Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke
Abstract
:1. Introduction
2. The Differences and Similarities between Microglia and Monocytes/Macrophages
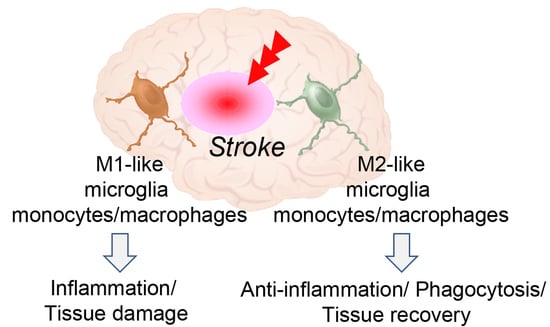
3. Microglia and Monocytes/Macrophages Act as Double-Edged Sword after Stroke
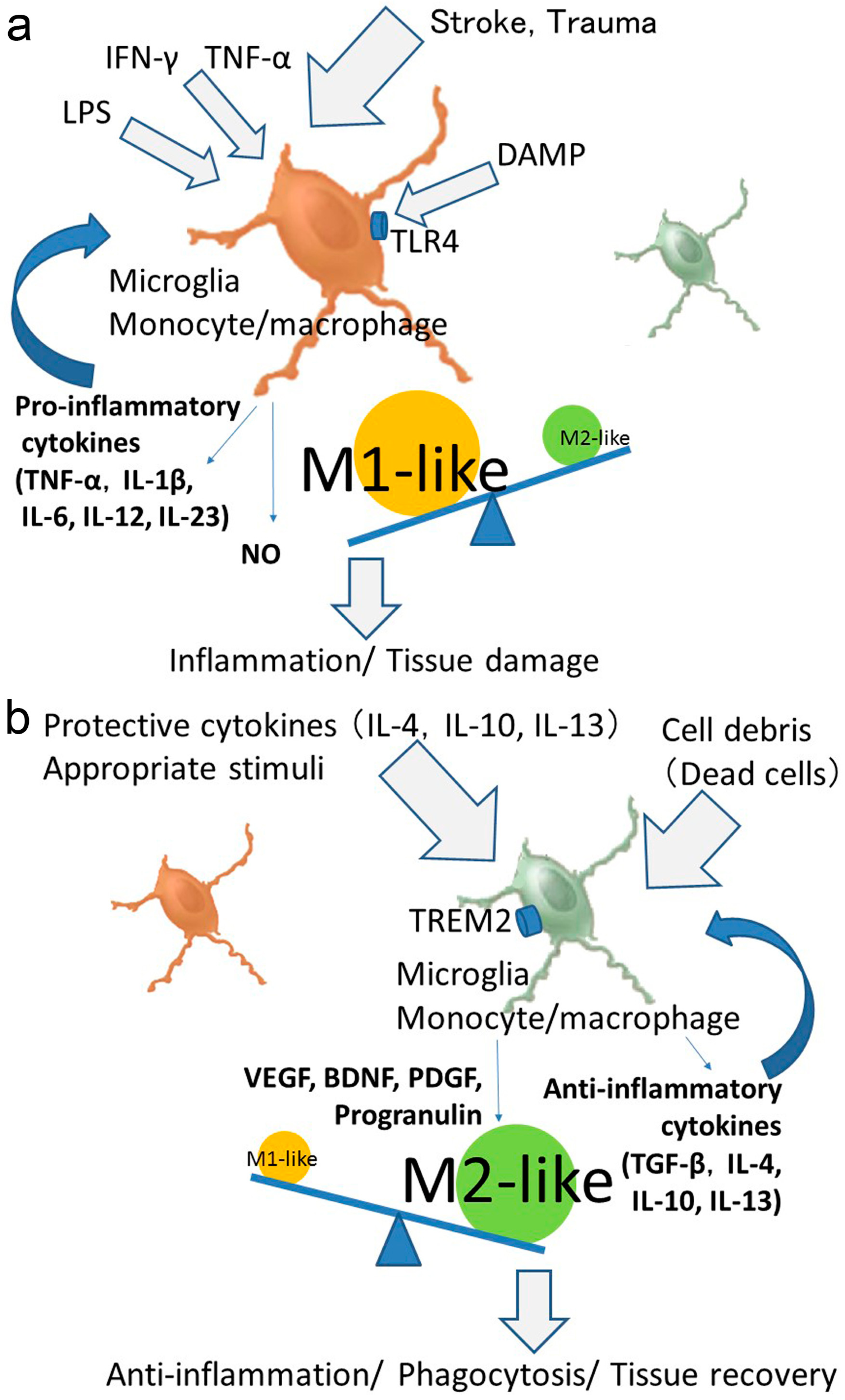
4. Phenotypic Polarization of Microglia and Monocytes/Macrophages after Stroke
5. Influence of Age and Sex on the Polarized Activation of Microglia and Monocytes/Macrophages
6. Polarized Activation of Microglia and Monocytes/Macrophages for Providing Therapeutic Strategies
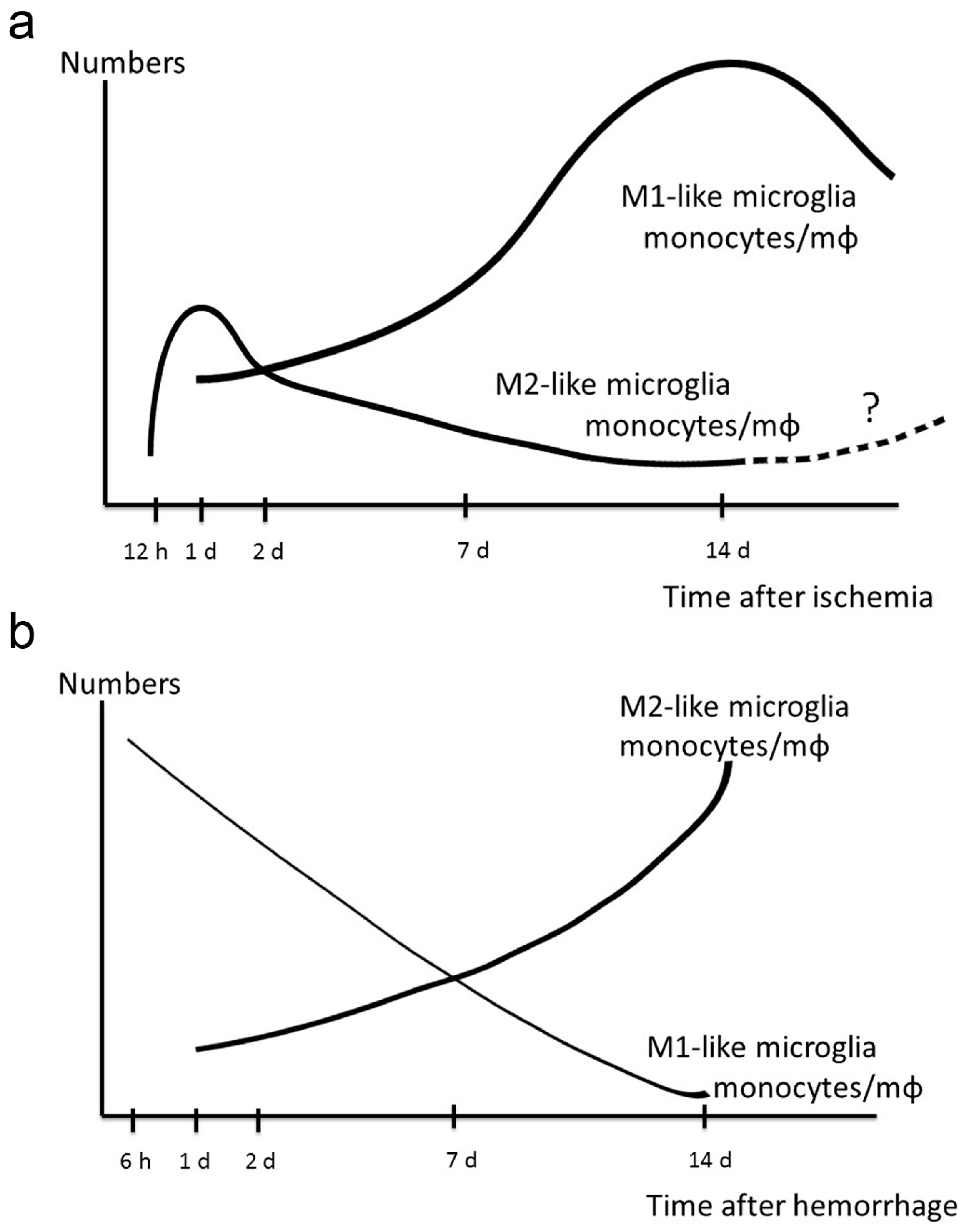
7. Dynamic Polarized Changes of Microglia and Monocytes/Macrophages after Stroke
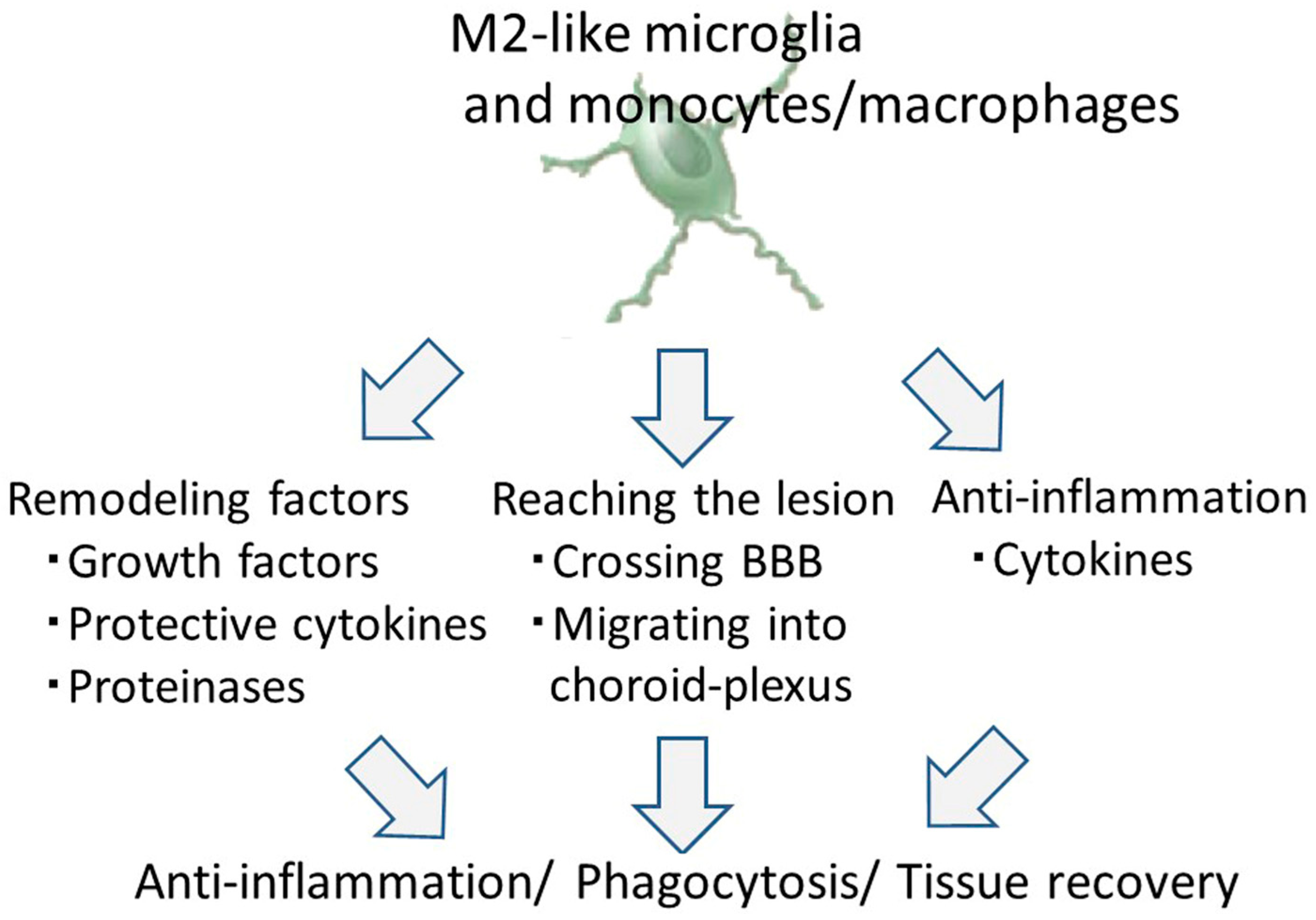
8. Therapeutic Potential of M2-Like Microglia and Monocytes/Macrophages against Ischemic Stroke
8.1. Tissue and Vascular Remodeling by M2-Like Microglia and Monocytes/Macrophages
8.2. Infiltrating Properties of M2-Like Microglia and Monocytes/Macrophages
8.3. The Anti-Inflammation Effect of M2-Like Microglia and Monocytes/Macrophages
9. Previous Reports on the Effects of M2-Like Microglia and Monocyte/Macrophage against Ischemic Stroke
10. Therapeutic Potential of M2-Like Microglia and Monocytes against Hemorrhagic Stroke
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| CCR2 | C-C chemokine receptor type 2 |
| CD | cluster of differentiation |
| CNS | central nervous system |
| CX3CR1 | CX3C chemokine receptor 1 |
| DAMP | damage-associated molecular pattern |
| ES | embryonic stem |
| GDNF | glial cell-derived neurotrophic factor |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| GSH | glutathione-SH |
| IFN | interferon |
| IGF | insulin-like growth factor |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| iPS | induced pluripotent stem |
| LPS | lipopolysaccharide |
| Mac-1 | macrophage-1 antigen |
| MMP-9 | matrix metalloproteinase-9 |
| MRI | magnetic resonance imaging |
| NO | nitrogen monoxide |
| OGD | oxygen-glucose deprivation |
| PDGF | platelet-derived growth factor |
| PET | positron emission tomography |
| P2Y12R | P2Y12 receptor |
| ROS | reactive oxygen species |
| SDF-1 | stromal-derived factor-1 |
| TGF-β | transforming growth factor-β |
| TLR4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor-α |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
| TSPO | translocator protein |
| USPIO | ultrasmall superparamagnetic particles of iron oxide |
| VEGF | vascular endothelial growth factor |
References
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. American Heart Association statistics committee; stroke statistics subcommittee. Executive summary: Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Guan, J.; Hawryluk, G.W. Targeting secondary hematoma expansion in spontaneous intracerebral hemorrhage-state of the art. Front. Neurol. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, R.; Yan, T.; Yu, S.P.; Wei, L.; Xu, G.; Fan, X.; Jiang, Y.; Stetler, R.A.; Liu, F.; et al. Cell based therapies for ischemic stroke: From basic science to bedside. Prog. Neurobiol. 2014, 115, 92–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ford, M.C.; Lavik, E.B.; Madri, J.A. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: An in vitro study. J. Neurosci. Res. 2006, 84, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Takagi, N.; Hashimura, K.; Itokawa, C.; Tanonaka, K. Intravenous injection of neural progenitor cells facilitates angiogenesis after cerebral ischemia. Brain Behav. 2013, 3, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, S.; Horie, N.; Satoh, K.; Fukuda, Y.; Nishida, N.; Nagata, I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013, 44, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren-Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Satani, N.; Savitz, S.I. Is immunomodulation a principal mechanism underlying how cell-based therapies enhance stroke recovery? Neurotherapeutics 2016, 13, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, M.; Gemma, C.; Pennypacker, K.R.; Bickford, P.C.; Sanberg, C.D.; Sanberg, P.R.; Willing, A.E. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp. Neurol. 2016, 199, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Kono, K.; Morimoto, K.; Inoue, Y.; Shintaku, H.; Hattori, H.; Matsuoka, O.; Yamano, T.; Tanaka, A. Brain magnetic resonance imaging in 23 patients with mucopolysaccharidoses and the effect of bone marrow transplantation. Ann. Neurol. 2001, 50, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Jander, S.; Schroeter, M. Inflammation and glial responses in ischemic brain lesions. Prog. Neurobiol. 1998, 56, 149–171. [Google Scholar] [CrossRef]
- Mabuchi, T.; Kitagawa, K.; Ohtsuki, T.; Kuwabara, K.; Yagita, Y.; Yanagihara, T.; Hori, M.; Matsumoto, M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 2000, 31, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Fumagalli, S.; De Simoni, M.G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflammation 2011, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Han, X.; Li, Q.; Yang, Q.W.; Wang, J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 2017, 13, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013, 91, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Imai, F.; Suzuki, H.; Oda, J.; Ninomiya, T.; Ono, K.; Sano, H.; Sawada, M. Neuroprotective effect of exogenous microglia in global brain ischemia. J. Cereb. Blood Flow Metab. 2007, 27, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef]
- Vivien, D.; Ali, C. Transforming growth factor-beta signalling in brain disorders. Cytokine Growth Factor Rev. 2006, 17, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, N.D.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M.H. Microglia-blood vessel interactions: A double-edged sword in brain pathologies. Acta Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Miura, M.; Toriyabe, M.; Koyama, M.; Hatakeyama, M.; Ishikawa, M.; Nakajima, T.; Onodera, O.; Takahashi, T.; Nishizawa, M.; et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep. 2017, 7, 42582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, G.; Zhang, J.; Strong, R.; Song, W.; Gonzales, N.; Grotta, J.C.; Aronowski, J. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor γ in microglia/macrophages. Ann. Neurol. 2007, 61, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Garton, T.; Keep, R.F.; Hua, Y.; Xi, G. Microglia/macrophage polarization after experimental intracerebral hemorrhage. Transl. Stroke Res. 2015, 6, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Kacimi, R.; Kauppinen, T.; Calosing, C.; Kim, J.Y.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Ttriggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 2015, 35, 3384–3396. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.M.; Allegrini, P.R.; Rudin, M.; Perry, V.H.; Mir, A.K.; Wiessner, C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J. Cereb. Blood Flow Metab. 2002, 22, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H. Microglia: A cellular vehicle for CNS gene therapy. J. Clin. Investig. 2006, 116, 2857–2860. [Google Scholar] [CrossRef] [PubMed]
- Wattananit, S.; Tornero, D.; Graubardt, N.; Memanishvili, T.; Monni, E.; Tatarishvili, J.; Miskinyte, G.; Ge, R.; Ahlenius, H.; Lindvall, O.; et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J. Neurosci. 2016, 36, 4182–4195. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β-dependent molecular and functional signature in microGlia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Webster, C.M.; Hokari, M.; McManus, A.; Tang, X.N.; Ma, H.; Kacimi, R.; Yenari, M.A. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS ONE 2013, 8, e70927. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.S.; Ase, A.R.; Kinsara, A.; Rao, V.T.; Michell-Robinson, M.; Leong, S.Y.; Butovsky, O.; Ludwin, S.K.; Séguéla, P.; Bar-Or, A.; et al. P2Y12 expression and function in alternatively activated human microGlia. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e80. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Merad, M.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.A.; Kauppinen, K.M.; Swanson, R.A. Microglial activation in stroke: Therapeutic targets. Neurotherapeutics 2010, 7, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.D.; Taylor, R.A.; Mullen, M.T.; Ai, Y.; Aguila, H.L.; Mack, M.; Kasner, S.E.; McCullough, L.D.; Sansing, L.H. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J. Neurosci. 2014, 34, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.M.; Arora, P.K. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992, 587, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Hu, S.; Molitor, T.W.; Shaskan, E.G.; Peterson, P.K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 1992, 149, 2736–2741. [Google Scholar] [PubMed]
- Lo, E.H. Degeneration and repair in central nervous system disease. Nat. Med. 2010, 16, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Miró-Mur, F.; Pérez-de-Puig, I.; Ferrer-Ferrer, M.; Urra, X.; Justicia, C.; Chamorro, A.; Planas, A.M. Immature monocytes recruited to the ischemic mouse brain differentiate into macrophages with features of alternative activation. Brain Behav. Immun. 2016, 53, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Owens, T.; Boddeke, E. What is microglia neurotoxicity (Not)? Glia 2014, 62, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.X.; Broughton, B.R.; Kim, H.A.; Lee, S.; Drummond, G.R.; Sobey, C.G. Evidence that Ly6C(hi) monocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. Stroke 2015, 46, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Kawamura, K.; Takahashi, T.; Miura, M.; Tanaka, Y.; Koyama, M.; Toriyabe, M.; Igarashi, H.; Nakada, T.; Nishihara, M.; et al. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain 2015, 138, 1932–1948. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.F.; Murray, H.W.; Wiebe, M.E.; Rubin, B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983, 158, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microGlia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jalabi, W.; Shpargel, K.B.; Farabaugh, K.T.; Dutta, R.; Yin, X.; Kidd, G.J.; Bergmann, C.C.; Stohlman, S.A.; Trapp, B.D. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J. Neurosci. 2012, 32, 11706–11715. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Rochford, C.D.; Neumann, H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005, 201, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Sachet, M.; Liang, Y.Y.; Oehler, R. The immune response to secondary necrotic cells. Apoptosis 2017, 22, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.J.L.P.; Ford, C.A.; Petrova, S.; Melville, L.; Paterson, M.; Pound, J.D.; Holland, P.; Giotti, B.; Freeman, T.C.; Gregory, C.D. Modulation of macrophage antitumor potential by apoptotic lymphoma cells. Cell Death Differ. 2017, 24, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Szulzewsky, F.; Pelz, A.; Feng, X.; Synowitz, M.; Markovic, D.; Langmann, T.; Holtman, I.R.; Wang, X.; Eggen, B.J.; Boddeke, H.W.; et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS ONE 2015, 10, e0116644. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Gottfried-Blackmore, A.C.; McEwen, B.S.; Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 2007, 55, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Cordeau, P., Jr.; Lalancette-Hébert, M.; Weng, Y.C.; Kriz, J. Estrogen receptors alpha mediates postischemic inflammation in chronically estrogen-deprived mice. Neurobiol. Aging 2016, 40, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Amor, S.; Brouwer, N.; Vinet, J.; Eggen, B.; Biber, K.; Boddeke, H.W. Identification of a microglia phenotype supportive of remyelination. Glia 2012, 60, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The dual role of microglia in ALS: Mechanisms and therapeutic approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Morimoto, E.T.; Goodarzi, H.; Liao, J.T.; O’Keeffe, S.; Phatnani, H.P.; Muratet, M.; Carroll, M.C.; Levy, S.; Tavazoie, S.; et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013, 4, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, T.L.; Schmitt, F.A.; Nelson, P.T.; Wilcock, D.M. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Chhor, V.; Le Charpentier, T.; Lebon, S.; Oré, M.V.; Celador, I.; Josserand, J.; Degos, V.; Jacotot, E.; Hagberg, H.; Sävman, K.; et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 2013, 32, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Mittelbronn, M. M1/M2 immune polarization concept in microglia: A fair transfer? Neuroimmunol. Neuroinflamm. 2014, 1, 6–7. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, M.; Leypoldt, F.; Steinbach, K.; Behrens, D.; Choe, C.U.; Siler, D.A.; Arumugam, T.V.; Orthey, E.; Gerloff, C.; Tolosa, E.; et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009, 40, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, H.; Yang, Q.; Wu, H.; Wang, J. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol. Neurobiol. 2017, 54, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Tornero, D.; Hirota, M.; Monni, E.; Laterza, C.; Lindvall, O.; Kokaia, Z. Choroid plexus-cerebrospinal fluid route for monocyte-derived macrophages after stroke. J. Neuroinflammation 2017, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Ozdinler, P.H.; Macklis, J.D. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat. Neurosci. 2006, 9, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Park, E.; You, B.; Jung, Y.; Park, A.R.; Park, S.G.; Lee, J.R. Neuronal synapse formation induced by microglia and interleukin 10. PLoS ONE 2013, 8, e81218. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Inoue, K.; Hashimoto, R.; Kumamoto, N.; Kosuga, A.; Tatsumi, M.; Kamijima, K.; Kunugi, H.; Iwata, N.; Ozaki, N.; et al. Tumor necrosis factor receptor-associated protein 1 regulates cell adhesion and synaptic morphology via modulation of N-cadherin expression. J. Neurochem. 2009, 110, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Wake, H.; Ishikawa, A.W.; Eto, K.; Shibata, K.; Murakoshi, H.; Koizumi, S.; Koizumi, S.; Moorhouse, A.J.; Yoshimura, Y.; et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016, 7, 12540. [Google Scholar] [CrossRef] [PubMed]
- Sipe, G.O.; Lowery, R.L.; Tremblay, M.E.; Kelly, E.A.; Lamantia, C.E.; Majewska, A.K. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 2016, 7, 10905. [Google Scholar] [CrossRef] [PubMed]
- Galtrey, C.M.; Fawcett, J.W. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 2007, 54, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.H.; Wells, J.E.; Stallcup, W.B.; Opdenakker, G.; Yong, V.W. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J. Neurosci. 2003, 23, 11127–11135. [Google Scholar] [PubMed]
- del Zoppo, G.J.; Frankowski, H.; Gu, Y.H.; Osada, T.; Kanazawa, M.; Milner, R.; Wang, X.; Hosomi, N.; Mabuchi, T.; Koziol, J.A. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J. Cereb. Blood Flow Metab. 2012, 32, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Kanazawa, M.; Hung, S.Y.; Wang, X.; Fukuda, S.; Koziol, J.A.; del Zoppo, G.J. Cathepsin L acutely alters microvessel integrity within the neurovascular unit during focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2015, 35, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.; Zhang, J.; Makinson, S.R.; Cahill, M.K.; Kelley, K.W.; Huang, H.Y.; Shang, Y.; Oldham, M.C.; Martens, L.H.; Gao, F.; et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 2016, 165, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Boutin, H.; Murray, K.; Pradillo, J.; Maroy, R.; Smigova, A.; Gerhard, A.; Jones, P.A.; Trigg, W. 18F-GE-180: A novel TSPO radiotracer compared to 11C-R-PK11195 in a preclinical model of stroke. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiart, M.; Davoust, N.; Pialat, J.B.; Desestret, V.; Moucharrafie, S.; Cho, T.H.; Mutin, M.; Langlois, J.B.; Beuf, O.; Honnorat, J.; et al. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke 2007, 38, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.; Coxon, A.; Wang, Y.F.; Frosch, M.P.; Lipton, S.A.; Hickey, P.R.; Mayadas, T.N. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke 1999, 30, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.O.; Tseng, D.; Liao, C.H.; Dorie, M.J.; Czechowicz, A.; Brown, J.M. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA 2010, 107, 8363–8368. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Tang, C.H.; Yeh, W.L.; Wong, K.L.; Lin, C.P.; Chen, Y.H.; Lai, C.H.; Chen, Y.F.; Leung, Y.M.; Fu, W.M. SDF-1alpha up-regulates interleukin-6 through CXCR4, PI3K/Akt, ERK, and NF-kappaB-dependent pathway in microGlia. Eur. J. Pharmacol. 2009, 613, 146–154. [Google Scholar] [CrossRef]
- Hill, W.D.; Hess, D.C.; Martin-Studdard, A.; Carothers, J.J.; Zheng, J.; Hale, D.; Maeda, M.; Fagan, S.C.; Carroll, J.E.; Conway, S.J.; et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: Association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 2004, 63, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.M.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Katakowski, M.; Zhang, L.; Wang, Y.; Zhang, C.; Chopp, M. Stromal cell-derived factor 1a mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Zhao, S.; Zhang, H.; Cai, W.; Cai, M.; Ji, X.; Leak, R.K.; Gao, Y.; Chen, J.; et al. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke 2016, 47, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Narantuya, D.; Nagai, A.; Sheikh, A.M.; Masuda, J.; Kobayashi, S.; Yamaguchi, S.; Kim, S.U. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS ONE 2010, 5, e11746. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, J.; Yu, L.; Ou, C.; Liu, X.; Zhao, X.; Wang, J. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav. Brain Res. 2013, 250, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Womble, T.A.; Green, S.; Shahaduzzaman, M.; Grieco, J.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Monocytes are essential for the neuroprotective effect of human cord blood cells following middle cerebral artery occlusion in rat. Mol. Cell Neurosci. 2014, 59, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Desestret, V.; Riou, A.; Chauveau, F.; Cho, T.H.; Devillard, E.; Marinescu, M.; Ferrera, R.; Rey, C.; Chanal, M.; Angoulvant, D.; et al. In vitro and in vivo models of cerebral ischemia show discrepancy in therapeutic effects of M2 macrophages. PLoS ONE 2013, 8, e67063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wang, H.; Sun, G.; Zhang, J.; Edwards, N.J.; Aronowski, J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J. Neurosci. 2015, 35, 11281–11291. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Hutchings, S.; Schlichter, L.C. Molecular and cellular responses to interleukin-4 treatment in a rat model of transient ischemia. J. Neuropathol. Exp. Neurol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Strecker, J.K.; Hucke, S.; Bruckmann, N.M.; Herold, M.; Mack, M.; Diederich, K.; Schäbitz, W.R.; Wiendl, H.; Klotz, L.; et al. Targeting different monocyte/macrophage subsets has no impact on outcome in experimental stroke. Stroke 2017, 48, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Khopkar, D.; Paranjape, A.; Sundaram, J.; Gandhi, S.; Badhe, P. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res. Treat. 2014, 2014, 234095. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, E.R.; Shevela, E.Y.; Starostina, N.M.; Morozov, S.A.; Davydova, M.N.; Menyaeva, E.V.; Ostanin, A.A. Safety and therapeutic potential of M2 macrophages in stroke treatment. Cell Transplant. 2016, 25, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Sakai, C.; Soma, T.; Kasahara, Y.; Stern, D.M.; Kajimoto, K.; Ihara, M.; Daimon, T.; Yamahara, K.; Doi, K.; et al. Intravenous autologous bone marrow mononuclear cell transplantation for stroke: Phase1/2a clinical trial in a homogeneous group of stroke patients. Stem Cells Dev. 2015, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.A.; Martins, M.P.; Araujo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; Sartori El Ammar, J.; Machado, D.C.; Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012, Suppl 1, S13–S21. [Google Scholar] [CrossRef]
- Honmou, O.; Houkin, K.; Matsunaga, T.; Niitsu, Y.; Ishiai, S.; Onodera, R.; Waxman, S.G.; Kocsis, J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011, 134, 1790–1807. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chen, J.; Lin, S.; Wang, P.; Wang, Y.; Xiong, X.; Yang, Q. CD36-mediated hematoma absorption following intracerebral hemorrhage: Negative regulation by TLR4 aignaling. J. Immunol. 2014, 192, 5984–5992. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Ozinsky, A.; Hajjar, A.M.; Stevens, A.; Wilson, C.B.; Bassetti, M.; Aderem, A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 1999, 401, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Abiega, O.; Shahraz, A.; Neumann, H. Janus-faced microglia: Beneficial and detrimental consequences of microglial phagocytosis. Front. Cell Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 5, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Faustino, J.V.; Wang, X.; Johnson, C.E.; Klibanov, A.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J. Neurosci. 2011, 31, 12992–13001. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Mao, S.; Xi, G.; Keep, R.F.; Hua, Y. Role of erythrocyte CD47 in intracerebral hematoma clearance. Stroke 2016, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- De Bilbao, F.; Arsenijevic, D.; Moll, T.; Garcia-Gabay, I.; Vallet, P.; Langhans, W.; Giannakopoulos, P. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J. Neurochem. 2009, 110, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Schaer, C.A.; Schoedon, G.; Imhof, A.; Kurrer, M.O.; Schaer, D.J. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ. Res. 2006, 99, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hao, J.; Zhang, N.; Ren, L.; Sun, N.; Li, Y.J.; Yan, Y.; Huang, D.; Yu, C.; Shi, F.D. Fingolimod for the treatment of intracerebral hemorrhage: A 2-arm proof-of-concept study. JAMA Neurol. 2014, 71, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, W.; Zhu, C.; Kong, Z.; Xu, Y.; Liu, G.; Gao, X. The clinical effect of deferoxamine mesylate on edema after intracerebral hemorrhage. PLoS ONE 2015, 10, e0122371. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zheng, K.; Su, Z.; Su, H.; Zhong, M.; He, X.; Zhou, C.; Chen, H.; Xiong, Q.; Zhang, Y. Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage. J. Neuroimmunol. 2016, 299, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.H.; Aubourg, P.; Ransohoff, R.M.; et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Parsha, K.; Schaar, K.; Satani, N.; Xi, X.; Aronowski, J.; Savitz, S.I. Cryopreservation of bone marrow mononuclear cells alters their viability and subpopulation composition but not their treatment effects in a rodent stroke model. Stem Cells Int. 2016, 5876836. [Google Scholar] [CrossRef] [PubMed]
- Ohgidani, M.; Kato, T.A.; Kanba, S. Introducing directly induced microglia-like (iMG) cells from fresh human monocytes: A novel translational research tool for psychiatric disorders. Front. Cell Neurosci. 2015, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 2017, 94, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Cox, C.S. Concise review: Cell therapies for stroke and traumatic brain injury: Targeting microGlia. Stem Cells 2016, 34, 537–542. [Google Scholar] [CrossRef] [PubMed]
| Reference | Source | Polarization | Stimuli | Comments |
|---|---|---|---|---|
| Kanazawa, et al. Sci Rep 2017 [28] | Primary microglia | M2-like microglia | OGD | Improving outcome by axonal outgrowth and angiogenesis Upregulation of VEGF, TGF-β and MMP-9 |
| Wattananit, et al. J Neurosci 2016 [34] | Monocyte-derived macrophages | M2-like macrophage | None | Improving outcome |
| Womble, et al. Molecular Cell Neurosci 2014 [97] | Umbilical cord blood mononuclear cells | No polar | None | Reduced infarct volume and improving outcome |
| Desestret, et al. PLoS ONE 2013 [98] | Bone marrow-derived monocytes | M2-like macrophage | IL-4 | Not reduced infarct volume and no improving outcome |
| Jiang, et al. Brain Res 2013 [96] | Bone marrow-derived mononuclear cells Primary microglia | No polar | None | Reduced infarct volume and improving outcome by mononuclear cells No improving outcome by microglia |
| Narantuya, et al. PLoS ONE 2010 [95] | Microglial cell line, HMO6 | No polar | None | Reduced infarct volume and improving outcome |
| Imai, et al. JCBFM 2007 [24] | Primary microglia | No polar | None | Inhibition of neuronal cell death Upregulation of BDNF and GDNF |
| Reference | ClinicalTrials.gov Identifier | Source | Polarization | Stimuli | Comments |
|---|---|---|---|---|---|
| Prasad, et al. Stroke 2016 [102] | NCT01501773 | Autologous bone marrow stem cell | No polar | None | No beneficial effect |
| Sharma, et al. Stroke Res Treat 2014 [103] | NCT02065778 | Autologous bone marrow mononuclear cell | No polar | None | Improving outcome |
| NCT00950521 | Autologous peripheral blood stem cell (CD34+) | No polar | None | Not reported results | |
| NCT00473057 | Autologous bone marrow cell | No polar | None | Not reported results | |
| Chernykh, et al. Cell Transplant 2016 [104] | - | Autologous blood mononuclear cell | M2-like macrophage | GM-CSF | Improving outcome |
| Taguchi, et al. Stem Cell Dev 2015 [105] | - | Autologous bone marrow mononuclear cell | No polar | None | Improving outcome |
| Friedrich, et al. Cell Transplant 2012 [106] | - | Autologous blood mononuclear cell | No polar | None | Improving outcome |
| Honmou, et al. Brain 2011 [107] | - | Autologous mesenchymal stem cell | No polar | None | Improving outcome |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. https://doi.org/10.3390/ijms18102135
Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. International Journal of Molecular Sciences. 2017; 18(10):2135. https://doi.org/10.3390/ijms18102135
Chicago/Turabian StyleKanazawa, Masato, Itaru Ninomiya, Masahiro Hatakeyama, Tetsuya Takahashi, and Takayoshi Shimohata. 2017. "Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke" International Journal of Molecular Sciences 18, no. 10: 2135. https://doi.org/10.3390/ijms18102135
APA StyleKanazawa, M., Ninomiya, I., Hatakeyama, M., Takahashi, T., & Shimohata, T. (2017). Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. International Journal of Molecular Sciences, 18(10), 2135. https://doi.org/10.3390/ijms18102135