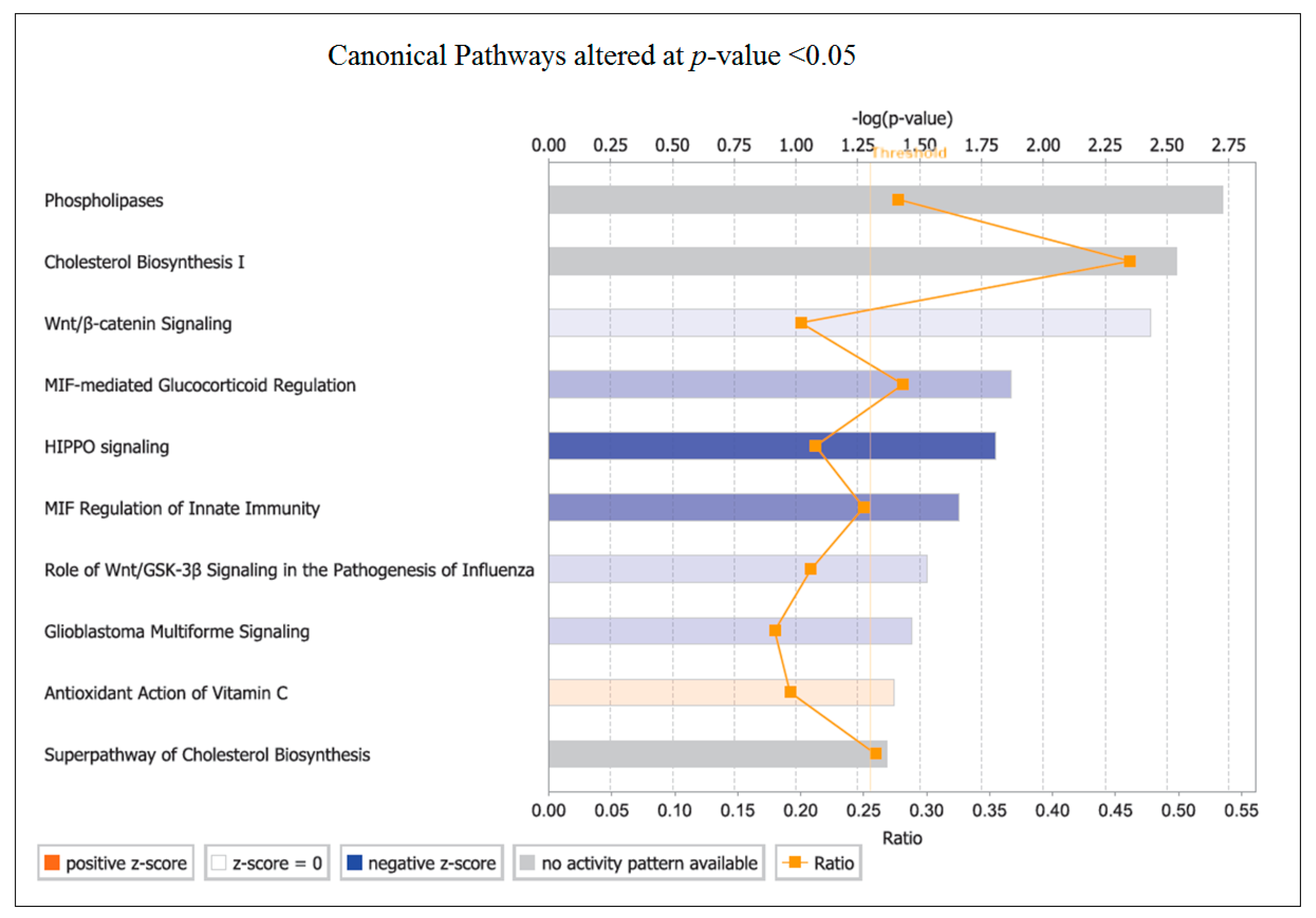
The top canonical pathway identified by pathway analysis was PLA. Phospholipases are enzymes that hydrolyse phospholipids into fatty acids and are categorized by the type of reaction they catalyse [
49]. The genes involved in changes with OLE were predominantly associated with phospholipase A2 (PLA
2) and then from within Phospholipase C (PLC). The PLC enzymes play an important role in cell signalling, proliferation and differentiation [
50].
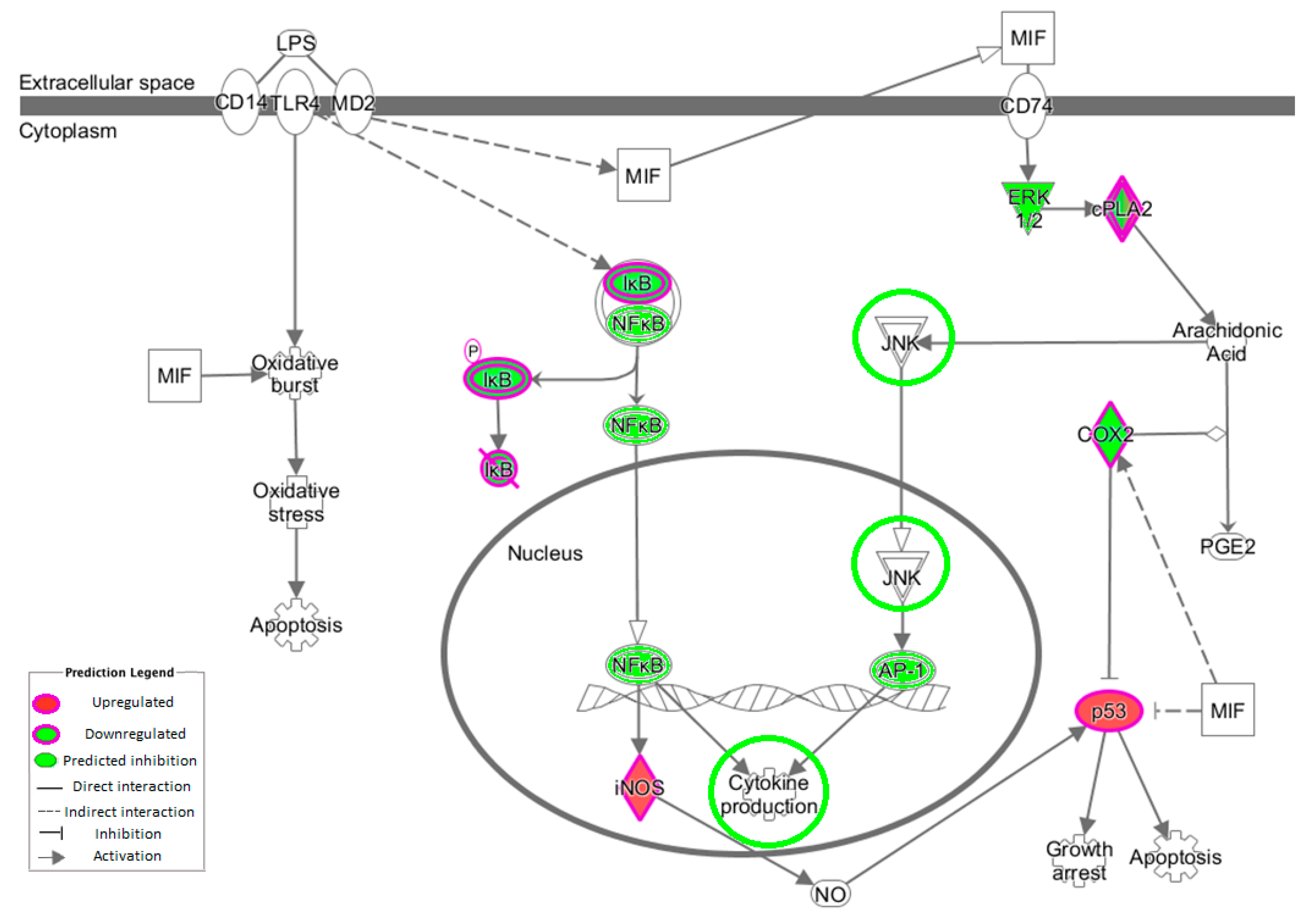
PLA
2 plays an important role in inflammation via the release of arachidonic acid (AA) from the phospholipid membrane [
51]. AA is transformed into prostaglandin (PGE2), which is converted by COXs to eicosanoids (including prostaglandins and leukotrienes), which influence both innate and adaptive immunity [
52,
53]. The downregulation of
COX-2 observed in this study could restrict prostanoid (prostaglandin, thromboxane and prostacyclin) production from AA. Many diseases and their related complications have been correlated to PLA
2 upregulation, including cardiovascular complications, atherosclerosis, vascular inflammation, cancer and obesity [
53,
54,
55,
56]. Inhibition of this pathway would partially explain the downregulation of inflammatory genes in this study.
G0S2 showed the strongest FC in expression after OLE consumption compared to all the other regulated genes, which is a novel finding. This gene has recently been highlighted for its ability to inhibit adipose triglyceride lipase (ATGL) which is key for the mobilization of triacylglycerol stores [
57]. We are not aware of any studies that have reported or investigated changes in
G0S2 with polyphenols.
G0S2 KO in high fat diet-fed mice protects from obesity and insulin resistance, and increases thermogenesis [
58,
59,
60]. Zhang et al. [
59] measured an increase in ketogenesis (measured by plasma ketones). Silencing of
G0S2 prevented adipocytes from differentiation, while overexpression activated progression from pre-adipocytes to mature adipocytes in vitro and in vivo.
G0S2 KO reduced fat mass, lipid droplet size and the adipogenesis pathway in mice [
57]. This suggests that
G0S2 is a key regulator of energy homeostasis, mediating lipolysis and fatty acid oxidation, and a downregulation of this gene could be beneficial in the treatment of both obesity and obesity-related diseases.
3.7. Gene Expression Change and Health Outcomes
The anti-inflammatory gene expression demonstrates that changes are not merely due to anti-oxidising capacity as was historically hypothesised. Downregulating the AA and NF-κB pathways could explain many of the health benefits attributed to OLE with traditional use. These include pain, inflammatory related diseases such as cancer, diabetes, cardiovascular disease, and an improved acute immune response.
Chronic inflammation is linked to many diseases including cancer and cardiovascular disease.
COX-2 is a strong driver of inflammation, and, therefore, has been an important area of focus [
61]. The AA pathway and PGE2 production have been correlated with cancer and are also known as the “inflammogenesis of cancer” [
53]. Regular intake of
COX-2 inhibitors such as aspirin has been associated with a reduced risk of death due to cancers including prostate and breast [
61,
62,
63]. Upregulation of
COX-2 is found in skin, breast, prostate, bladder and pancreatic carcinomas [
64].
Inhibiting
COX-2 and thereby reducing prostaglandins could also explain improvements in Blood Pressure (BP) measured in humans after OLE consumption [
9]. HT administered to rats was able to reduce thromboxane B
2 and platelet aggregation in blood [
65]. In a model using hypertensive rats, COX-2 inhibitors reduced Prostaglandin F2α (PGF2α) expression, improved endothelial relaxation and reduced BP [
66]. In humans, intake of high phenolic enriched OO has also shown improved BP with reduced chemokine (C-X-C motif) receptor 2 (
IL8RA) and other genes related to BP [
67]. This study explained that the reduction in
IL8RA could influence the renin–angiotensin–aldosterone system II, which plays an important role in blood pressure and thereby hypertension. Although
IL8RA was not identified in our study, it is a specific receptor for
IL-8. In studies investigating postprandial effects of olive oil phenolic consumption in humans, the anti-inflammatory profile was evident but an upregulation in cholesterol efflux was also demonstrated [
68,
69]. Cholesterol accumulation in immune cells leads to inflammatory responses, which, in turn, can lead to decreased cholesterol efflux and further inflammation [
70]. A reduction in oxidised cholesterol could help explain the anti-inflammatory profile measured with OLE in our study.
In downregulating acute inflammation, OLE would be hypothesized to compromise the immune system and conceivably facilitate infection. More recently, it has been suggested that bacteria may benefit from and further stimulate PGE2 synthesis in order to compromise the gut and lung linings of the host [
71]. In human macrophages, an inhibition of COX-2 reduced H5N1 infected pro-inflammatory response and virus replication, the work indicated the virus required COX-2 pathway activation in order to replicate [
72]. IL-8 production is also activated by influenza [
73]. A downregulation of inflammatory related genes as observed in this study with OLE could improve immune function and defence against pathogen attack.
This study demonstrated downregulation in G0S2 with OLE supplementation. If G0S2 was able to downregulate free fatty acid (FFA) or adipogenesis, this could contribute to the reduced inflammatory profile measured in participants taking OLE in this study.